Abstract
Two key characteristics of the inflammatory response are the recruitment of leukocytes to inflamed tissue as well as changes in vessel permeability. We explored the relationship between these two processes using intravital confocal microscopy in cremasters of anesthetized (65 mg/kg Nembutal ip) mice. We provide direct evidence that intercellular adhesion molecule-1 (ICAM-1) links leukocyte-endothelial cell interactions and changes in solute permeability (Ps). Importantly, we show that arterioles, not just venules, respond to proinflammatory stimuli, thus contributing to microvascular exchange. We identified two independent, ICAM-1-mediated pathways regulating Ps. Under control conditions in wild-type (WT) mice, there is a constitutive PKC-dependent pathway (Ps = 1.0 ± 0.10 and 2.2 ± 0.46 × 10−6 cm/s in arterioles and venules, respectively), which was significantly reduced in ICAM-1 knockout (KO) mice (Ps = 0.54 ± 0.07 and 0.77 ± 0.11 × 10−6 cm/s). The PKC inhibitor bisindolylmaleimid l (1 μmol/l in 0.01% DMSO) decreased Ps in WT mice to levels similar to those in ICAM-1 KO mice. Likewise, a PKC activator (phorbol-12-myristate-acetate; 1 μmol/l in 0.01% DMSO) successfully restored Ps in ICAM-1 KO vessels to be not different from that of the WT controls. On the other hand, during TNF-α-induced inflammation, Ps in WT mice was significantly increased (2-fold in venules and 2.5-fold in arterioles) in a Src-dependent and PKC-independent manner. The blockade of Src (PP2; 2 μmol/l in 0.01% DMSO) but not PKC significantly reduced the TNF-α-dependent increase in Ps. We conclude that ICAM-1 plays an essential role in the regulation of Ps in microvessels and that there are two separate (constitutive and inducible) signaling pathways that regulate permeability under normal and inflamed conditions.
Keywords: adhesion molecules, microvascular exchange
an inflammatory response is characterized by two major occurrences: leukocyte recruitment to the inflamed tissue and an increase in molecular and hydraulic permeability of the vessel wall. It is established that following proinflammatory stimuli such as TNF-α, leukocyte-endothelial interactions with the endothelium and subsequent leukocyte transmigration are dramatically increased (2, 5, 8). Moreover, we and others (25, 39) have shown that with TNF-α stimulation, leukocyte-endothelial cell (EC) interactions are increased not only in venules but also in arterioles.
A number of studies have also shown that following TNF-α treatment, microvascular permeability is dramatically increased, allowing greater amounts of water and solutes to leave the microvasculature (9, 11, 17). This is a critical event underlying numerous pathological conditions such as the formation of tissue edema (16) and multiple organ failure (4); thus, not surprisingly, a great effort has been dedicated to understanding the mechanisms that regulate microvascular permeability.
Although biophysical measurements of solute permeability (Ps) have been successfully completed in different in vitro and isolated vessel systems, the signaling pathways and the mechanisms involved in the regulation of microvascular permeability remain unclear. Multiple models have been proposed to explain the changes in Ps. Many intracellular signaling molecules such as Rac and Rho GTPases, Src kinases, and myosin light chain kinases, which in turn affect junctional integrity or cytoskeletal rearrangement via molecules such as paxillin, β-catenin, or focal adhesion kinase, have been implicated in the regulation of a paracellular permeability pathway (15, 26, 30, 43). On the other hand, other molecules such as PKC, Ca2+, nitric oxide, and cAMP play a regulatory role in vesicular or receptor-mediated transcellular pathways (30, 35, 37). Not surprisingly, there is no clear separation between these different mechanisms, and the same molecules have often been shown to play a role in both para- and transcellular mechanisms.
Until recently, the venular microcirculation has been the main focus in understanding leukocyte-EC interactions and vessel barrier function, but with increasing evidence of leukocyte-EC interactions in arterioles (25, 38, 40), the upregulation of key regulatory molecules (38, 39) and the capability of arterioles to change barrier function (20, 44), a role for arterioles during inflammation has become of great interest. Importantly, the differences in leukocyte behavior between arterioles (where no leukocyte-EC interactions are observed under control conditions) and venules (which support leukocyte-EC interactions under control conditions) can be exploited to help us understand the connection between leukocyte-EC interactions and vessel Ps. Moreover, despite the dramatic increase in the expression of intercellular adhesion molecule-1 (ICAM-1) in arterioles following TNF-α treatment, rolling leukocytes do not adhere and transmigrate in arterioles as they do in venules (39). These findings raise the question as to what is the role of ICAM-1 in arterioles if it is not to mediate leukocyte adhesion and transmigration. We recently showed that ICAM-1 mediates leukocyte rolling in inflamed arterioles and confirmed that this is separate from its contribution to leukocyte adhesion and transmigration in venules (38). Continuing this line of thought, in the current work we address the physiological consequences of ICAM-1-mediated leukocyte rolling and the upregulation of ICAM-1 in arterioles.
Leukocyte-EC interactions have been implicated in vascular permeability increases (14, 42), thus it is reasonable to speculate that there might be common signaling pathways involved in the regulation of Ps and leukocyte emigration. A potential candidate that might link these two processes is the key adhesion molecule ICAM-1. ICAM-1 mediates leukocyte-endothelial interactions via binding to the family of β2-integrins and plays a major role in leukocyte adhesion in venules (24, 27, 48) and rolling in arterioles (39). It is constitutively expressed at low levels under control conditions and is highly upregulated following TNF-α treatment in both arterioles and venules (39). Moreover, in vitro studies indicate that the ligation of ICAM-1 on the EC surface results in outside-in signaling, necessary for leukocyte transmigration. For example, ICAM-1 ligation affects EC Ca2+ levels, PKC activation, myosin contractility, and tyrosine-phosphorylation of cytoskeletal proteins, (e.g., Src-kinases) that are involved in the rearrangement of the interendothelial junctions (7, 18, 48). In addition, the expression levels of ICAM-1 can affect Ps (3). There is also evidence that the activation of β2-integrins by using either crosslinking antibodies in vitro (13, 29) or TNF-α treatment in isolated venules (14) with consequent leukocyte adhesion to the endothelium can increase Ps, suggesting that β2-integrin-mediated signaling could play a role in regulating vessel barrier function.
