Abstract
MyD88 is an adaptor protein critical for innate immune response against microbial infection and in certain noninfectious tissue injury. The present study examined the role of MyD88 in myocardial inflammation and injury after ischemia-reperfusion (I/R). I/R was produced by coronary artery ligation for 30 min followed by reperfusion. The ratios of area at risk to left ventricle (LV) were similar between wild-type (WT) and MyD88-deficient (MyD88−/−) mice. However, 24 h after I/R, the ratios of myocardial infarction to area at risk were 58% less in MyD88−/− than in WT mice (14 ± 2% vs. 33 ± 6%, P = 0.01). Serial echocardiographic studies demonstrated that there was no difference in baseline LV contractile function between the two groups. Twenty-four hours after I/R, LV ejection fraction (EF) and fractional shortening (FS) in WT mice were reduced by 44% and 62% (EF, 51 ± 2%, and FS, 22 ± 1%, P < 0.001), respectively, and remained depressed on the seventh day after I/R. In comparison, EF and FS in MyD88−/− mice were 67 ± 3% and 33 ± 2%, respectively, after I/R (P < 0.001 vs. WT). Similarly, LV function, as demonstrated by invasive hemodynamic measurements, was better preserved in MyD88−/− compared with WT mice after I/R. Furthermore, when compared with WT mice, MyD88−/− mice subjected to I/R had a marked decrease in myocardial inflammation as demonstrated by attenuated neutrophil recruitment and decreased expression of the proinflammatory mediators keratinocyte chemoattractant, monocyte chemoattractant protein-1, and ICAM-1. Taken together, these data suggest that MyD88 modulates myocardial inflammatory injury and contributes to myocardial infarction and LV dysfunction during I/R.
Keywords: myocardial infarction, inflammation, echocardiography, innate immune system
myocardial ischemia-reperfusion (I/R) leads to significant myocardial inflammation and injury. Evidence from several lines of investigation suggests that inflammation may be an important functional contributor to the pathogenesis of ischemic myocardial injury (11, 15, 48) although some anti-inflammatory interventions have yielded disappointing results (38, 48).
Following myocardial I/R, there is a significant inflammatory response that leads to downstream activation of multiple soluble and cellular factors such as the activation of endothelial cells, release of chemoattractants (e.g., reactive oxygen species, cytokines, and the activated complements) and adhesion molecules, increased vascular permeability, and rapid recruitment of neutrophils into ischemic myocardium. Although many of the downstream events leading to inflammatory injury have been identified (48), the proximal signaling mechanisms that control the critical events during I/R remain incompletely defined.
Innate immune systems such as those mediated via Toll-like receptors (TLRs) represent the first line of defense against microbial infection. There are at least 10 TLRs identified so far, and they recognize and specifically bind to a variety of pathogenic agonists such as lipopolysaccharide (LPS) (via TLR4), lipopeptide (via TLR2), flagellin (via TLR5), cytosine-phosphorothioate-guanine DNA (via TLR9), and dsRNA (via TLR3) by “molecular pattern recognition” (22). In addition to their pivotal role in host immune defense, recent studies have demonstrated that TLRs can recognize endogenous mediators and modulate tissue inflammation and injury in response to noninfectious injury in the lung (20), liver (37), and heart (7, 35, 42, 46).
The heart expresses at least three receptors involved in TLR signaling, CD14, TLR2, and TLR4 (12, 13, 25, 34, 51, 52). These receptors are in part responsible for cardiac dysfunction in some pathological conditions such as endotoxemia (25, 34) and peptidoglycan-associated lipoprotein (51). The role of TLRs in ischemic myocardial injury is, however, incompletely defined, and prior studies seem conflicting. For example, systemic TLR4 activation by LPS reduced subsequent ischemic myocardial infarction (MI) and improved cardiac functions both in vivo and in isolated hearts (3, 5, 26, 29, 31, 43, 47, 49, 50). The activation of TLR4-MyD88 signaling also protects cardiomyocytes against apoptosis and improved cardiomyocyte functions (6, 52). However, in the absence of systemic TLR4 stimulation, mice deficient for TLR4 exhibited reduced myocardial inflammation and infarction in an in vivo model of I/R injury compared with WT mice, suggesting that TLR4 may mediate ischemic injury in the heart (7, 35). Interestingly, TLR2 deficiency led to a reduced myocardial remodeling with improved LV function but had no impact on the infarct size and degree of inflammation in the heart after ischemia (42).
MyD88, originally isolated as one of the 12 myeloid differentiation primary response genes (27), is an adaptor protein that is critical for transducing signals from all 10 TLR family members (19, 22, 30, 33), except TLR3, and interleukin-1 (IL-1) receptor family members, including IL-1R and IL-18R (1, 10). MyD88 has a NH2-terminal death domain (DD) and a COOH-terminal Toll/IL-1R-related domain, which interacts with another Toll/IL-1R-related domain found in TLRs. Upon binding to TLRs and IL-1R, MyD88 recruits the downstream kinase IL-1 receptor-associated kinases via their DD-DD interaction. Like TLR4-deficient mice (36), MyD88−/− mice (21, 52) lack the ability to respond to LPS although MyD88-independent pathways (e.g., Toll/IL-1 receptor domain containing adaptor inducing IFN-β production) exist in TLR4 signaling (23).
