Abstract
The role of anaerobic glycolysis and oxidative substrate selection on contractile function and mechanical efficiency during moderate severity myocardial ischemia is unclear. We hypothesize that 1) preventing anaerobic glycolysis worsens contractile function and mechanical efficiency and 2) increasing glycolysis and glucose oxidation while inhibiting free fatty acid oxidation improves contractile function during ischemia. Experiments were performed in anesthetized pigs, with regional ischemia induced by a 60% decrease in left anterior descending coronary artery blood flow for 40 min. Three groups were studied: 1) no treatment, 2) inhibition of glycolysis with iodoacetate (IAA), or 3) hyperinsulinemia and hyperglycemia (HI + HG). Glucose and free fatty acid oxidation were measured using radioisotopes and anaerobic glycolysis from net lactate efflux and myocardial lactate content. Regional contractile power was assessed from left ventricular pressure and segment length in the anterior wall. We found that preventing anaerobic glycolysis with IAA during ischemia in the absence of alterations in free fatty acid and glucose oxidation did not adversely affect contractile function or mechanical efficiency during myocardial ischemia, suggesting that anaerobic glycolysis is not essential for maintaining residual contractile function. Increasing glycolysis and glucose oxidation with HI + HG inhibited free fatty acid oxidation and improved contractile function and mechanical efficiency. In conclusion, these results show a dissociation between myocardial function and anaerobic glycolysis during moderate severity ischemia in vivo, suggesting that metabolic therapies should not be aimed at inhibiting anaerobic glycolysis per se, but rather activating insulin signaling and/or enhancing carbohydrate oxidation and/or decreasing fatty acid oxidation.
Keywords: angina, fatty acids, glucose, insulin
acute myocardial ischemia reduces the rate of aerobic ATP formation and activates anaerobic glycolysis, resulting in an accumulation of lactate in the myocardium and a switch from net lactate uptake to lactate efflux into the blood. ATP generated by anaerobic glycolysis during moderate severity ischemia may help sustain membrane function and relaxation (12, 32); however, it is unclear whether it is important for the generation of external myocardial power. This issue is of clinical relevance, since an infusion of insulin and glucose, which stimulates myocardial glucose uptake and glycolysis (25, 33), can be effective treatment for acute ischemic events (24). Accelerating anaerobic glycolysis and lactate production with an infusion of insulin and glucose during ischemia improves systolic function (3, 7, 35). Conversely, pharmacologically decreasing lactate production through the inhibition of glycolysis during severe ischemia (1, 18) or moderate severity ischemia (10) lowers ATP content and worsens myocardial contractile function. These observations suggest that anaerobic glycolysis and lactate production during ischemia help sustain systolic function and that stimulating or inhibiting this process causes parallel changes in mechanical function.
On the other hand, there is evidence to suggest that anaerobic glycolysis and lactate production contribute to contractile dysfunction during and immediately after ischemia, since high tissue lactate concentration can slow the rate of glycolysis and anaerobic ATP production (6), and is associated with a decrease in intracellular pH, Ca2+ overload, and contractile dysfunction (14–16, 28). Improved cardiac function during ischemia has been observed in response to the inhibition of free fatty acid (FFA) oxidation or the activation of pyruvate dehydrogenase (13, 14, 26, 28), both of which accelerate glucose uptake and pyruvate oxidation in mitochondria but also decrease anaerobic glycolysis by diverting more of the glycolyticly derived pyruvate toward oxidation in the mitochondria and less toward lactate formation. (26). Evidence for this mechanism comes from studies in models of demand-induced ischemia (4, 29, 34) or ischemia-reperfusion (14–16, 28). The benefit of metabolic therapies, like glucose-insulin infusions or partial inhibition of fatty acid oxidation, appears to be due to the switching oxidative metabolism away from fatty acids toward greater glucose oxidation, which provides more mechanical power generation by the myocardium for a given oxygen consumption (i.e., greater mechanical efficiency). The premise here is that during ischemia, oxygen is wasted when glucose oxidation is low and fatty acid oxidation is high due to the lower ATP-to-O2 ratio for fatty acids than pyruvate and the greater ATP requirement for maintenance of Na+/Ca2+ homeostasis as a result of greater lactate and H+ production (14–16, 28). On the other hand, the acceleration of glycolysis with hyperinsulinemia + hyperglycemia (HI + HG) during severe low-flow ischemia and reperfusion in isolated rat hearts increased anaerobic glycolysis and improved contractile recovery during reperfusion compared with normal glucose and insulin levels (31). In addition, Zhu et al. (35) showed that HI + HG accelerated glucose uptake and net lactate efflux from the myocardium and improved contractile function in a pig model of acute coronary syndrome induced by a 50% decrease in coronary blood flow (35). To our knowledge, the effects of HI + HG on glucose and FFA oxidation have not been reported during moderate severity ischemia.