Hence, the major goals of this work were to determine whether ICAM-1 serves as a link between leukocyte-EC interactions and vessel permeability in arterioles and venules and to identify key elements in ICAM-1-mediated signaling pathways leading to changes in Ps. To our knowledge this is the first study that provides insights into the ICAM-1-dependent signaling pathways regulating Ps in intact, blood-perfused microvessels during inflammation.
MATERIALS AND METHODS
Animal preparation.
All procedures were approved by the Institutional Review Board of the University of Rochester. Male wild-type (WT; C57BL6J; Jackson Laboratories), ICAM-1 knockout (KO) mice, (B6.129S4-Icam1tm1Jcgr/J; Jackson Laboratories) or CD18 KO mice (B6.129S7-Itgb2tm1Bay/J; Jackson Laboratories) between 12 and 15 wk old were anesthetized with pentobarbital sodium (65 mg/kg ip) and maintained on supplemental anesthetic as needed via a jugular catheter. An endotracheal tube was inserted to insure a patent airway during the experiment, and body temperature was maintained by placing the animal on a warmer. The cremaster muscle was prepared for intravital microscopy as previously described (22, 38, 39). Briefly, the right cremaster muscle was exteriorized and gently pinned over a quartz pedestal for vizualization by microscopy. During preparation and observation, the tissue was continuously superfused with warmed physiological salt solution (PSS) with the following composition (in mM): 131.9 NaCl, 4.7 KCl, 2.0 CaCl, 1.2 MgSO4, 18 NaHCO3, pH 7.4 at 36°C, and equilibrated with gas containing 0% O2, 5% CO2, and 95% N2 to maintain tissue PO2 <15 torr (22, 38, 39). Upon completion of the protocols, the animal was euthanized by anesthetic overdose.
Drug application.
To induce inflammation, selected animals were locally treated by intrascrotal injection of mouse recombinant TNF-α (0.5 μg TNF-α in 0.25 ml saline; Sigma-Aldrich) 3 h before the start of the surgical preparation (38, 39). Microcirculatory observations were made between 4 and 5 h after TNF-α injection. Where called for in the protocols, rat anti-mouse antibodies (ICAM-1 YN-1/1.7.4, 100 μg in 100 μl PBS; eBioscience; CD18 GAME-46, 100 μg in 100 μl PBS; BD Pharmingen; and rat IgG control isotope R3-34, 100 μg in 100 μl PBS; BD Pharmingen) were administered via a second jugular catheter. Data were collected 30 min after antibody administration. Agonists/antagonists were phorbol 12-myristate 13-acetate (PMA; 1 μM in 0.01% DMSO; Calbiochem), bisindolylmaleimid l (BIM; 1 μM in 0.01% DMSO; Calbiochem), and PP2 (2 μM in 0.01% DMSO; Calbiochem) were added to PSS and superfused onto the tissue for 10 min. PMA and BIM were selected to broadly explore whether there is a general role for PKCs in Ps regulation in these microvessels. Many PKC isomers are expressed in ECs (10, 12, 21). Both of these drugs are not specific to any one of the PKC isomers and do not affect the activity of atypical PKC proteins (10, 12, 21). For permeability measurements, microvessels were perfused with 10 mg/ml BSA in PSS in which 10% was BSA conjugated with Alexa 488 as described in detail previously (34).
Intravital confocal microscopy.
All images were acquired using intravital confocal microscopy as described previously (23, 34, 38, 39). In summary, images were acquired on an Olympus BX61WI microscope through an Olympus PlanF1 immersion objective (10×; 0.65 numerical aperture), allowing a spatial resolution of 1.8 μm. Images were recorded using a DVD recorder (SONY DVO100MD) for offline analysis. Fluorescence images were collected from tissue that was illuminated with a 20-mW Argon laser and were acquired through a Nipkow disk-scanning confocal head (CSU 10; Yokogawa) connected to an intensified CCD camera (XR Mega10; Stanford Photonics). Laser power and camera gain settings were held constant throughout all the experiments. We also verified that the relationship between measured intensity and the concentration of fluorochrome was linear using this acquisition system (r2 was between 0.996 and 0.999 for the range of gain settings used in our acquisition system).
Permeability measurement.
Ps in intact cremaster muscle microvessels was measured using the approach previously published from our laboratory and fully detailed elsewhere (34). Briefly, the Ps measurement technique is based on the expectation that fluorescently tagged albumin will move from the lumen of the vessel into the tissue in proportion to the movement of unlabeled albumin. Fluorescent intensity from the selected microvessel and surrounding tissue are measured and used to calculate the solute flux (Js) per unit surface area (S) and concentration gradient (ΔC) using the relation: Ps = Js/SΔC = 1/ΔI0(dIf/dt)i(D/4), where ΔI0 is the fluorescence intensity of the test solute filling the vessel, (dIf/dt) is the initial change in fluorescence intensity in the measured conditions as labeled BSA moves across the vessel, and D is the microvessel diameter (19). The measured vessel volume that acts as a source of BSA-488 was corrected from an assumed cylindrical blood vessel to that appropriate to the measured confocal slice as previously described (34).
To measure Ps in blood-perfused mouse microvessels, a feed arteriole to the microvascular network was cannulated (using a triple-beveled micropipette to facilitate the penetration of the tissue and microvessel wall) and perfused with the fluorescent BSA solution. The perfusion of selected portions of the network downstream of the cannulation site was achieved by raising the pressure in the pressure reservoir connected to the cannulating pipette and by positioning a second, blunted glass microoccluding rod upstream of the cannulation site to control the direction of the flow in the network. In each preparation, the targeted vessel was visualized using confocal fluorescent imaging; it was recorded continuously for 3–6 s (baseline), during 1 min perfusion with BSA-488, and for 3–6 s after the perfusion was stopped and blood flow was reestablished.
Analyses and statistics.
Recorded sequences were analyzed with National Institutes of Health (NIH) image software (v6.1). A region of interest (ROI) was identified, and the total fluorescence intensity was measured in the ROI in each sequence of acquired fields, as described previously (34), starting with baseline and throughout the minute of perfusion with BSA. To control for local variations in the perfusion pressure in the selected regions of intact network, the intensity in the vessel itself was also quantified. Measurements in vessels exhibiting a drop in fluorescent intensity or oscillation during the measured period were discarded.