The role of MyD88 in cell death and tissue injury is incompletely understood, and prior results have been conflicting (2, 20). Using MyD88−/− mice, Jiang et al. (20) have recently found that MyD88 mediates an survival signal in lung epithelial cells and plays a critical role in tissue repair as well as inflammation in an acute lung injury model. In cardiomyocytes, we found that although MyD88 deficiency has no impact on cardiomyocyte death in either normal or apoptosis-inducing conditions, it is essential for TLR4-activated survival in mouse cardiomyocytes (52). Moreover, although adenovirus-mediated expression of MyD88 modulates TLR2-induced cytokine production, MyD88 overexpression without upstream TLR2 activation is not sufficient to produce survival benefit (unpublished data) or cytokine production in cardiomyocytes (51). However, other investigators have reported that the overexpression of dominant negative mutant of MyD88 leads to reduced cardiomyocyte apoptosis and tissue injury (16).
Given its critical role in innate immune signaling and the demonstrated roles of TLR4 and TLR2 in ischemic myocardial injury and remodeling, we set out to test the hypothesis that signaling via MyD88 is an important determinant of consequences after ischemic myocardial injury and contributes critically to myocardial inflammatory injury and LV dysfunction after I/R.
METHODS
Animal Models
All animal experiments were performed with the approval of the Animal Care Committee of Massachusetts General Hospital. MyD88−/− mice were provided by Dr. Mason Freeman at Massachusetts General Hospital and backcrossed at least 6 generations into the C57BL/6J strain.
I/R injury model.
Male mice (8–12 wk old; 25–30 g) were anesthetized with pentobarbital sodium, intubated, and ventilated in a volume-control mode (inspired O2 fraction = 1.0, 10 μl/g, and 120/min). Mouse body temperature was maintained within normal limit (35–37°C) with a heating pad. The left hemi-thorax was shaved. A left thoracotomy was then performed, and the left anterior descending coronary artery (LAD) was ligated with 1 silk suture (7.0) 1 to 2 mm from its origin with a slipknot. Ischemia was confirmed by myocardial blanching and lead II ECG changes. After 30 min of ischemia, the LAD ligature was released, and reperfusion was visually confirmed. The overall mortality rates of the I/R injury model were <10% and were similar between the WT and MyD88−/− groups. To calculate the area at risk (AAR) 24 h after reperfusion, the LAD was religated using the same sutures. Fluorescent microspheres (Molecular Probes) were injected into the LV cavity with the aorta transiently clamped to increase coronary artery flow and reduce microsphere circulating into systemic circulation. In sham-operated animals, a suture was passed under the LAD but not tied.
Ex vivo model of I/R injury.
Mice were heparinized (1,000 IU/kg ip) and euthanized with pentobarbital sodium. The hearts were excised and immersed in ice-cold perfusion buffer. Aortae were cannulated and retrograde perfused at a constant rate (3 ml/min) with modified Krebs-Henseleit buffer containing (in mM) 118 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11.1 glucose, and 2 CaCl2, equilibrated with 95% O2-5% CO2 at a pH of 7.4 at 37°C. A 1.4-Fr Millar pressure transducer catheter (SPR-671) was directly inserted into the LV after a left atrial incision was made to expose the mitral annulus for recording the LV end-systolic pressure (LVESP), the LV end-diastolic pressure (LVEDP), and heart rate (PowerLab, ADInstruments). LV developed pressure (LVDP) was calculated as LVDP = LVESP − LVEDP. The maximum rate of rise of the LV pressure (dP/dtmax) and the maximum isovolumetric rate of relaxation (dP/dtmin) were calculated. After 10 min of control perfusion in a Langendorff perfusion system, the hearts were exposed to 20 min of global ischemia followed by 40 min of reperfusion.
Measurement of AAR and MI
Twenty-four hours after I/R, mice were euthanized and the hearts isolated and sectioned from apex to base into four 2-mm sections. Under a fluorescent microscope, the perfused myocardium was identified as the areas filled with red microsphere and the AAR identified as areas devoid of red microsphere. To examine MI, the sections were incubated in 1% (wt/vol) triphenyltetrazolium chloride (TTC) in Tris·HCl (pH 7.4) at 30°C for 10 min followed by fixation with 4% paraformadehyde. For each section, the AAR and MI areas were measured from enlarged digital microscopic images using Adobe Photoshop. The percentage of MI/AAR was calculated as the infarction area (MI/LV × 100%) divided by the AAR(AAR/LV × 100%).