The present study investigated the role of nonoxidative glycolysis and oxidative substrate selection on the regulation of myocardial contractile function and mechanical efficiency during moderate severity ischemia. We hypothesized that 1) preventing anaerobic glycolysis during ischemia in the absence of alterations in FFA and glucose oxidation would worsen contractile function and mechanical efficiency during myocardial ischemia and 2) increasing nonoxidative glycolysis and glucose oxidation while inhibiting FFA oxidation improves contractile function during ischemia. Experiments were performed in a well-established swine model of moderate severity ischemia that is similar to the clinical condition of acute coronary syndrome (19, 22, 27). Glycolysis was inhibited with iodoacetate (IAA), a sulfhydryl-oxidizing agent that selectively inhibits the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (10, 11). An infusion of insulin and glucose were used to stimulate glycolysis and glucose oxidation and inhibit FFA oxidation. Regional myocardial ischemia was induced by a 60% decrease in left anterior descending coronary artery (LAD) blood flow, and glucose and FFA oxidation was measured using radioisotopic tracers. Anaerobic glycolysis was assessed from serial measurements of the arterial-coronary venous concentration differences and analysis of myocardial biopsies. Myocardial contractile power was assessed from the left ventricular (LV) pressure (LVP)-anterior wall segment length loop area measured with sonomicrometry.
METHODS
Experiments were performed on domestic swine (23 total, 37.1 ± 1.1 kg mean wt) in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, Revised 1996) and with the approval of the Institutional Animal Care and Use Committee at Case Western Reserve University.
Surgical preparation.
Studies were performed in an open-chest swine model that allows for simultaneous measurements of myocardial substrate utilization, metabolite concentrations in arterial and coronary venous blood, and tissue substrate concentrations as previously described in detail (20). After an overnight fast, animals were sedated with Telazol (6 mg/kg im), anesthetized with isoflurane (5% by mask), intubated via a tracheotomy, and maintained with isoflurane (0.75–1.25%) and ketamine (4 mg·kg−1·h−1) throughout the remainder of the experiment. Animals were mechanically ventilated with 100% O2, and blood gases were maintained in the normal range (Po2, >100 mmHg; Pco2, 35–45 mmHg; and pH 7.35–7.45). The heart was exposed via a midline sternotomy with a left-side rib resection. A femoral vein was cannulated, and the animals were heparinized to prevent clotting (200 U/kg bolus, followed by 100 U·kg−1·h−1). The LAD was cannulated above the first diagonal branch and perfused with blood supplied from the femoral artery via the extracorporal perfusion circuit (20). The anterior interventricular vein was catheterized for sampling the venous effluent from the LAD perfusion bed, and arterial blood samples were obtained from the perfusion line supplied by the femoral artery. Heart rate, aortic pressure, LVP, and systolic thickening were continuously recorded using an online data acquisition system (BioPac Acknowledge). LVP was measured with a 7-Fr high-fidelity transducer catheter (Millar Instruments, Houston, TX), and segmental shortening in the anterior LV free wall was measured by sonimicrometry (Triton Technologies, San Diego, CA) (20) in duplicate using two pairs of piezoelectric crystals placed at midmyocardial depth.
Experimental protocol.
Three groups were studied under baseline and ischemic conditions. The first group served as the control group (CTRL; n = 8, 35.1 ± 2.8 kg). The second group (IAA) was treated with an intracoronary infusion of IAA to inhibit glycolysis (n = 7, 36.9 ± 2.1 kg). A concentrated solution of IAA (15.4 mM) was infused into the LAD perfusion circuit at a rate set to give a 100-μM step increase in the concentration of IAA in LAD blood (0.0065 ml of IAA solution infused per ml of LAD blood flow). This concentration has been shown to inhibit glycolysis in nonischemic hearts without effecting contraction (1). The LAD flow is only 1% to 2% of the cardiac output (∼30 ml/min vs. ∼2000 ml/min), ensuring minimal recirculation of IAA into the general circulation. The third group (n = 8, 38.1 ± 1.5 kg mean wt) was treated with an intravenous infusion of insulin (0.3 U/kg bolus, followed by 1 U·kg−1·hr−1 iv) and glucose (100 mg/kg bolus, followed by 700 mg·kg−1·h−1 iv) (HI + HG). Each group underwent a 60-min baseline period followed by a 40-min ischemia period (60% reduction in blood flow). After completion of the surgical preparation, the tracer infusion was initiated at −60 min (time = 0 was set to the onset of ischemia) with a bolus [14C]glucose (20 μCi) followed by a constant infusion of [U-14C]glucose (13.3 μCi/h), and [9,10-3H]oleate (40 μCi/h) at a rate of 4.5 ml/h. An infusion of insulin and glucose or IAA was initiated at −50 min (10 after initiation of tracer infusion). All three groups had the same sampling protocol. For measurement of lactate exchange across the myocardium in the LAD perfusion bed, paired blood samples were simultaneously drawn from the coronary artery perfusion circuit and the anterior interventricular vein at −10 and −1 min (baseline) and at 1, 3, 5, 7, 10, 20, 30, and 40 min of ischemia. Small myocardial biopsies (∼20 mg) for measurement of tissue lactate were taken from the LAD perfusion territory at −7 min, and at 4, 12 and 41 min of ischemia using a 14-gauge biopsy needle. Blood samples for measurement of glucose and FFA uptake and oxidation with analysis of 14CO2 and 3H2O concentrations were taken at −10 and −1 min and 30 and 40 min of ischemia. At the conclusion of the experiment, an additional needle biopsy was taken for the measurement of glycogen concentration. All biopsies were immediately freeze clamped on aluminum blocks precooled in liquid nitrogen and stored at −80°C for later analysis.