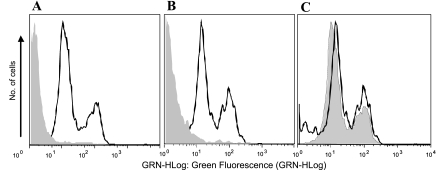
For the flow cytometric analysis of CD18 expression in WT and ICAM-1 KO neutrophils, mouse blood was treated with diluted PBS to lyse red cells and washed in 10 mM HEPES-buffered PBS with 0.1% BSA. To assay total CD18 expression, cells were labeled with either rat anti-mouse FITC-conjugated anti-CD18 monoclonal antibody (clone M18/2; AbD Serotec) or the corresponding isotype FITC-conjugated rat IgG2a (clone YTH71.3; AbD Serotec). Samples (5,000 cells) were analyzed by flow cytometry (Guava EasyCyte Mini; Guava Technologies).
Statistical tests were performed using GraphPad Prism (v4.0) for t-tests, ANOVA, linear regression, or correlation analyses as appropriate. Significance was set at P < 0.05.
RESULTS
Observations were made in both arterioles and venules (diameter range, 20–60 μm) in control or TNF-α-activated tissue conditions in WT and KO mice models as described in materials and methods.
ICAM-1 is essential for TNF-α-induced increase in Ps.
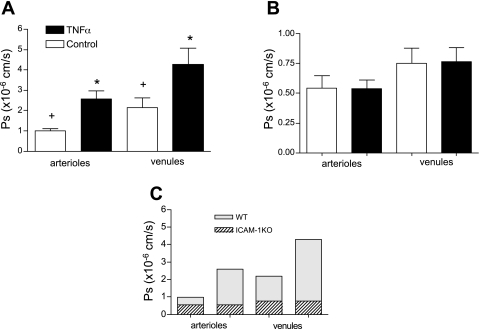
One of the major events characterizing the inflammatory response is a change in vessel permeability to water and solutes that in turn may lead to tissue edema. In our study, we measured permeability to albumin as an indicator of barrier function of the microvascular wall. Confirming earlier work in isolated cells and tissues (9, 17), we found that following TNF-α stimulation, there was a dramatic twofold increase in venular Ps (Fig. 1A). Intriguingly, a relatively greater, 2.5-fold increase in Ps was measured in arterioles. Although the concept of leakier venules during inflammation is widely accepted (including both macromolecular permeation across the wall and leukocyte transmigration), it is a novel finding that arterioles under the same conditions respond in a similar way. Importantly, we found that both arterioles and venules under control conditions had a measurable basal Ps with average values of 1.0 ± 0.10 and 2.2 ± 0.46 × 10−6 cm/s, respectively. Thus, under these conditions, control venules were leakier, with twofold higher absolute Ps values than arterioles. Interestingly, these results directly correlate with our recent finding that the expression levels of ICAM-1 under control conditions were twofold greater in venules compared with arterioles (39). Moreover, in that same study, we showed that although the expression of ICAM-1 significantly increased in arterioles following TNF-α treatment, the expression was comparable with that of ICAM-1 in control venules. In the present study, we report that Ps levels in arterioles following TNF-α treatment were also similar to control venules, whereas TNF-α-treated venules had a further increase in Ps (Fig. 1A).
Fig. 1.
TNF-α-mediated increase in solute permeability (Ps) is ICAM-1 dependent. A: Ps in wild-type (WT) arterioles and venules significantly increased following TNF-α treatment (n = 17 vessels). B: in ICAM-1 knockout (KO) mice, TNF-α-induced increase in Ps was abolished in arterioles and venules (n = 12). C: overlay of average Ps values from WT and ICAM-1 KO mice. ICAM-1 KO vessels were much less permeable than WT vessels, and the difference between arteriolar and venular Ps was abolished. *Significantly different from controls and each other; +significantly different from each other (P < 0.05).
Intracellular signaling pathways associated with the ligation of ICAM-1 [e.g., increased EC Ca2+ followed by cytoskeletal rearrangement (31)] have been shown in other systems to be a prerequisite for changes in vessel permeability (30). Together with the findings presented above, this led us to hypothesize that ICAM-1 might influence the observed Ps increase in our system.
To test this, we measured Ps in ICAM-1 KO mice and compared it with Ps in WT mice. Supporting our hypothesis, we found that in ICAM-1 KO mice the TNF-α-induced increase in Ps was abolished in both arterioles and venules (Fig. 1B), suggesting that ICAM-1 is essential for mediating the effect of TNF-α on Ps. To our surprise, not only was the TNF-α-dependent increase in Ps abolished in KO animals, but both arterioles and venules were significantly less permeable to BSA (control Ps decreased to 0.54 ± 0.07 and 0.77 ± 0.11 × 10−6 cm/s, respectively, compared with WT; Fig. 1C). The fact that without ICAM-1, baseline Ps was significantly lower than in WT in both arterioles and venules suggests that there is a constitutive Ps pathway in both types of vessels that is mediated by ICAM-1, and which in WT vessels is normally expressed and active. We verified that in the ICAM-1 KO mice, the expression of CD18 on neutrophils was not different from WT animals (Fig. 2), indicating that the observed changes in Ps are specific to the expression of ICAM-1. Thus our results suggest that the TNF-α-mediated increase in Ps is dependent upon ICAM-1-mediated downstream signaling.
Fig. 2.
β2-integrins (CD18) expression is not different in WT and ICAM-1 KO mice. To measure total CD18 expression on mouse leukocytes, flow cytometry was performed on white cells from whole blood sample, which were labeled with rat anti-mouse FITC-conjugated anti-CD18 monoclonal antibody (clone M18/2). Corresponding isotype FITC-conjugated rat IgG2a (clone YTH71.3) was used as a negative control. n = 5,000 cells for each sample. A: WT. B: ICAM-1 KO. Solid line, CD18-positive cells; shaded areas, isotype control. C: overlay of CD18-positive WT and ICAM-1 KO mice. Solid line, KO; shaded area, WT.
ICAM-1 ligation by rolling or adhered leukocytes increases Ps.