Echocardiography in Mice
On days 1, 3, and 7 after I/R, mice were lightly anesthetized with ketamine (0.016 mg/g). Ultrasonic transmission gel was applied to the thorax. Transthoracic echocardiograms were obtained and interpreted by an echocardiographer (X. Xu) blinded to the experimental groups using a 13.0-MHz linear probe (Vivid 7, GE Medical System, Milwaukee, WI). LV end-diastolic and -systolic dimensions (LVID and LVIS, respectively) were measured on an M-mode obtained from a parasternal short-axis view at midpapillary level. The fractional shortening (FS) was defined as (LVID − LVIS)/LVID. The ejection fraction (EF) was calculated from the M-mode (LVID3 − LVIS3) (9, 14).
Measurements of Tail-Cuff Blood Pressure in Awake Mice
Systolic blood pressure was measured with a noninvasive tail-cuff machine (XBP 1000, Kent Scientific, Torrington, CT) in awake WT and MyD88−/− mice. Mice were first subjected to one or two practice sessions to acclimate to the device. Briefly, the mouse was initially placed in a restrainer for a short period (approximately <1 min to start) and then maintained in the restrainer for longer times to acclimate to the device. The degree of acclimatization was judged by the absence of agitation in the device. After a few days of practice sessions, mice remained comfortably restrained for longer periods in the restraint device (Kent Scientific).
Invasive Cardiac Hemodynamic Measurements
Three days after I/R, mice were anesthetized by intraperitoneal injection with ketamine (0.1 mg/g), fentanyl (50 ng/g), and pancuronium (0.002 mg/g); intubated; and mechanically ventilated. An SPR-839 pressure-volume Millar catheter (Millar Instruments, Houston, TX) was inserted into the left carotid artery and advanced into the LV. Heart rate and LVESP and LVEDP were measured, and dP/dtmax and dP/dtmin were calculated (14, 40).
Immunohistochemistry
Twenty-four hours after I/R or sham operation, hearts were immersed in freeze medium in plastic cubes, frozen in liquid N2, and cut from apex to base into 12-μm thin sections. The sections were stained with hematoxylin and eosin to examine myocardial morphology and any evidence of inflammatory cell infiltration. Immunohistochemical staining of neutrophils was performed using immunoperoxidase detecting systems (Vector). Myocardial neutrophils were detected using a biotin-labeled-specific rat anti-mouse Ly-6G/Ly-6C antibody (Gr-1, Clone RB6–8C5, BD Pharmingen, 1:500). Biotin signals were then detected using horseradish peroxidase-coupled streptavidin (ABC reagent, Vector) and AEC peroxidase substrate (32).
Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was measured with a MPO assay kit according to the manufacturer's recommendations (Cytostore, Calgary, Alberta, Canada).
RNA Extraction and Quantitative RT-PCR Analysis
Total RNA was extracted from mouse myocardial tissues using TRIzol reagent, and cDNA was synthesized by reverse-transcriptase reaction. cDNA gene sequences corresponding to 18S rRNA were amplified by PCR and quantitated using an ABI Prism 7000 (Applied Biosystems) with the forward primer 5′-TCATGTGGTGTTGAGGAAAGC-3′ and the reverse primer 5′-TGGCGTGGATTCTGCATAATG-3′ (52). To detect keratinocyte chemoattractant (KC) transcripts (45), forward primer: 5′-CCG CGCCTATCGCCAATGAGCTGCGC-3′, reverse primer: 5′-CTTGGGGACACCCTT TTAGCATCTTTTGG-3′ were used. For monocyte chemoattractant protein-1 (MCP-1) transcripts, forward primer: 5′-TTAAAAACCTGGATCGGAACCAA-3′, reverse primer: 5′-GCATTAGCTTCAGATTTACGGGT-3′ were used. For ICAM-1 transcripts, forward primer: 5′-GTGATGCTCAGGTATCCATCCA-3′, reverse primer: 5′-CACAGTTCTCAAAGCACAGCG-3′ were used. For macrophage inflammatory protein-1 (MIP-1) transcripts (45), forward primer: 5′-CACCCTCTGTCACCTGCTCAACATC-3′, reverse primer: 5′-GGTTCCTCGCTGCCTCCAAGACTC T-3′ were used. For MIP-2 transcripts (45), forward primer: 5′-CCGCTGTTGTGGCCAGTGAACTGCG-3′, reverse primer: 5′-TTAGCCTTGCCTTTGTTCAGTAT-3′ were used. Changes in relative gene expression normalized to 18S rRNA levels were determined using the relative threshold cycle method (52).
Statistical Analysis
Unless stated otherwise, all data are expressed as means ± SE and analyzed with two-way ANOVA for statistic significance. For the serial echocardiographic studies where multiple measurements were performed, a two-way ANOVA for repeated measures with Bonferroni post hoc tests were used to analyze for statistical significance. The null hypothesis was rejected for P < 0.05.
RESULTS
MyD88−/− Mice Have Smaller MI Sizes Compared with WT Mice after I/R
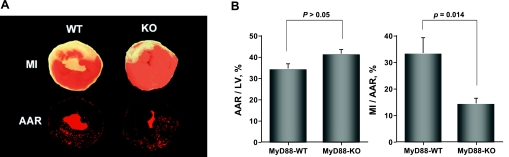
Figure 1A shows representative photographs of myocardial tissues staining with fluorescent microsphere to delineate AAR and with TTC to show myocardial infarct area in WT and MyD88−/− mice subjected to 30 min of ischemia and 24 h of reperfusion. As indicated in Fig. 1B, the ratios of ischemic area (AAR) to LV are similar between WT and MyD88−/− mice. However, the ratios of MI to AAR are decreased by 58% in MyD88−/− mice compared with that in WT mice (14 ± 2% vs. 33 ± 6%, respectively, P < 0.05).