Analytic methods.
Arterial and venous oxygen saturation and hemoglobin were determined on a hemoximeter (Avoximeter; San Antonio, TX) and pH, Pco2, and Po2 measured using a blood-gas analyzer (NOVA Profile Stat 3, NOVA Biomedical; Waltham, MA). Blood samples for glucose and lactate analysis were immediately deproteinized in ice-cold 1 M perchloric acid (1:2 vol/vol), weighed, centrifuged, and analyzed in quadruplicate for glucose and triplicate for lactate using previously described spectrophotometric methods (5). Blood samples for [14C]glucose measurements were neutralized with K2CO3 and passed through ion-exchange resin columns (Bio-Rad AG 50W-X8 Resin and Bio-Rad AG1-X8 Formate Resin) to separate [14C]glucose as previously described in detail (5). Total glucose concentration and 14C activity were then measured in the eluate to calculate [14C]glucose specific activity. Blood 14CO2 concentration was measured using 1 ml of blood by expelling 14CO2 with the addition of concentrated lactic acid and trapping it in hyamine hydroxide as previously described (5). Plasma FFA concentration was measured using a commercial kit (Wako Chemicals, Richmond, VA). Plasma [3H]oleate concentration was measured by extracting FFA from 0.5 ml of plasma in 3 ml of heptane-isopropanol (3:7) and counting the organic phase as previously described (5). 3H2O was measured by determining the difference in disintegrations per minute per milliliter of water distilled from plasma using a Hickman still. Tissue lactate was measured in perchloric acid extracts using a spectrofluorometric assay and glycogen using the amyloglucosidase method as previously described (23).
Calculations.
Myocardial blood flow was measured from the calibrated pump flow of the perfusion circuit and normalized by dividing by the weight of the heart being perfused by the LAD. The net uptakes (in μmol·min−1·g wet mass−1) for glucose, lactate, and FFA as well as the myocardial oxygen consumption (MV̇o2) were calculated based on the product of the arterial and coronary venous substrate difference and the normalized myocardial blood flow. The rates of exogenous glucose and FFA oxidation (in μmol·min−1·g wet mass−1) were calculated as the product of the release of either 14CO2 or 3H2O (in dpm/ml) and myocardial blood flow, divided by the arterial specific radioactivity of glucose or FFA (in dpm/μmol), as previously described (5, 23). Values for the baseline and ischemic periods were taken as the averages of −10 and −1 min and 30 and 40 min of ischemia. The LVP-segment length loop area was calculated off-line from ∼30 consecutive heartbeats using Matlab software and was used as the measure of external work of the anterior free wall of the LV (4, 5, 22). The external power of the anterior wall was calculated as the product of the LVP-segment length loop area and heart rate as previously described (4, 5).
Statistical analysis.
Hemodynamic and metabolic parameters were compared using a two-way ANOVA for repeated measures, with the Fisher least significant difference post hoc test. Anterior wall external power and mechanical efficiency were compared with a one-way ANOVA. Differences at the 0.05 level were considered significant. All values are reported as means ± SE.
RESULTS
Hemodynamics.
There were no differences among the three groups in heart rate at baseline or during ischemia. Peak LVP decreased significantly in the CTRL group under ischemic conditions but did not change in the IAA or HI + HG groups (Table 1). Peak positive and negative LV dP/dt were similar among groups and were unaffected by ischemia. MV̇o2 was significantly lower (P < 0.01) in the IAA group than in the CTRL group at baseline and decreased significantly during ischemia in all groups (P < 0.001). (Table 1). There were no differences in MV̇o2 among treatments during ischemia.
Table 1.