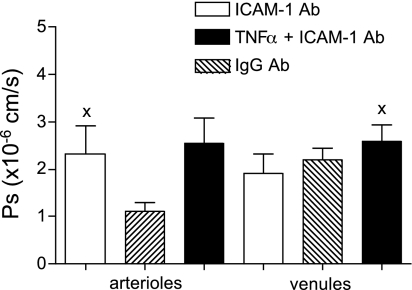
Using the knowledge that leukocyte-EC interactions are absent in control arterioles but could be induced by TNF-α treatment (39), we asked whether in arterioles, ligation of ICAM-1 by rolling leukocytes was responsible for the observed increase in Ps. To mimic ICAM-1 ligation by leukocytes, the microvasculature was perfused with anti-ICAM-1 antibody (YN-1/1.7.4; 100 μg iv), which specifically binds to the leukocyte binding site on ICAM-1. Ligation of ICAM-1 in control arterioles (where rolling leukocytes are absent) significantly increased arteriolar Ps (Fig. 3). Confirming our hypothesis, we found that Ps in control arterioles during ICAM ligation was similar to the Ps measured in TNF-α-activated arterioles where ICAM-1 was naturally ligated by rolling leukocytes (2.3 ± 0.58 and 2.6 ± 0.37 × 10−6 cm/s, respectively). Since the antibody causes clustering, our results also suggest that ICAM-1 clustering resulting from rolling leukocytes is a key element in ICAM-1 activation and the consequent increase in arteriolar Ps. Moreover, these results imply that the degree of ICAM-1 clustering and activation by antibody ligation (reflected by consequent changes in Ps) is similar to that resulting from native rolling leukocytes. TNF-α treatment in combination with ICAM-1 ligation did not produce any further increase in arteriolar Ps (2.5 ± 0.15 × 10−6 cm/s; Fig. 3), arguing that the TNF-α-mediated increase in Ps in arterioles is indeed due to the induction of leukocyte rolling and subsequent ICAM-1 ligation.
Fig. 3.
ICAM-1 ligation increased Ps in arterioles but not in venules. In control WT arterioles, but not WT venules, antibody (Ab) ligation of ICAM-1 (YN-1/1.7.4; 100 μg iv) significantly increased Ps to levels similar to those following TNF-α activation (P < 0.05; n = 10 vessels). x is significantly different from WT without ICAM-1 ligation (Fig. 1A).
In control WT venules, ligation of ICAM-1 with the same antibody used in arterioles did not produce any significant change in Ps (1.9 ± 0.41 × 10−6 cm/s; Fig. 3). Moreover, TNF-α treatment in combination with ICAM-1 ligation failed to produce the expected TNF-α-induced increase in Ps. We speculate that the difference in the outcome of the antibody ligation between venules and arterioles could be explained by higher ICAM-1 expression and the presence of leukocyte rolling in venules (39). As previously mentioned, Ps in control arterioles during ICAM-1 ligation with the antibody was similar to the Ps measured in TNF-α-activated arterioles, where ICAM-1 was ligated by rolling leukocytes (2.3 ± 0.58 and 2.6 ± 0.37 × 10−6 cm/s, respectively). In venules on the other hand, rolling leukocytes are present under control conditions, and Ps is higher compared with that of control arterioles (2.2 ± 0.46 vs. 1.0 ± 0.10 × 10−6 cm/s, respectively; Fig. 1). Importantly, Ps in control venules is not different from Ps in either ligated or TNF-α-activated arterioles. We conclude that the increase in Ps following ICAM-1 ligation with the antibody in arterioles was not seen in venules because it had been already accomplished by rolling leukocytes (interacting with ICAM-1), thus masking the potential effect of the antibody. The lack of an effect of TNF-α on venular Ps when the TNF-α treatment was combined with ligation of ICAM-1 can be explained by previous work (38), where we showed that the same antibody as was used here decreased leukocyte adhesion events by >50% in these vessels. Importantly, our findings suggest that leukocyte rolling and adhesion have different effects on Ps; specifically leukocyte adhesion, rather than rolling, is needed to produce the TNF-α-mediated increase in Ps in venules.
We also conclude that the changes in Ps that we observed are specific to ICAM-1-mediated signaling. This is based on the finding that VCAM-1 ligation (using a similar protocol to the one used to ligate ICAM-1 and using rat anti-mouse CD106 429MVCAM.A, 100 μg iv; Pharmingen) did not result in increased Ps in either arterioles or venules (1.1 ± 0.22 and 2.1 ± 0.53 × 10−6 cm/s, respectively).
TNF-α-induced increase in Ps is mediated via ICAM-1/β2-integrin interactions.
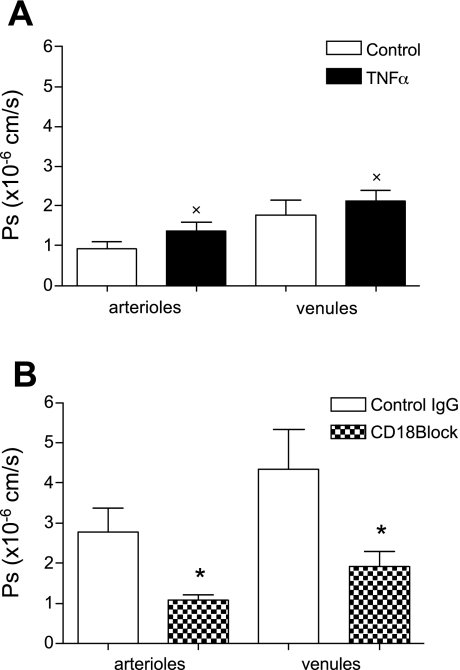
ICAM-1/β2-integrin-mediated leukocyte-EC interactions are a key event in the leukocyte recruitment cascade. Here we asked whether leukocyte rolling in arterioles and leukocyte adhesion in venules are necessary and/or sufficient to mediate changes in Ps following TNF-α treatment. To answer this, we measured Ps in TNF-α-activated microvesssels (to induce rolling in arterioles and adhesion in venules) of CD18 KO mice and compared this with Ps in WT animals. In arterioles of CD18 KO mice, the number of rolling leukocytes decreased by ∼40%, confirming a previous finding in ICAM-1 KO mice (38). In venules of CD18 KO mice, the number of adhered leukocytes was also decreased by ∼60% (data not shown) compared with that of WT mice.