Fig. 1.
Decreased myocardial infarction (MI) in MyD88-knockout (KO) mice subjected to ischemia-reperfusion (I/R) injury. A: representative of triphenyltetra zolium chloride staining (top) and fluorescent microsphere distribution (bottom) of myocardial sections from wild-type (WT) and MyD88-KO mice at 24 h of reperfusion. The nonischemic area is indicated by red fluorescent staining, area at risk (AAR) by area devoid of red fluorescent light, and infarct area by white. B: cumulative data of AAR and MI (n = 11 to 12 in each group). LV, left ventricle.
MyD88−/− Mice Have Better Preserved LV Function Compared with WT Mice after I/R
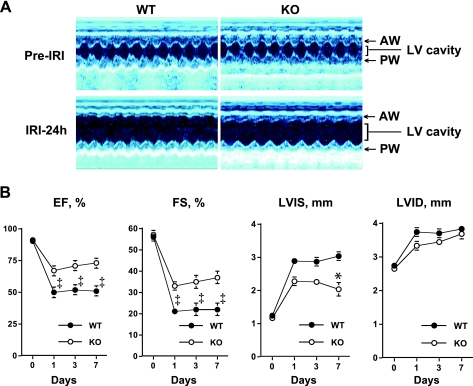
We tested whether or not systemic MyD88 deficiency has an impact on cardiac function after I/R injury in vivo. We first examined the baseline of cardiac function in WT and MyD88−/− mice before I/R. At the cellular level, our previous studies have shown that cardiomyocytes isolated from adult WT and MyD88−/− mice shared similar morphology and sarcomere length and had similar cellular function as measured by calcium transients and sarcomere shortening in response to pacing (52). Consistent with these findings, transthoracic echocardiographic studies indicated that cardiac contractile function was similar between WT and MyD88−/− mice before I/R injury with the EF at 90 ± 1% and 91 ± 1% and the FS at 55 ± 2% and 57 ± 2%, respectively. The LVIS and LVID were similar between the two groups of mice as well (LVIS, 1.2 ± 0.1 vs. 1.1 ± 0.1 mm, and LVID, 2.7 ± 0.1 vs. 2.6 ± 0.1 mm, respectively) (Fig. 2). After 24 h of I/R, the EF and FS in WT mice were decreased by 44% and 62%, respectively (EF, 51 ± 2%, and FS, 22 ± 1%, P < 0.001 vs. baseline) and remained depressed for up to 7 days after reperfusion (EF, 51 ± 1% and FS, 22 ± 1%, P < 0.001). The LVIS and LVID were significantly increased by 133% and 37%, respectively, at 24 h (P < 0.001) and by 150% and 40%, respectively, 7 days (P < 0.001) after reperfusion. In comparison, MyD88−/− mice showed much less ventricular depression after I/R. EF and FS were decreased by 27% and 42%, respectively, at 24 h (EF, 67 ± 3% and FS, 33 ± 2%, P < 0.001 vs. WT) and by 20% and 35% (EF, 73 ± 1%, and FS, 37 ± 1%, P < 0.001 vs. WT), respectively, on day 7 of I/R. LVIS was significantly smaller in the MyD88−/− compared with the WT mice after 7 days of reperfusion (P < 0.01), whereas LVID was similar between the two groups of animals within the same period of time (Fig. 2). These echocardiographic data suggest that cardiac contractile function was better preserved in MyD88−/− mice compared with that in WT mice after I/R. We also examined mouse cardiac function using invasive hemodynamic measurements after I/R. As shown in Table 1, the baseline cardiac functions of WT and MyD88−/− mice were similar. Three days after I/R, LV dP/dtmax and dP/dtmin were remarkably reduced indicating attenuated LV function. However, dP/dtmax and dP/dtmin were significantly higher in mice deficient for MyD88 than that in WT mice, suggesting a significant improvement in both LV systolic and diastolic function in the MyD88−/− mice. The baseline of mean arterial blood pressure was similar between the two mouse strains (Table 2). Six hours after surgery, the mean arterial blood pressure was significantly reduced in both sham-operated and I/R mice and to a similar degree in both WT and knockout mice, suggesting that the reduction in the mean blood pressure was mainly due to the thoracotomy procedure and not I/R injury.
Fig. 2.
MyD88-deficient (MyD88−/−) mice have better preserved cardiac function after I/R injury (IRI). All mice underwent 30 min of myocardial ischemia and various periods of reperfusion (1, 3, and 7 days). A: representative M-mode echocardiogram of WT and KO mice before and 24 h after I/R injury. B: quantitative data of echocardiographic measurements. Serial echocardiograms were measured before and 1, 3, and 7 days after I/R (n = 12 to 13). *P < 0.01 and ‡P < 0.001 vs. WT mice. EF, ejection fraction; FS, fraction shortening; LVIS, left ventricular dimensions at end systole; LVID, left ventricular dimensions at end-diastole; AW, anterior wall; PW, posterior wall.