Hemodynamic variables in the CTRL, IAA, and HI + HG groups under aerobic conditions and during 60% myocardial ischemia
| CTRL |
IAA | HI + HG | ||||
|---|---|---|---|---|---|---|
| Baseline | Ischemia | Baseline | Ischemia | Baseline | Ischemia | |
| Heart rate, beats/min | 118±6 | 128±9 | 129±8 | 129±7 | 123±8 | 131±5 |
| Peak LVP, mmHg | 93±4 | 84±2† | 86±5 | 83±5 | 90±3 | 89±2 |
| dP/dtmax, mmHg/s | 1,870±156 | 1,799±107 | 1,902±183 | 1,959±154 | 2,102±134 | 1,999±182 |
| dP/dtmin, mmHg/s | −1,305±99 | −1,086±106 | −1,206±133 | −1,093±84 | −1,212±125 | −1,163±106 |
| MV̇o2, μmol·g−1·min−1 | 3.5±0.2 | 1.3±0.2‡ | 2.3±0.2* | 1.0±0.1‡ | 3.1±0.2 | 1.4±0.2‡ |
| Rate pressure product, mmHg·beat −1·min−1 | 11,000±900 | 10,800±1,000 | 11,000±800 | 10,200±600 | 11,300±1100 | 11,500±800 |
Values are means ± SE. CTRL, control; IAA, iodoacetate, HI + HG, hyperinsulinemia + hyperglycemia; LVP, left ventricular pressure; dP/dtmax and dP/dtmin, maximum and minimum first derivative of pressure, respectively; MV̇o2, myocardial oxygen consumption.
P < 0.01 vs. CTRL;
P < 0.01 vs. baseline;
P < 0.001 vs. baseline.
Circulating substrate and insulin levels.
There were no differences in arterial insulin concentration between CTRL and IAA groups (5.5 ± 0.9 vs. 5.8 ± 3.9 pM); however, the insulin concentrations in the HI + HG group were dramatically increased (4,219 ± 1,028 pM) (Fig. 1). Arterial FFA concentration was not significantly different under baseline conditions among groups but was lower in the HI + HG group during ischemia (Table 2). Arterial lactate and glucose concentration were also not different between CTRL and IAA groups at baseline and during ischemia (Table 2). Arterial glucose and lactate concentrations were significantly higher in the HI + HG group than the CTRL group (Table 2). In addition, in the HI + HG group, the arterial lactate concentration increased from baseline to 40 min of ischemia (Table 2).
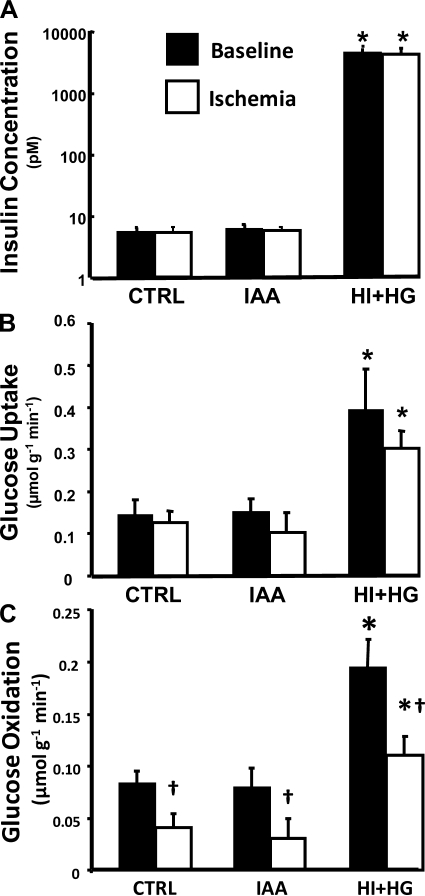
Fig. 1.
Arterial insulin concentration (A), myocardial glucose uptake (B), and glucose oxidation (C) for the control (CTRL), iodoacetate (IAA), and hyperinsulinemia and hyperglycemia (HI + HG) groups under baseline and during ischemia. *P < 0.001 vs. CTRL and IAA under the same condition; †P < 0.05 vs. baseline within the same treatment group.
Table 2.
Arterial substrate concentrations in the CTRL, IAA, and HI + HG groups under baseline conditions and during 60% myocardial ischemia
| CTRL |
IAA | HI + HG | ||||
|---|---|---|---|---|---|---|
| Baseline | Ischemia | Baseline | Ischemia | Baseline | Ischemia | |
| Glucose | 3.33±0.38 | 3.18±0.41 | 4.15±0.3 | 3.67±0.31 | 5.98±0.27* | 6.74±0.3* |
| Lactate | 1.77±0.13 | 1.93±0.19 | 2.00±0.21 | 2.38±0.22 | 2.30±0.11* | 2.89±0.15*† |
| FFA | 0.41±0.07 | 0.52±0.10 | 0.43±0.09 | 0.54±0.11 | 0.33±0.05 | 0.29±0.03‡ |
Values are means ± SE (in mM). FFA, free fatty acid.
P < 0.05 vs. CTRL;
P < 0.05 vs. baseline,
P < 0.05 vs. CRTL and IAA during ischemia.
Myocardial glucose and fatty acid metabolism.