Control Ps in CD18 KO microvessels was not significantly different from that of WT (0.92 ± 0.18 vs. 1.0 ± 0.10 × 10−6 cm/s in arterioles and 1.8 ± 0.18 vs. 2.2 ± 0.46 × 10−6 cm/s in venules; Fig. 4A). In the absence of leukocyte-EC interactions in control arterioles, and with the majority of leukocyte interactions in venules being rolling interactions, which are minimally mediated by β2-integrins (1, 38), these results further support our hypothesis. Indeed, the finding that control Ps in CD18 KO mice is similar to that in WT strengthens the conclusion that the decrease in Ps observed in ICAM-1 KO mice under control conditions was indeed a constitutive, ICAM-1-mediated permeability pathway that was not present in ICAM-1 KO mice.
Fig. 4.
CD18/ICAM-1 interactions are essential for TNF-α-mediated Ps increase. A: Ps in control and TNF-α-activated microvessels in CD18 KO mice. Ps in control vessels was similar to that in WT, but Ps did not increase with TNF-α. B: CD18 blocking antibody (CD18 GAME-46; 100 μg iv), but not IgG control (R3-34; 100 μg iv), significantly reduced the TNF-α-induced increase in Ps in WT arterioles and venules. x is significantly different from Ps measured in TNF-α-treated WT mice (n = 10 vessels; Fig. 1A); *significantly different (P < 0.05) from controls (n = 9 vessels).
Importantly, we show that ICAM-1/β2-integrin interactions are essential to induce changes in Ps in both arterioles and venules following TNF-α activation (Fig. 4), since TNF-α treatment did not increase Ps in either vessel type in CD18 KO mice. With TNF-α, Ps in these mice was significantly different from that in TNF-α-treated WT vessels (1.4 ± 0.22 vs. 2.6 ± 0.37 × 10−6 cm/s in arterioles and 2.1 ± 0.26 vs. 4.3 ± 0.79 × 10−6 cm/s in venules, respectively) but was not different from Ps measured in WT controls (see Fig. 1A).
Because of the possibility that compensatory alteration in the expression of other adhesion molecules or their counter receptors may have occurred in KO mice (6, 38), we conducted additional experiments where WT mice were treated with TNF-α and then treated with either CD18 blocking or IgG nonspecific (control) antibodies (100 μg iv). Similar to those of CD18 KO mice, leukocyte-EC interactions in these vessels were diminished (data not shown) and Ps was significantly decreased with CD18 blocking antibody (but not the nonspecific IgG) in TNF-α-activated arterioles and venules (1.1 ± 0.13 and 1.9 ± 0.38 × 10−6 cm/s, respectively; Fig. 4B).
Constitutive permeability under control conditions is PKC dependent.
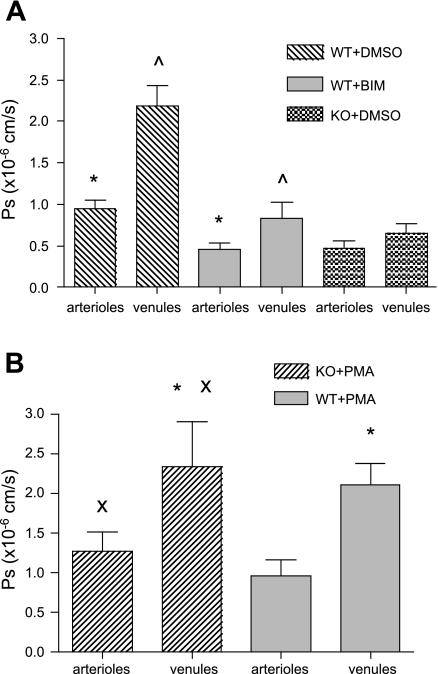
The finding that ICAM-1 mediates changes in Ps led us to our next question, which was to identify key signaling molecules involved in this response. ICAM-1 can activate a PKC-dependent signaling pathway (7), and PKC can alter Ps (30, 51), hence we asked whether in our system the ICAM-1-mediated changes in Ps were PKC dependent. To do this, we topically applied BIM (1 μM, 10 min in superfusate), which nonselectively inhibits all isoforms of PKC (7). When control WT vessels were treated with BIM, Ps was significantly decreased in both arterioles and venules (to 0.46 ± 0.07 and 0.84 ± 0.19 × 10−6 cm/s, respectively; Fig. 5A). Intriguingly, these values were similar to Ps measured in arterioles and venules in ICAM-1 KO mice (0.54 ± 0.07 and 0.77 ± 0.11 × 10−6 cm/s, respectively). These results strongly support the idea that under control conditions there is a constitutively active permeability pathway, working via an ICAM-1-induced, PKC-dependent signal.
Fig. 5.
Ps under control conditions is PKC dependent. A: control WT vessels treated with DMSO alone, bisindolylmaleimid l (BIM; PKC blocker in 0.01% DMSO, 1 μmol/l for 10 min in superfusate), and ICAM-1 KO mice with DMSO alone. DMSO had no effect in both WT and ICAM-1 KO (Ps similar to Fig. 1, A and B). Ps significantly decreased with BIM to levels similar to ICAM-1 KO mice. B: control ICAM-1 KO and WT vessels treated with PMA (PKC activator in 0.01% DMSO, 1 μM for 10 min in superfusate). Ps in ICAM-1 KO vessels was restored to WT levels, whereas Ps in WT vessels was unchanged. * and ^, Significantly different (P < 0.05) from each other (n = 10 vessels); x is significantly different (P < 0.05) from Ps measured in ICAM-1 KO mice (n = 10 vessels; Fig. 1B).
In further support, control Ps in ICAM-1 KO mice was restored from 0.54 ± 0.07 to 1.28 ± 0.24 × 10−6 cm/s in arterioles and from 0.77 ± 0.11 to 2.22 ± 0.57 × 10−6 cm/s in venules (Fig. 5B) by the PKC activator PMA (1 μM for 10 min in 0.01% DMSO). Interestingly, Ps in ICAM-1 KO arterioles and venules exposed to PMA was not different from Ps in WT vessels, suggesting that the further increase observed in WT arterioles and venules following TNF-α treatment was not mediated by PKC but must be due to a different signaling mechanism.
