Abstract
Amiloride, injected into the popliteal artery, has been reported to attenuate the reflex pressor response to static contraction of the triceps surae muscles. Both mechanical and metabolic stimuli arising in contracting skeletal muscle are believed to evoke this effect, which has been named the exercise pressor reflex. Amiloride blocks both acid-sensing ion channels, as well as epithelial sodium channels. Nevertheless, amiloride is thought to block the metabolic stimulus to the reflex, because this agent has been shown to attenuate the reflex pressor response to injection of lactic acid into the arterial supply of skeletal muscle. The possibility exists, however, that amiloride may also block mechanical stimuli evoking the exercise pressor reflex. The mechanical component of the reflex can be assessed by measuring renal sympathetic nerve activity during the first 2–5 s of contraction. During this period of time, the sudden tension developed by contraction onset briskly discharges mechanoreceptors, whereas it has little effect on the discharge of metaboreceptors. We, therefore, examined the effect of amiloride (0.5 μg/kg) injected into the popliteal artery on the renal sympathetic and pressor responses to static contraction of the triceps surae muscles in decerebrated cats. We found that amiloride significantly attenuated the pressor and renal sympathetic responses to contraction; for the latter variable, the attenuation started 10 s after the onset of contraction. Our findings lead us to conclude that acid-sensing ion channels and epithelial sodium channels play little, if any, role in evoking the mechanical component of the exercise pressor reflex.
Keywords: group III and IV muscle afferents, renal sympathetic nerves, neural control of the circulation, cats, autonomic nervous system
static exercise increases mean arterial pressure and minute volume of ventilation to maintain an adequate supply of oxygen and blood to metabolically active muscles. The exercise pressor reflex is believed to be one cause of these cardiovascular and ventilatory responses to exercise (2, 23, 25). The afferent arm of the reflex is composed of group III and IV muscle afferents (23), the endings of which are stimulated by both mechanical and metabolic stimuli arising in contracting muscles (16, 17). Considered together, group III and IV afferents have been termed thin-fiber afferents, a name used to distinguish them from thick-fiber afferents or group I and II afferents (i.e., muscle spindles and Golgi tendon organs). For the most part, group III afferents are thought to be responsive to mechanical stimuli, whereas group IV afferents are thought to be responsive to metabolic stimuli arising in the contracting muscles (15).
The metabolite or metabolites that stimulate thin-fiber afferents during contraction are under investigation. Bradykinin, ATP, prostaglandins, and lactic acid have each been suggested to play a role in evoking the metabolic component of the exercise pressor reflex (9, 20, 27, 36). Of interest, lactic acid injected into the arterial supply of skeletal muscle reflexly increased arterial pressure, heart rate, and ventilation (33). Similarly, lactic acid increased the discharge rate of group III and IV muscle afferents (32). In addition, presumptive blockade of acid-sensing ion channels (ASICs) and epithelial sodium channels (ENaCs) with amiloride attenuated the exercise pressor reflex (11). Taken together, lactic acid, working through ASICs or ENaCs, may play an important role in evoking the metabolic component of the exercise pressor reflex.
In the experiments described above (11), amiloride, injected into the arterial supply of the triceps surae muscles, had no effect on the pressor response to tendon stretch. This finding led to the conclusion that ASICs and ENaCs are not involved in the pressor response to tendon stretch, which has been used to represent the muscle mechanoreceptor reflex (34). Hayes and colleagues (10) discovered that the group III mechanoreceptors responsive to tendon stretch often were not responsive to static contraction. Therefore, the finding that the pressor response to muscle stretch is unaffected by amiloride may not be applicable to the mechanoreceptor component of the exercise pressor reflex, the stimulus for which is muscle contraction and is not muscle stretch.
Nevertheless, ASICs and ENaCs have been suggested to be involved in the transduction of mechanical stimuli. For example, amiloride reduced the responses of baroreceptor neurons to mechanical stimulation (4). In addition, mechanosensory dorsal root ganglion cells were found to express ASIC subunits (8). Futhermore, transgenic expression of the ASIC3 subunit in mice leads to an increased sensitivity to mechanical stimuli (26).
The mechanical component of the exercise pressor reflex can be assessed during the first 2–5 s of static contraction, because group III mechanoreceptors often discharge explosively in response to the sudden tension developed during the first seconds of this maneuver (16). In contrast, group IV metaboreceptors respond minimally to contraction during the first 2–5 s of its onset (16, 17). Arterial blood pressure is not a useful index of the mechanical component of the exercise pressor reflex, because vascular smooth muscle has a slow time constant, making it uncertain whether mechanoreceptors were responsible for a pressor effect. In contrast, renal sympathetic nerve activity (RSNA) is a useful index of this component because it increases within the first 2–5 s of static contraction (19, 21, 37). In the studies to be described, we have used amiloride to determine the effect of blockade of ASICs and ENaCs on thin-fiber afferents innervating the triceps surae muscles on the renal sympathetic response to static contraction and tendon stretch in decerebrated cats. We tested the hypothesis that ASICs and ENaCs play a role in evoking the mechanical component of the exercise pressor reflex.
METHODS
All procedures were reviewed and approved by the Institutional Care and Use Committee of the Pennsylvania State University, Hershey Medical Center.