Glucose uptake was significantly higher in the HI + HG group than the CTRL and IAA groups under both baseline and ischemic conditions (Fig. 1). Glucose oxidation decreased during ischemia in all groups (by 40%, 62%, and 44% compared with respective baseline for the CTRL, IAA, and HI + HG groups) and was significantly higher in the HI + HG group under both baseline and ischemic conditions compared with the that in the CTRL and IAA groups (Fig. 1).
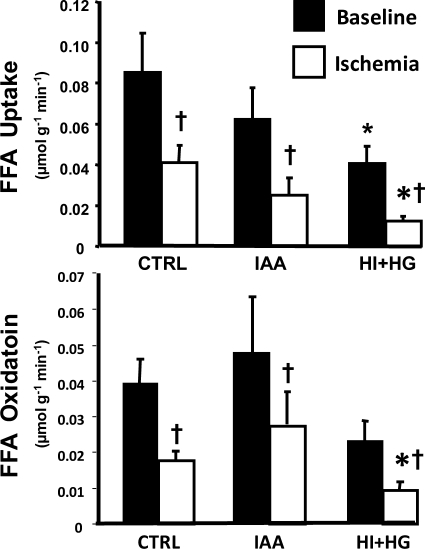
FFA uptake was significantly decreased in response to ischemia in all groups and was further inhibited in the HI + HG group compared with the CTRL and IAA groups (Fig. 2). Under baseline conditions, FFA oxidation was similar among treatment groups and decreased significantly during ischemia in all groups (Fig. 2). FFA oxidation was lower in the HI + HG group under ischemic conditions compared with that in the CTRL group (Fig. 2).
Fig. 2.
Myocardial free fatty acid (FFA) uptake (top) and oxidation (bottom) for the CTRL, IAA, and HI + HG groups under baseline and during ischemia. *P < 0.05 vs. CTRL under the same condition; †P < 0.05 vs. baseline within the same treatment group.
Glycolytic metabolism.
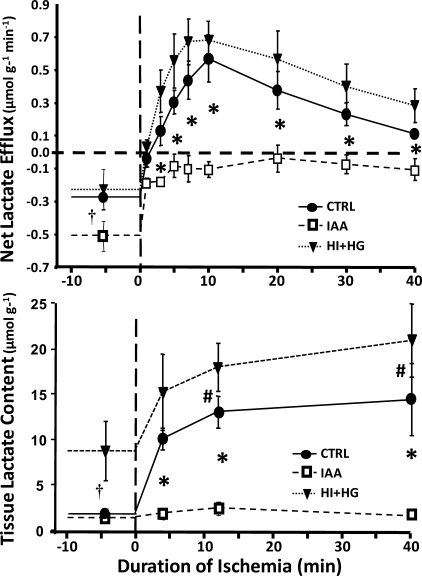
Myocardial lactate production was decreased in the IAA group compared with the CTRL and HI + HG groups. Under baseline conditions, there was a net lactate uptake in all the groups, which was greater in the IAA group than in the CTRL and HI + HG groups (Fig. 3). During ischemia, there was a rapid switch to net lactate production in the CTRL and HI + HG groups, but not in the IAA group, which maintained net lactate uptake throughout the ischemic period. Lactate efflux during ischemia was not different between the HI + HG and CTRL group. The cumulative net lactate efflux was significantly lower in the IAA group over the 40-min period of ischemia (−3.7 ± 1.8 μmol·g−1·40 min−1) compared with both the CTRL group (13.9 ± 3.4 μmol·g−1·40 min−1) or the HI + HG group (19.6 ± 4.7 μmol·g−1·40 min−1) (P < 0.01). Myocardial lactate concentration during the preischemic period was significantly higher in the HI + HG group compared with the CTRL and IAA groups (Fig. 3). During ischemia, treatment with IAA resulted in significantly lower myocardial lactate concentrations at all time points compared with the CTRL and HI + HG groups, and lactate concentration was significantly higher in the HI + HG group compared with the CTRL at 12 and 40 min of ischemia. The cumulative increase above the preischemic baseline in myocardial lactate concentration from 0 to 40 min of ischemia was less in the IAA group (16 ± 8 μmol·g−1·40 min−1) compared with the CTRL or HI + HG groups (280 ± 69 and 177 ± 99 μmol·g−1·40 min−1, respectively). Tissue glycogen content at the end of the ischemic period were 4.3 ± 1.0 and 3.0 ± 1.1 μmol/g in the CTRL and HI + HG groups, respectively, and 10.2 ± 2.3 μmols/g in the IAA group (P < 0.05), consistent with the inhibition of glycolysis with IAA treatment.
Fig. 3.