To explore whether the TNF-α-induced increase in Ps was indeed via a different signaling pathway, control WT microvessels were treated with PMA. This failed to induce any Ps increase in either arterioles or venules (0.97 ± 0.19 and 2.1 ± 0.26 × 10−6 cm/s, respectively; Fig. 5B). Thus, in control WT microvessels, Ps is maintained at a basal, constitutive level by a PKC-dependent mechanism. Moreover, PMA increased Ps in ICAM-1 KO mice to control WT levels but failed to elevate Ps in WT mice above control levels; hence, we conclude that during TNF-α treatment, a different mechanism must be responsible for the inflammatory increase in Ps.
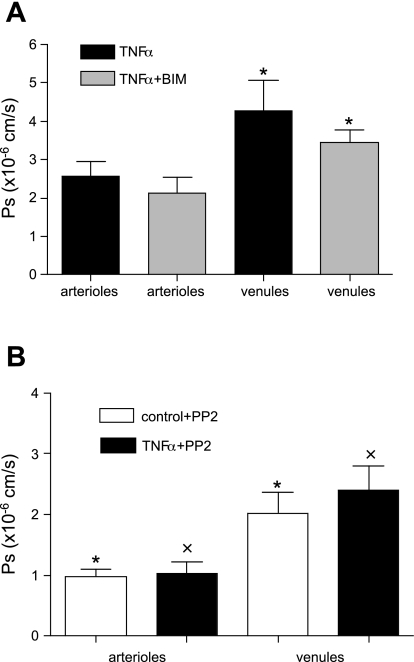
TNF-α treatment switches Ps from a PKC-dependent constitutive permeability to a Src-dependent inducible response.
To confirm that the TNF-α-induced increase in Ps was not PKC dependent, WT mice were treated with TNF-α and then subjected to PKC inhibition with BIM. TNF-α treatment increased Ps as expected from results shown in Fig. 1A. In agreement with our hypothesis, the TNF-α-induced increase in Ps in arterioles was not significantly affected by the PKC blocker [2.6.0 ± 0.37 × 10−6 cm/s (TNF-α alone) vs. 2.2 ± 0.40 × 10−6 cm/s (TNF-α + BIM); Fig. 6A]. In venules, although the decrease in Ps due to BIM was significant [4.3.0 ± 0.79 × 10−6 cm/s (TNF-α alone) vs. 3.5 ± 0.34 × 10−6 cm/s (TNF-α + BIM); Fig. 6A], the TNF-α effect was still present, producing significantly higher Ps in venules compared with controls (2.2 ± 0.46 × 10−6 cm/s; Fig. 1A). With BIM, the expected increase in Ps with TNF-α still occured but was less than with TNF-α alone, likely due to the blockade of the PKC-dependent constitutive pathway (as demonstrated in Fig. 5). Furthermore, the PKC blocker had no effect on the increase in Ps mediated by ligation of ICAM-1 in control arterioles [2.1 ± 0.42 × 10−6 cm/s (ICAM-1 ligation) vs. 2.1 ± 0.38 × 10−6 cm/s (ICAM-1 ligation + BIM)]. These results thus strongly argue that treatment with TNF-α caused the system to switch from the constitutively active PKC-dependent Ps pathway to a different signaling pathway.
Fig. 6.
TNF-α-mediated increase in Ps is Src dependent. A: control WT vessels treated with TNF-α or TNF-α + BIM (in 0.01% DMSO, 1 μmol/l for 10 min in superfusate). In arterioles, the increase in Ps with TNF-α was not significantly different from Ps with TNF-α + BIM. In venules, the increase in Ps with TNF-α was attenuated significantly by BIM. B: WT vessels (control or TNF-α activated) treated with PP2 (Src blocker in 0.01% DMSO, 2 μmol/l for 10 min in superfusate). Ps in controls was unchanged with PP2, whereas PP2 significantly inhibited the TNF-α-induced increase in Ps. *Significantly different (P < 0.05) from each other; x is significantly different from Ps in TNF-α-treated WT mice (n = 9 vessels; Fig. 1A).
Other potential candidates for linking ICAM-1-mediated signaling to changes in Ps are Src-family kinases, due to their important role in cytoskeletal rearrangement (49, 50). Thus we utilized a selective Src-family inhibitor, PP2 (2 μM in 0.01% DMSO, 10 min), to explore whether the TNF-α-induced increase in Ps might be dependent upon Src activation. Indeed, in the presence of PP2, the TNF-α-mediated increase in Ps in both arterioles and venules was reversed (to 1.0 ± 0.19 and 2.4 ± 0.38 × 10−6 cm/s, respectively; Fig. 5B) to levels not significantly different from WT controls (see Fig. 1A). Together with the data presented earlier, these results strongly suggest that while baseline Ps is PKC dependent, during inflammation the system switches to a Src-dependent mechanism. Moreover, Src inhibition had no effect on Ps in control WT arterioles and venules (0.98 ± 0.11 and 2.0 ± 0.34 × 10−6 cm/s, respectively; Fig. 6B), further supporting the conclusion that different Ps regulatory pathways are active during control versus inflammation.
DISCUSSION
The current work to our knowledge is the first to provide a direct link, via a specific adhesion molecule, between leukocyte-EC interactions and changes in Ps. We show that the molecule connecting these two processes is ICAM-1. Importantly, we show that arterioles respond to proinflammatory stimuli in a very similar way to venules, thus contributing to microvascular exchange. While the ability to regulate solute exchange has been recognized in larger coronary arterioles (20), in the current work we demonstrate for the first time that smaller arterioles also regulate Ps. Moreover, we identified two independent pathways, a constitutive (PKC dependent) pathway and a TNF-α-mediated, inducible (Src dependent) pathway, both leading to changes in Ps in an ICAM-1-dependent manner.
In arterioles, similarly to venules, ICAM-1 is expressed under control conditions and is upregulated by TNF-α (39, 47). In venules, increased ICAM-1 expression is directly implicated in leukocyte adhesion and transmigration, whereas in arterioles it supports leukocyte rolling with no subsequent leukocyte adhesion or transmigration (38). Other studies have established in isolated ECs that ligation or overexpression of ICAM-1 on the EC surface initiates multiple signaling events (e.g., increased EC Ca2+, reactive oxygen species production, and tyrosin phosphorylation of cytoskeletal and junctional proteins) (18, 45, 46). Interestingly, similar signaling events are implicated in the regulation of vascular permeability (13, 41, 51). Thus we hypothesized that the direct physiological consequence of leukocyte rolling in arterioles and adhesion in venules is ligation of ICAM-1, induction of these signaling events, and consequent regulation of Ps. Consistent with our hypothesis, and with the outcomes of other inflammatory agents such as VEGF, histamine, and thrombin (30), TNF-α activation increased Ps in venules. Uniquely, we show that Ps in arterioles was also significantly upregulated. With the consideration of the large surface area of resistance arterioles, their contribution to maintenance of tissue homeostasis will be more than negligible.