Table 1.
Invasive hemodynamic measurements of WT and MyD88−/− mice at baseline and 3 days after I/R
| Baseline |
I/R | |||
|---|---|---|---|---|
| WT | MyD88−/− | WT | MyD88−/− | |
| LV mass/BW, mg/g | 3.0±0.1 | 2.9±0.1 | 3.5±0.2 | 3.4±0.1 |
| HR, beats/min | 625±13 | 619±20 | 647±12 | 648±22 |
| LVESP, mmHg | 118±2 | 115±1 | 63±2† | 68±2 |
| LVEDP, mmHg | 4±1 | 4±1 | 4±0.3 | 4±0.3 |
| LV dP/dtmax, mmHg/s | 14,600±1,300 | 15,000±1,800 | 5,447±514† | 7,853±524* |
| LV dP/dtmin, mmHg/s | −15,100±800 | −14,700±700 | −5,370±424† | −7,239±526* |
Values are means ± SE; n = 5 mice in each group. The baseline cardiac functions were measured in 12–16 wk-old mice without left anterior descending coronary artery (LAD) ligation. Separate groups of mice were subjected to LAD ligation for 30 min followed by days of reperfusion {ischemia-reperfusion (I/R)} as described in methods. LV, left ventricular; BW, body weight; HR, heart rate; LVESP, LV end-systolic pressure; LVEDP, LV end-diastolic pressure; +dP/dtmax, the maximum first derivative of developed LV pressure; −dP/dtmax, the minimum first derivative of developed LV pressure.
P < 0.05 vs. wild-type (WT) I/R;
P < 0.01 vs. WT baseline.
Table 2.
Mean arterial BP of WT and MyD88−/− mice before and after surgery
| Sham, mmHg |
I/R, mmHg | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| WT | 106±8 | 59±5* | 99±6 | 57±5* |
| MyD88−/− | 101±12 | 65±2* | 105±7 | 61±6* |
Values are means ± SE; n = 5–7 mice. Blood pressure (BP) was measured in awake WT and MyD88−/− mice before and 6 h after either sham operation or I/R injury using tail-cuff method (see methods)
P < 0.01 vs. baseline BPs before surgery.
MyD88 Deficiency Has No Impact on MI Sizes in Isolated Mouse Hearts after Global I/R
To avoid the potential systemic contributors associated with extracardiac MyD88 deficiency in the setting of in vivo I/R, we isolated and subjected mouse hearts to I/R in a Langendorff perfusion system. Again, similar to the in vivo model of I/R, there was no difference in the baseline LV functions between isolated WT and MyD88−/− hearts (Table 3). Global I/R in isolated hearts induced 30 ± 4% (MI/LV) MI in WT mice and 26 ± 3% MI in MyD88−/− mice (n = 8, P > 0.05) and similar levels of cardiac contractile dysfunction in both groups. After 20 min ischemia and 40 min reperfusion, the cardiac function of the hearts isolated from WT and MyD88 were significantly reduced but similar between the two groups of mice (Table 3).
Table 3.
Cardiac function of ex vivo perfused hearts from WT and MyD88−/− mice
| Pre-I/R |
I/R | |||
|---|---|---|---|---|
| WT | MyD88−/− | WT | MyD88−/− | |
| HR, beats/min | 390±11 | 380±13 | 375±6 | 367±8 |
| LVESP, mmHg | 68±6 | 72±7 | 49±5 | 49±4 |
| LVEDP, mmHg | 2±2 | 3±1 | 7±2 | 3±2 |
| LVDP, mmHg | 66±6 | 70±7 | 42±3 | 45±2 |
| LVRPP, mmHg/min | 25,740±2,080 | 26,600±2,350 | 15,750±1,159 | 16,515±980 |
| LV dP/dtmax, mmHg/s | 2,470±265 | 2,430±290 | 1,439±86 | 1,513±123 |
| LV dP/dtmin, mmHg/s | 2,046±207 | 2,102±240 | 1,171±138 | 1,043±146 |
Values are means ± SE; n = 8 mice in each group. After 10 min of control perfusion in a Langendorff perfusion system, the baseline cardiac functions were recorded (Pre-I/R). The isolated hearts were then exposed to 20 min of global ischemia followed by 40 min of reperfusion. LVDP, LV developing pressure (LVESP − LVEDP); LVRPP, LV rate-pressure product (HR × LVDP).