Net myocardial lactate uptake (top) and myocardial lactate concentration (bottom) plotted as a function of time in the CTRL, IAA, and HI + HG groups under normal flow conditions and during 40 min with a 60% decrease coronary blood flow. *P < 0.01 for IAA vs. both CTRL and HI + HG at given time point; †P < 0.05 for HI + HG vs. both CTRL and IAA at preischemic baseline; #P < 0.05 for HI + HG vs. CTRL at given time point.
Regional myocardial external power and myocardial efficiency.
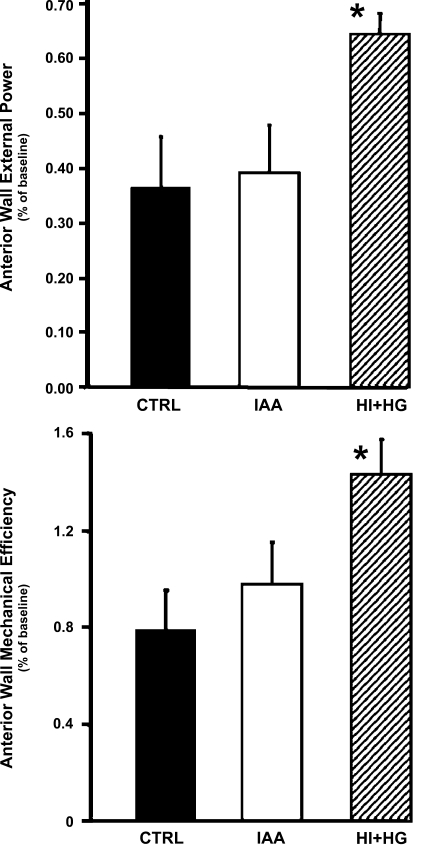
There were no differences at baseline in anterior wall fractional systolic shortening (0.217 ± 0.018, 0.208 ± 0.012, and 0.231 ± 0.0181 for the CTRL, IAA, and HI + HG groups, respectively) or rate-pressure product (Table 1). During myocardial ischemia, the anterior wall external power calculated from [(LVP − anterior wall segment length loop) × heart rate] was significantly higher in the HI + HG group than the CTRL and IAA groups (Fig. 4). Similarly, myocardial mechanical efficiency during ischemia was not different between the CTRL and IAA groups but was significantly greater in the HI + HG group (Fig. 4).
Fig. 4.
Comparison of regional external power (top) and myocardial mechanical efficiency (bottom) among the CTRL, IAA, and HI + HG groups in the anterior left ventricular free wall during ischemia expressed as a fraction of the values obtained under normal flow baseline conditions. *P < 0.05 vs. CTRL and IAA.
DISCUSSION
The present study investigated the role of anaerobic glycolysis and oxidative substrate selection on the regulation of myocardial contractile function and mechanical efficiency during moderate severity ischemia. There are two main findings from this study. First, we found that preventing anaerobic glycolysis during ischemia in the absence of alterations in FFA and glucose oxidation did not adversely affect contractile function or mechanical efficiency during myocardial ischemia. This suggests that anaerobic glycolysis during the transition from normal flow to moderate severity ischemia is not essential for maintaining residual contractile function. The second finding is that the insulin-induced acceleration of glucose uptake and oxidation, with a concomitant reduction in FFA oxidation, improved myocardial contractile function, and mechanical efficiency during ischemia. HI + HG accelerated glycolysis during ischemia, as evidenced by the greater rate of glucose uptake and the low myocardial glycogen content. Thus a smaller fraction of the glycolyticly derived pyruvate was diverted toward lactate formation (anaerobic glycolysis) and more toward oxidation in the mitochondria (aerobic glycolysis). Taken together, these results indicate that anaerobic glycolysis during the early transition to reduced coronary flow is not a major regulator of contractile function or mechanical efficiency of the myocardium. Rather, oxidative substrate selection affected systolic power and mechanical efficiency, as seen in the improved function with the increase in glucose oxidation and decrease in FFA acid oxidation in the HI + HG group.