We found that in the absence of ICAM-1 (ICAM-1 KO), the TNF-α-mediated increase in Ps was abolished. Moreover, both arterioles and venules had significantly lower baseline Ps and were not different from each other as they were in WT mice (Fig. 1). In previous work we have demonstrated that the expression of VCAM-1 was altered in ICAM-1 KO mice (38), hence we confirmed in this study that the differences in basal Ps levels, and the ability of the vessels in ICAM-1 KO mice to respond to the proinflammatory stimulus, could not be attributed to the altered expression of CD18 in the ICAM-1 KO animals (Fig. 2). While the inability of TNF-α to increase Ps in ICAM-1 KO mice suggests that ICAM-1 is required for the regulation of Ps, the significantly lower control Ps in these animals implies that in WT mice there is a constitutive Ps pathway dependent on the presence of ICAM-1. We showed that this constitutive pathway is PKC dependent. Furthermore, we showed, in agreement with others (14), that regulation of Ps does not always require interactions between leukocytes and EC. In control arterioles there are no leukocyte-EC interactions, yet a constitutively active Ps pathway was detected. Moreover, we successfully blocked that pathway using the PKC blocker, in the absence of rolling leukocytes. Together, our findings suggest that Ps in both arterioles and venules is tightly regulated not only during inflammation but also under control (physiologically normal) conditions and that one of the key molecules involved is PKC.
In control venules, on the other hand, both leukocyte rolling and adhesion interactions were observed, and the Ps was twofold greater than in control arterioles. This difference in Ps between venules and arterioles could be explained by the activation state of ICAM-1. In its multidimeric form, the half-life of an ICAM-1/β2-integrin bond is four times longer than in the monomeric form (33). We thus speculate that under control conditions, with no leukocytes present, the majority of ICAM-1 molecules expressed on the EC surface are monomers in a low activity state, producing the ICAM-1-mediated constitutive permeability measured in arterioles. Under similar conditions, venules express significantly higher levels of ICAM-1 (39), with, presumably, higher density on the EC surface. In these vessels, leukocyte ligation of ICAM-1 could induce clustering (dimerization) and activation of ICAM-1, leading to more activated venules and higher permeability. ICAM-1 clustering in vitro induces signaling (7), but this speculation remains to be addressed in an in vivo system in future studies.
Supporting the idea that leukocyte-EC interactions are responsible for higher Ps in control venules is our observation that the low Ps in control arterioles increased to levels similar to control venules following the initiation of leukocyte rolling by TNF-α treatment (Fig. 1A). The possibility that TNF-α treatment could increase Ps independent of leukocyte-EC interactions can be ruled out because in both CD18 KO and WT mice with CD18 blocking antibody, leukocyte-EC interactions were significantly diminished and the TNF-α-mediated increase in Ps was also significantly abrogated (Fig. 4).
Finally, we identified Src as a key signaling molecule in the TNF-α-mediated increase in Ps in both microvessel types. The role of the Src kinase family is well characterized in isolated cell systems. For example, it is regulated by ICAM-1 activation (46), can mediate vascular endothelial-cadherin disruption (28), and intercellular gap formation (32). On the other hand, Src inhibitors can prevent transcellular albumin transport and reduce the number of vesicles in ECs (36). Thus there is evidence that Src-dependent signaling may be important for alterations in Ps via both paracellular and transcellular routes. In our system, the TNF-α-mediated increase in Ps was not significantly affected by the PKC inhibitor (Fig. 6A), arguing that once the system shifted to the inflammatory state, signaling was no longer PKC dependent. On the other hand, the TNF-α-mediated increase in Ps was reversed by the inhibition of Src (Fig. 6B), suggesting that during TNF-α the increase in Ps is Src dependent.
Our data thus suggest that rolling and firmly adhered leukocytes trigger different signaling pathways mediating different levels of Ps, as schematized in Fig. 7. Under different conditions (control venules vs. TNF-α-treated arterioles), but with similar leukocyte behavior [the majority of leukocyte-EC interactions being rolling interactions (38)], Ps was similar and dependent upon PKC activation. Furthermore, in inflamed venules, where most of the interacting leukocytes are firmly adhered (38), Ps is significantly higher and mediated not by PKC but by Src. Interpreting these data, we suggest that the difference in the degree of ICAM-1 ligation and clustering by either rolling or firmly adhered leukocytes induces different signaling mechanisms and consequent differences in Ps. We have no information about whether different Ps pathways (para- or transcellular) are regulated by the different signaling pathways; this is clearly an important question that is beyond the scope of the present study.
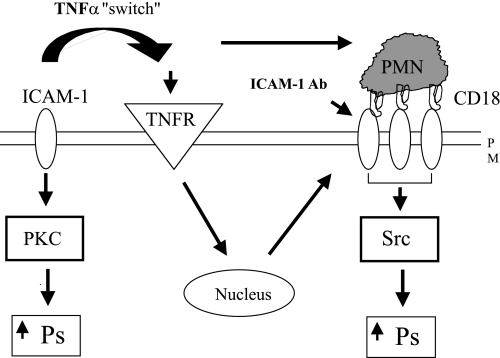
Fig. 7.
Proposed model of ICAM-1-mediated changes in Ps in microvasculature. Under control conditions, there is a constitutive permeability pathway mediated via basally expressed ICAM-1 (independently of interactions with leukocytes; left) in a PKC-dependent manner. Following TNF-α treatment, the expression of ICAM-1 and consequent interactions with β2-integrins increase, resulting in ICAM-1 clustering and, hence, increasing Ps via a switch to a different, Src-dependent pathway. PMN, neutrophil; TNFR, TNF receptors; PM, plasma membrane.