Myocardial Neutrophil Recruitment Is Impaired in MyD88−/− Mice Subjected to I/R
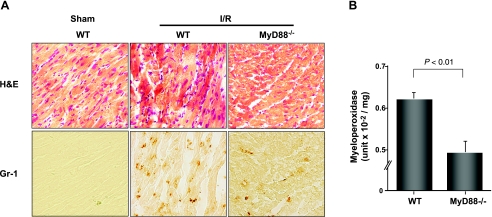
Thirty minutes of ischemia followed by 24 h of reperfusion induced significant neutrophil infiltration in myocardium in WT mice compared with the sham-operated mice as demonstrated by hematoxylin and eosin staining and immunohistochemical staining with GR-1, a specific antibody for neutrophils. However, in MyD88−/− mice subjected to the same I/R protocol, the level of neutrophil recruitment was much lower compared with that in WT mice (Fig. 3A). Moreover, MPO activity, a marker of neutrophil function, was significantly decreased in the hearts of MyD88−/− mice compared with WT mice after I/R (Fig. 3B). The decrease in the myocardial neutrophil recruitment in MyD88−/− mice after I/R was not due to the altered level of circulating neutrophils since the numbers of blood neutrophils were similar between WT and MyD88−/− mice (data not shown). Together, these data suggest that myocardial neutrophil recruitment was significantly impaired in MyD88−/− mice compared with WT mice subjected to I/R.
Fig. 3.
MyD88−/− mice have a marked reduction in neutrophil recruitment after I/R. Mice were subjected to 30 min ischemia and 24 h of reperfusion. A: representative photomicrographs (×400) of myocardial sections labeled with hematoxylin and eosin (H&E) stain and anti-Gr-1 mAb (immunoperoxidase method). B: myeloperoxidase (MPO) activity (units × 10−2/mg; n = 8). MPO values for sham-operated hearts were 0.46 ± 0.03 for WT and 0.33 ± 0.07 units × 10−2/mg for MyD88−/− mice. P = 0.14 (not significant).
MyD88 Mediates the Myocardial Production of Proinflammatory Mediators after I/R
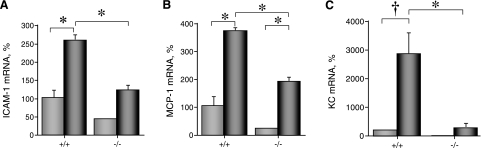
We examined the myocardial levels of ICAM-1, MCP-1, KC, and MIP-1/2, key proinflammatory mediators critical for neutrophil recruitment, in WT and MyD88−/− mice. The baseline levels of ICAM-1, MCP-1, KC, and MIP-1/2 were similar in sham-operated WT and MyD88−/− mice (P > 0.05). Six hours after I/R, there was a significant increase in the levels of MCP-1 and KC transcripts in WT mice compared with those in sham-operated control WT mice (432 ± 103% and 508 ± 124%, respectively, P < 0.05, n = 6). However, MyD88−/− mice had a significantly lower level of KC (174 ± 37%, P < 0.05, n = 6). Twenty-four hours after I/R, there was a significant increase in the level of ICAM-1, MCP-1, and KC transcripts in WT mice compared with the level in sham-operated control WT mice (258 ± 17%, 381 ± 92%, and 2,859 ± 753%, respectively) (Fig. 4). In contrast, MyD88−/− mice had significantly lower levels of ICAM-1, MCP-1, and KC expression after I/R (120 ± 17%, 180 ± 16%, and 267 ± 190%, respectively) compared with those of WT mice subjected to I/R (Fig. 4). There was no significant increase in the level of myocardial MIP-1/2 transcripts after 24 h of I/R or any difference between the two groups of animals (data not shown).
Fig. 4.
MyD88 deficiency attenuates expression of proinflammatory mediators in the hearts after I/R injury. Both WT and MyD88−/− mice were subjected to either sham operation (light gray bars) or I/R (30 min of ischemia and 24 h of reperfusion) (dark gray bars). At the end of reperfusion, the animals were euthanized and the cardiac tissues processed for RNA extraction as described in methods. ICAM-1 (A), monocyte chemoattractant protein-1 (MCP-1; B), and keratinocyte chemoattractant (KC; C) transcripts were analyzed with quantitative RT-PCR (n = 3–5). *P < 0.05; †P < 0.01.
DISCUSSION
It is well documented that inflammation is an important functional contributor to the pathogenesis of ischemic myocardial injury (11, 15, 48) and that the innate immune response to I/R is, by far, the most common cause of myocardial inflammation. Given its critical role in the signal transduction of many innate immune receptors (22), we hypothesized that MyD88 signaling is an important determinant in ischemic myocardial injury. Using a mouse model of I/R injury, we demonstrate that MyD88 is critical for myocardial neutrophil recruitment and proinflammatory mediator production after I/R and significantly contributes to ischemic MI and LV dysfunction.