Since the introduction of glucose and insulin infusion for treatment of acute ischemic events in 1962 by Sodi-Palares et al. (24), there have been extensive animal and clinical studies with this approach for treating acute myocardial infarction (8, 17, 28). Less is known about the effects of glucose-insulin therapy on cardiac function and metabolism during moderate severity ischemia, such as occurs clinically with acute coronary syndrome. The present study is the first to show that HI + HG causes a dramatic switch away from fatty acid oxidation toward glucose oxidation without a significant change in lactate efflux and improves contractile power and mechanical efficiency in the ischemic region. Previous human studies found that hyperinsulinemia lowers plasma FFA concentration and oxidation by the heart and increases glucose uptake and oxidation (9, 25, 28, 33). Cardiac glucose uptake is increased in an additive fashion by hyperinsulinemia and moderate severity ischemia (∼30% flow reduction) in canine myocardium, which is attributable to a parallel increase in the translocation of glucose transporters (mainly GLUT4) into the sarcolemmal membrane (21a, 34a). The present study extends these observations by demonstrating that during moderate severity ischemia, HI + HG decreases FFA oxidation, increases glucose oxidation, and improves mechanical power generation for a given rate of myocardial energy expenditure (i.e., improved myocardial mechanical efficiency). The improved efficiency could be due to greater anaerobic ATP production in the HI + HG group; however, lactate accumulation and efflux were not different between the CTRL and HI + HG groups during ischemia. The beneficial effect of HI + HG on contractile function was more likely the result of the clear switch in oxidative substrate from fatty acids to carbohydrates (Figs. 1 and 2). When compared with fatty acids, oxidation of carbohydrate is more oxygen efficient, with pyruvate yielding 11% more ATP than palmitate for a given amount of mitochondrial oxygen consumption (30). In addition, high fatty acid levels uncouple mitochondria, further contributing to oxygen wasting (2, 21). Another possibility is that the activation of insulin signaling pathways improves the efficiency of ATP synthesis and/or the use of ATP for contraction and ion homeostasis, although it is difficult to postulate specific mechanisms for such effects.
Inhibition of glycolysis with IAA prevented lactate production during ischemia but did not affect LV power or mechanical efficiency, providing a strong support for the concept that lactate production is not a major determinant of cardiac mechanical function during moderate severity ischemia. Few studies have examined the effects of IAA on cardiac metabolism and function in vivo. Jennings et al. (11) subjected dog hearts to total no-flow ischemia following treatment with a high concentration of IAA (∼1,500 μM) (11) and observed an accelerated adenine nucleotide depletion and prevention of lactate formation but no effects on ultrastructural damage. Hacker et al. (10) injected a similar bolus dose of IAA in pigs 40 min into an 85 min bout of ischemia (40% reduction in LAD flow) and observed a decrease in glycolysis from exogenous glucose, a switch from net lactate efflux to lactate uptake, and a sharp decline in systolic segment shortening in the ischemic territory (10). In the present study we employed a much lower dose (100 μM) and observed no association between anaerobic glycolysis and mechanical dysfunction, thus rejecting the concept that anaerobic glycolysis is a major determinant of contractile function and mechanical efficiency during ischemia.
Facilitated lactate transport and the mechanism of lactate efflux may have played an important role in the present study, particularly in the HI + HG group during ischemia. The HI + HG group had high tissue lactate under aerobic conditions (Fig. 3), which may exert a beneficial effect during ischemia by providing a source for pyruvate formation and subsequent oxidation by the mitochondria. Although we did not address the mechanisms of lactate transport in the present study, additional work is needed before the role of lactate metabolism in the overall scheme of these studies can be understood.
In conclusion, the present study found that reducing anaerobic glycolysis during ischemia in the absence of activation of glucose oxidation and/or suppression of fatty acid oxidation does not affect contractile function. Furthermore, contractile function and mechanical efficiency during ischemia were improved by hyperinsulinemia and hyperglycemia, which was associated with inhibition of fatty acid oxidation and stimulation of glucose oxidation but not greater anaerobic glycolysis. These finding suggest that metabolic therapies applied to moderate severity ischemia should not be aimed at inhibiting anaerobic glycolysis per se but rather activating insulin signaling and/or enhancing carbohydrate oxidation and decreasing fatty acid oxidation.
GRANTS
This work was supported by National Institutes of Health Grants HL-074237 and GM-66309.
Acknowledgments
Current address for L. Zhou: Div. of Cardiology, Dept. of Medicine, The Johns Hopkins Univ., 720 Rutland Ave., Ross 1055, Baltimore, MD 21205.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Apstein CS, Deckelbaum L, Hagopian L, Hood WB Jr. Acute cardiac ischemia and reperfusion: contractility, relaxation, and glycolysis. Am J Physiol Heart Circ Physiol 235: H637–H648, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Borst P, Loos JA, Christ EJ, Slater EC. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta 62: 509–518, 1964. [DOI] [PubMed] [Google Scholar]