In summary, we have identified a unique role for ICAM-1 in linking leukocyte-EC interactions and vascular permeability and have highlighted the important role arterioles play in inflammation by contributing to Ps changes. We identified a constitutive, ICAM-1-mediated, PKC-dependent Ps pathway in control microvessels that switches to a Src-dependent Ps pathway following TNF-α treatment. Future work will have to address whether these two separate signaling pathway also result in different paths for solutes to leave the microvasculature. It is possible, for example, that under control conditions the major pathway for albumin to leave the vessel is via an active vesicular or receptor mediated pathway, whereas during inflammation the activation of contractile machinery and cytoskeletal rearrangement (which are greatly affected by Src kinases) results in gap formation and a paracellular route for albumin to leave the vessels.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL75186 and PO1-HL18208 and American Heart Association Grant 0615677T.
Acknowledgments
We thank J. M. Kuebel for superb technical contributions and Dr. V. H. Huxley for critical discussion.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbassi O, Kishimoto TK, McIntire LV, Anderson DC, Smith CW. E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest 92: 2719–2730, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgstrom P, Hughes GK, Hansell P, Wolitsky BA, Sriramarao P. Leukocyte adhesion in angiogenic blood vessels. Role of E-selectin, P-selectin, and beta2 integrin in lymphotoxin-mediated leukocyte recruitment in tumor microvessels. J Clin Invest 99: 2246–2253, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol 127: 762–774, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Czabanka M, Peter C, Martin E, Walther A. Microcirculatory endothelial dysfunction during endotoxemia—insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol 5: 266–275, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood 99: 336–341, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Eppihimer MJ, Russell J, Anderson DC, Wolitzky BA, Granger DN. Endothelial cell adhesion molecule expression in gene-targeted mice. Am J Physiol Heart Circ Physiol 273: H1903–H1908, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol 165: 3375–3383, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med 187: 903–915, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero E, Zocchi MR, Magni E, Panzeri MC, Curnis F, Rugarli C, Ferrero ME, Corti A. Roles of tumor necrosis factor p55 and p75 receptors in TNF-α-induced vascular permeability. Am J Physiol Cell Physiol 281: C1173–C1179, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Ferro T, Neumann P, Gertzberg N, Clements R, Johnson A. Protein kinase C-α mediates endothelial barrier dysfunction induced by TNF-α. Am J Physiol Lung Cell Mol Physiol 278: L1107–L1117, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MF, Alexander HR. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood 100: 1334–1339, 2002. [PubMed] [Google Scholar]
- 12.Gaudreault N, Perrin R, Guo M, Clanton C, Wu M, Yuan SY. Counter regulatory effects of PKCbetaII and PKCdelta on coronary endothelial permeability. Arterioscler Thromb Vasc Biol 28: 1527–1533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta2 integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med 191: 1829–1839, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He P, Wang J, Zeng M. Leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1686–H1694, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Hippenstiel S, Tannert-Otto S, Vollrath N, Krull M, Just I, Aktories K, von Eichel-Streiber C, Suttorp N. Glucosylation of small GTP-binding Rho proteins disrupts endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 272: L38–L43, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hocking DC, Ferro TJ, Johnson A. Dextran sulfate inhibits PMN-dependent hydrostatic pulmonary edema induced by tumor necrosis factor. J Appl Physiol 70: 1121–1128, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Hocking DC, Phillips PG, Ferro TJ, Johnson A. Mechanisms of pulmonary edema induced by tumor necrosis factor-alpha. Circ Res 67: 68–77, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med 28: 1379–1386, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: α-lactalbumin transport. Am J Physiol Heart Circ Physiol 252: H188–H197, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol 293: H1196–H1205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ Res 92: 1089–1097, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kim MB, Sarelius IH. Regulation of leukocyte recruitment by local wall shear rate and leukocyte delivery. Microcirculation 11: 55–67, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kim MB, Sarelius IH. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am J Physiol Heart Circ Physiol 287: H2705–H2711, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel EJ, Jung U, Bullard DC, Norman KE, Wolitzky BA, Vestweber D, Beaudet AL, Ley K. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J Exp Med 183: 57–65, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunkel EJ, Jung U, Ley K. TNF-α induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am J Physiol Heart Circ Physiol 272: H1391–H1400, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13: 1175–1189, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65: 859–873, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 64: 1029–1036, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lindbom L Regulation of vascular permeability by neutrophils in acute inflammation. Chem Immunol Allergy 83: 146–166, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Millan J, Ridley AJ. Rho GTPases and leucocyte-induced endothelial remodelling. Biochem J 385: 329–337, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mucha DR, Myers CL, Schaeffer RC Jr. Endothelial contraction and monolayer hyperpermeability are regulated by Src kinase. Am J Physiol Heart Circ Physiol 284: H994–H1002, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sarantos MR, Raychaudhuri S, Lum AF, Staunton DE, Simon SI. Leukocyte function-associated antigen 1-mediated adhesion stability is dynamically regulated through affinity and valency during bond formation with intercellular adhesion molecule-1. J Biol Chem 280: 28290–28298, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Sarelius IH, Kuebel JM, Wang J, Huxley VH. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol 290: H474–H480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1−/− knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, l-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277: 40091–40098, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem 279: 20392–20400, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell 15: 3114–3122, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol 293: H2786–H2798, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Sumagin R, Sarelius IH. TNF-α activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 291: H2116–H2125, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Physiol Heart Circ Physiol 272: H1725–H1729, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Tinsley JH, Teasdale NR, Yuan SY. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol 286: L841–L847, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Valeski JE, Baldwin AL. Effect of early transient adherent leukocytes on venular permeability and endothelial actin cytoskeleton. Am J Physiol Heart Circ Physiol 277: H569–H575, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, Kowalczyk AP. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am J Physiol Cell Physiol 283: C811–C821, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Whitt SP, Rubin LJ, Huxley VH. Differential coronary microvascular exchange responses to adenosine: roles of receptor and microvessel subtypes. Microcirculation 12: 313–326, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Doerschuk CM. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J Immunol 164: 6487–6494, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Pfeiffer GR 2nd, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem 278: 47731–47743, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Wung BS, Ni CW, Wang DL. ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J Biomed Sci 12: 91–101, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106: 584–592, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol 177: 6440–6449, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res 98: 394–402, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Yuan SY Signal transduction pathways in enhanced microvascular permeability. Microcirculation 7: 395–403, 2000. [PubMed] [Google Scholar]