Emerging evidence from various animal models of tissue injury suggest that TLR-MyD88 signaling plays a critical role in modulating tissue inflammation and injury in the absence of microbial infection. For example, in an acute lung injury model, hyaluronan, produced in response to lung injury, induces a proinflammatory and an antiapoptotic effect in lung epithelial cells via both TLR2- and TLR4-MyD88-dependent mechanisms (20), suggesting a role for TLR-MyD88 signaling in tissue repair of acute lung injury. However, the role of TLR signaling in myocardial I/R injury is complex and incompletely defined. Systemic administration of LPS, which signals through TLR4 and MyD88, reduces subsequent ischemic MI and improves cardiac function in both in vivo and ex vivo models of I/R injury (3, 5, 26, 29, 31, 43, 47, 49, 50). TLR4 signaling also protects cardiomyocytes from apoptosis via MyD88- and inducible nitric oxide synthase-dependent mechanisms in vitro (6, 52). However, given the multiple systemic reactions in response to in vivo administration of LPS, it is unclear whether the observed cardiac benefits are direct results of TLR4 signaling in the heart or due to other events secondary to systemic activation of the innate immune system. In contrast, in the absence of systemic TLR4 stimulation, mice deficient for TLR4 (7, 35) exhibited reduced myocardial inflammation and infarction compared with WT, suggesting that TLR4 may mediate ischemic injury in the heart. Consistent with these findings, the present study demonstrates that systemic MyD88 deficiency leads to a significant reduction in MI size and impairment in myocardial neutrophil recruitment and proinflammatory mediator production after I/R. Using serial transthoracic echocardiographic and invasive hemodynamic measurements, the present studies also demonstrate that the LV contractile function is better preserved in MyD88−/− mice compared with WT mice as early as 24 h and up to 7 days after I/R. Moreover, in an effort to determine the potential impact of extracardiac MyD88 deficiency on cardiac injury associated with the in vivo model of I/R, we tested whether or not MyD88 deficiency has any effect on myocardial injury in isolated mouse hearts. We found that in isolated hearts subjected to global I/R, MyD88 deficiency has no direct impact on MI and cardiac function following ex vivo I/R. This finding suggests that in the in vivo condition, MyD88 may play a pivotal role in mediating myocardial inflammation that is critical for I/R injury and is consistent with the hypothesis that the cardiac benefits observed in MyD88−/− mice in vivo may require circulating blood components during I/R.
Evidence from several lines of investigation suggests that inflammation is an important functional contributor to the pathogenesis of ischemic MI (11, 15). In animal models, neutrophil depletion with antibodies (39) or physical filtering (8, 18) as well as inhibition of neutrophil adhesion with anti-CD18 mAb (44), all substantially reduce injury after reperfusion. In addition, interventions targeted at a variety of specific inflammatory mediators such as complement depletion (28) or lipoxygenase inhibitors (41) or antibodies to the proinflammatory cytokine, IL-1 (17), have demonstrated benefits in I/R injury. However, so far, clinical studies investigating the efficacy of anti-inflammatory therapies noted above have failed to show any meaningful cardioprotective effect (48). Moreover, some anti-inflammatory interventions, such as corticosteroids, have yielded disappointing results (38). Failure to translate experimentally effective cardioprotective interventions into clinical therapies is multifactorial. One of these factors could be the use of animal models that do not adequately approximate the clinical setting (4). Interestingly, other investigators have suggested that inflammation may actually play a beneficial role in the healing process after infarction (24). To define the role of MyD88 signaling in controlling the inflammatory processes in the ischemic myocardium, we tested the impact of MyD88 deficiency on myocardial neutrophil recruitment as well as the expression of the NF-κB-dependent chemokine, KC, MCP-1, and MIPs, and the adhesion molecule, ICAM-1, after I/R. We found that both neutrophil recruitment and the expression of KC, MCP-1, and ICAM-1 were attenuated in MyD88−/− mice compared with WT mice after I/R injury. It is unclear, however, whether the decreased myocardial inflammation contributes to, or is the result of, smaller myocardial injury in the MyD88−/− mice. Our finding in isolated perfused heart, a system devoid of circulatory blood cells, that MyD88 deficiency has no impact on myocardial infarction and cardiac function following I/R is consistent with the hypothesis that MyD88 signaling mediates ischemic myocardial injury via mechanisms that are dependent on circulatory blood components such as neutrophils. We speculate that the lack of MyD88 in extracardiac tissues and cells, such as inflammatory cells, could have contributed to the reduction of both myocardial inflammatory injury and LV dysfunction after I/R.
The role of MyD88 in cell survival or death is incompletely understood, and prior results have been conflicting (2, 20). The expression of high levels of exogenous MyD88 in transfected 293 cell line mediates TLR2-induced cell death (2). The overexpression of dominant negative MyD88 led to reduced cardiomyocyte death and injury (16). However, in most cells, the activation of TLR/IL-1R family members does not result in cell death but rather triggers the expression of cell survival and inflammatory genes. For example, MyD88 mediates a cell survival signal in lung epithelial cells and plays a critical role in pulmonary tissue repair (20). Consistent with this observation, we previously reported that although MyD88 deficiency is not sufficient to alter the level of cardiomyocyte apoptosis in vitro, MyD88 is essential for TLR4-activated antiapoptotic signaling in mouse cardiomyocytes (52).
In summary, the present study demonstrates an important role of MyD88 signaling in the pathogenesis of myocardial inflammation, infarction, and cardiac dysfunction after I/R. Systemic MyD88 deficiency leads to attenuated neutrophil recruitment and diminished proinflammatory mediator production, reduced MI, and dramatically improved LV contractile function after I/R injury. The observation that isolated MyD88-deficient hearts are not protected from I/R injury suggests that MyD88 may contributes to ischemic myocardial injury via mechanisms involving systemic components. These studies identify MyD88 as a potential therapeutic target in the management of ischemic myocardial injury.