- 3.Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, Eberli FR. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation 101: 2090–2096, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Chandler MP, Chavez PN, McElfresh TA, Huang H, Harmon CS, Stanley WC. Partial inhibition of fatty acid oxidation increases regional contractile power and efficiency during demand-induced ischemia. Cardiovasc Res 59: 143–151, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chandler MP, Huang H, McElfresh TA, Stanley WC. Increased nonoxidative glycolysis despite continued fatty acid uptake during demand-induced myocardial ischemia. Am J Physiol Heart Circ Physiol 282: H1871–H1878, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cross HR, Clarke K, Opie LH, Radda GK. Is lactate-induced myocardial ischaemic injury mediated by decreased pH or increased intracellular lactate? J Mol Cell Cardiol 27: 1369–1381, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res 68: 466–481, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation 96: 1152–1156, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Santoro D, Bonadonna R, Natali A, Parodi O, Camici PG. Metabolic and hemodynamic effects of insulin on human hearts. Am J Physiol Endocrinol Metab 264: E308–E315, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Hacker TA, Renstrom B, Nellis SH, Liedtke AJ. The role of glucose metabolism in a pig heart model of short-term hibernation. Mol Cell Biochem 180: 75–83, 1998. [PubMed] [Google Scholar]
- 11.Jennings RB, Reimer KA, Steenbergen C Jr, Schaper J. Total ischemia III: effect of inhibition of anaerobic glycolysis. J Mol Cell Cardiol 21, Suppl 1: 37–54, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Jeremy RW, Koretsune Y, Marban E, Becker LC. Relation between glycolysis and calcium homeostasis in postischemic myocardium. Circ Res 70: 1180–1190, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Liedtke AJ, Nellis SH, Mjos OD. Effects of reducing fatty acid metabolism on mechanical function in regionally ischemic hearts. Am J Physiol Heart Circ Physiol 247: H387–H394, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res 79: 940–948, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 39: 718–725, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 264: 135–144, 1993. [PubMed] [Google Scholar]
- 17.Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 293: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Kusuoka H, Ambrosio G, Becker LC. Glycolysis is necessary to preserve myocardial Ca2+ homeostasis during beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 264: H670–H678, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Panchal AR, Comte B, Huang H, Dudar B, Roth B, Chandler M, des RC, Brunengraber H, Stanley WC. Acute hibernation decreases myocardial pyruvate carboxylation and citrate release. Am J Physiol Heart Circ Physiol 281: H1613–H1620, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol 279: H2390–H2398, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Pressman BC, Lardy HA. Effects of surface active agents on the latent ATPase of mitochondria. Biochim Biophys Acta 21: 458–466, 1956. [DOI] [PubMed] [Google Scholar]
- 21a.Russell RR, Yin R, Caplan MJ, Hu X, Ren J, Shulman GI, Sinusas AJ, Young LH. Addictive effects of hyperinsulinemia and ischemia on myocardial GLUT1 and GLUT4 translocation in vivo. Circulation 98: 2180–2186, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Salem JE, Cabrera ME, Chandler MP, McElfresh TA, Huang H, Sterk JP, Stanley WC. Step and ramp induction of myocardial ischemia: comparison of in vivo and in silico results. J Physiol Pharmacol 55: 519–536, 2004. [PubMed] [Google Scholar]
- 23.Sharma N, Okere IC, Brunengraber DZ, McElfresh TA, King KL, Sterk JP, Huang H, Chandler MP, Stanley WC. Regulation of pyruvate dehydrogenase activity and citric acid cycle intermediates during high cardiac power generation. J Physiol 562: 593–603, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodi-Pallares D, Testelli MR, Fishleder BL, Bisteni A, Medrano GA, Friedland C, de Micheli A. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction. A preliminary clinical report. Am J Cardiol 9: 166–181, 1962. [DOI] [PubMed] [Google Scholar]
- 25.Stanley AW, Moraski RE, Russell RO, Rogers WJ, Mantle JA, Kreisberg RA, McDaniel HG, Rackley CE. Effects of glucose-insulin-potassium on myocardial substrate availability and utilization in stable coronary artery disease. Studies on myocardial carbohydrate, lipid and oxygen arterial-coronary sinus differences in patients with coronary artery disease. Am J Cardiol 36: 929–937, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Stanley WC Partial fatty acid oxidation inhibitors for stable angina. Expert Opin Investig Drugs 11: 615–629, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Stanley WC, Hernandez LA, Spires D, Bringas J, Wallace S, McCormack JG. Pyruvate dehydrogenase activity and malonyl CoA levels in normal and ischemic swine myocardium: effects of dichloroacetate. J Mol Cell Cardiol 28: 905–914, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 33: 243–257, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, Lopaschuk GD. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol Heart Circ Physiol 289: H2304–H2309, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Lloyd SG, Chatham JC. Impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery after low-flow ischemia and reperfusion in the isolated perfused rat heart. Circulation 111: 2066–2072, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest 75: 436–447, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisneski JA, Stanley WC, Neese RA, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J Clin Invest 85: 1648–1656, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L, Belardinelli L, Fraser H. A novel partial fatty acid oxidation inhibitor decreases myocardial oxygen consumption and improves cardiac efficiency in demand-induced ischemic heart. J Cardiovasc Pharmacol 51: 372–379, 2008. [DOI] [PubMed] [Google Scholar]
- 34a.Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, Shulman GI, Sinusas AJ. Low flow ischemia leads to translocation of canine heart GLUT4 and GLUT1 glucose transporters to the sarcolemma in vivo. Circulation 95: 415–422, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Zhu P, Lu L, Xu Y, Greyson C, Schwartz GG. Glucose-insulin-potassium preserves systolic and diastolic function in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol 278: H595–H603, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]