GRANTS
This study was supported in part by National Institutes of Health Grants HL-04336 and GM-080906 (to W. Chao) and grants from the William Milton Foundation of Harvard University Grant (to W. Chao) and the American Heart Association (to E. S. Buys and W. Chao).
Acknowledgments
We thank Dr. Y. Nagasaka for help with mouse model of I/R injury, Dr. H. Beppu for advice on immunohistochemisry, and Dr. E. Bittner for assistance on statistic analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9: 143–150, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19: 3325–3336, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belosjorow S, Schulz R, Dorge H, Schade FU, Heusch G. Endotoxin and ischemic preconditioning: TNF-α concentration and myocardial infarct development in rabbits. Am J Physiol Heart Circ Physiol 277: H2470–H2475, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Becker L, Gross G, Mentzer R Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA 86: 2516–2520, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem 280: 21997–22005, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol Heart Circ Physiol 251: H314–H323, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Feigenbaum H, Popp RL, Wolfe SB, Troy BL, Pombo JF, Haine CL, Dodge HT. Ultrasound measurements of the left ventricle. A correlative study with angiocardiography. Arch Intern Med 129: 461–467, 1972. [PubMed] [Google Scholar]
- 10.Fitzgerald KA, O'Neill LA. The role of the interleukin-1/Toll-like receptor superfamily in inflammation and host defence. Microbes Infect 2: 933–943, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 276: 5197–5203, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 291: H379–H384, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol 146: 419–428, 1995. [PMC free article] [PubMed] [Google Scholar]
- 16.Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun 338: 1118–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol 38: 1546–1553, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ito BR, Engler RL, del Balzo U. Role of cardiac mast cells in complement C5a-induced myocardial ischemia. Am J Physiol Heart Circ Physiol 264: H1346–H1354, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 27: 474–482, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. TLR signaling. Cell Death Differ 13: 816–825, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167: 5887–5894, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Kloner RA, Fishbein MC, Lew H, Maroko PR, Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation 57: 56–63, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation 106: 2608–2615, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Lipton BP, Delcarpio JB, McDonough KH. Effects of endotoxin on neutrophil-mediated ischemia/reperfusion injury in the rat heart in vivo. Exp Biol Med (Maywood) 226: 320–327, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 5: 1095–1097, 1990. [PubMed] [Google Scholar]
- 28.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest 61: 661–670, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazenot C, Gobeil F, Ribuot C, Regoli D, Godin-Ribuot D. Delayed myocardial protection induced by endotoxin does not involve kinin B(1)-receptors. Br J Pharmacol 131: 740–744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253–258, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Meng X, Ao L, Brown JM, Meldrum DR, Sheridan BC, Cain BS, Banerjee A, Harken AH. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-α. Am J Physiol Heart Circ Physiol 273: H1894–H1902, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24: 79–91, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278: 1612–1615, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol 282: H2316–H2323, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg 202: 407–417, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation 53: I204–I206, 1976. [PubMed] [Google Scholar]
- 39.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67: 1016–1023, 1983. [DOI] [PubMed] [Google Scholar]
- 40.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Shappell SB, Taylor AA, Hughes H, Mitchell JR, Anderson DC, Smith CW. Comparison of antioxidant and nonantioxidant lipoxygenase inhibitors on neutrophil function. Implications for pathogenesis of myocardial reperfusion injury. J Pharmacol Exp Ther 252: 531–538, 1990. [PubMed] [Google Scholar]
- 42.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108: 2905–2910, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Song W, Furman BL, Parratt JR. Delayed protection against ischaemia-induced ventricular arrhythmias and infarct size limitation by the prior administration of Escherichia coli endotoxin. Br J Pharmacol 118: 2157–2163, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka M, Brooks SE, Richard VJ, FitzHarris GP, Stoler RC, Jennings RB, Arfors KE, Reimer KA. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation 87: 526–535, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol 159: 3595–3602, 1997. [PubMed] [Google Scholar]
- 46.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102: 257–264, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Wang YP, Sato C, Mizoguchi K, Yamashita Y, Oe M, Maeta H. Lipopolysaccharide triggers late preconditioning against myocardial infarction via inducible nitric oxide synthase. Cardiovasc Res 56: 33–42, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart: a comparison with endotoxin. Arterioscler Thromb Vasc Biol 20: 1521–1528, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Zacharowski K, Otto M, Hafner G, Chatterjee PK, Thiemermann C. Endotoxin induces a second window of protection in the rat heart as determined by using p-nitro-blue tetrazolium staining, cardiac troponin T release, and histology. Arterioscler Thromb Vasc Biol 19: 2276–2280, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, Hellman J, Schmidt U. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med 35: 886–892, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol 291: H1900–H1909, 2006. [DOI] [PubMed] [Google Scholar]