Surgical preparation.
Adult cats (n = 19, 3.7 ± 0.2 kg, range 2.8–5.0 kg) of either sex (8 males; 11 females) were anesthetized with a mixture of 5% isoflurane and oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham) to monitor blood pressure. Heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within normal range by adjusting either ventilation or intravenous administration of sodium bicarbonate (8.5%). A temperature probe was passed through the mouth to the stomach. Temperature was continuously monitored and maintained at 37–38°C by a water-perfused heating pad.
The left common iliac artery and vein were isolated, and snares were placed around these vessels to trap the amiloride in the circulation of the leg (see below). The left triceps surae muscles, left popliteal artery, and tibial nerve were isolated. The cat was placed in a Kopf stereotaxic frame and spinal unit. The left calcaneal bone was cut, and its tendon was attached to a force transducer (model FT-10C, Grass) for measurement of the tension developed during stretch and static contraction of the left triceps surae muscles. The knee joint was secured to a post.
The cats were decerebrated at the midcollicular level under isoflurane anesthesia. Dexamethasone (4 mg) was injected intravenously just before the decerebration procedure to minimize brain edema. The left common carotid artery was tied off to reduce bleeding. All neural tissue rostral to the midcollicular section was removed, and the cranial vault was filled with agar.
The left renal nerve was exposed using a retroperitoneal approach, while the cat was positioned in the Kopf stereotaxic frame and spinal unit. The nerve was cut; its central end was draped over a monopolar hook electrode attached in series with a high-impedance probe (model HIP 511, Grass) and then amplified (model P511, Grass). Petroleum jelly was applied to the nerve. RSNA was displayed on a storage oscilloscope (Hewlett-Packard) and made audible. The amplifier was filtered between 100 Hz and 3 kHz. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air.
Experimental protocol.
The effect of amiloride (0.5 μg/kg) on the reflex pressor, cardioaccelerator, and renal sympathetic nerve responses to three stimuli were assessed: 1) static contraction of the left triceps surae muscles; 2) stretch of the left calcaneal tendon, which, in turn, stretched the triceps surae muscles; and 3) injection of lactic acid (24 mM; 0.5–1.0 ml) into the left popliteal artery. Static contraction is a combined mechanical and metabolic stimulus, tendon stretch is a mechanical stimulus, and lactic acid injection is a metabolic stimulus. Contraction and tendon stretch were applied for 60 s. The triceps surae muscles were contracted statically by electrical stimulating of the left tibial nerve (40 Hz, 25 μs, 2 times motor threshold); stretch was induced by turning a rack and pinion that was attached to the calcaneal tendon. Baseline tension was set at 0.3–0.5 kg. Arterial blood pressure, heart rate, and RSNA were recorded for 60 s before and during static contraction or tendon stretch. Injections of lactic acid were accomplished by gently inserting a 30-gauge needle into the popliteal artery and then injecting the compounds over ∼10 s into the vasculature of the triceps surae muscles. Before injecting lactic acid into the popliteal artery, we paralyzed the cat by injecting intravenously rocuronium bromide (0.5–0.7 mg/kg). The three maneuvers were repeated at 30 and 60 min after injection of amiloride (0.5 μg/kg) into the left popliteal artery. Immediately before injecting amiloride, we tightened the snare placed around the left common iliac artery and vein. Amiloride was then injected into the popliteal artery, and the snares were maintained for 10 min, after which they were released and the hindlimb was freely perfused. At this point, the triceps surae muscles were contracted, followed by stretch and injection of lactic acid. Amiloride exhibits peak attenuation of the exercise pressor response by 30 min and is no longer effective 60 min after injection at this dose (0.5 μg/kg) (11). Thus we ran the protocol again to ensure that any attenuation in mean arterial pressure, heart rate, or RSNA was from the effect of amiloride and not from deterioration of the preparation. The renal postganglionic sympathetic activity was confirmed by intravenous injection of hexamethonium (20 mg/kg) at the end of data collection.
We choose the dose of amiloride used for this protocol (0.5 μg/kg) based on the findings of Hayes et al. (11), who used a low (0.5 μg/kg) and high dose of amiloride (5.0 μg/kg) to investigate the pressor responses to contraction and tendon stretch and to injections of lactic acid and capsaicin. They found that both doses of amiloride blocked the pressor responses to contraction and lactic acid. In addition, the high dose of amiloride (5.0 μg/kg) blocked the pressor responses to both stretch and capsaicin injection, maneuvers in which ASICs and ENaCs probably played no significant role. Thus the high dose of amiloride not only blocked ASICs and ENaCs but probably also blocked voltage-gated sodium channels, thereby impairing the ability of the afferents to conduct action potentials. In contrast, the low dose did not block the pressor responses to stretch and to capsaicin injection and, therefore, seemed to block selectively ASICs and ENaCs.
Data analysis.
Mean arterial blood pressure, heart rate, and RSNA values are expressed as means ± SE. Baseline mean arterial blood pressure and heart rate were measured immediately before a maneuver, and peak mean arterial blood pressure and heart rate were measured during injection of lactic acid, as well as during the 60 s of tendon stretch or static muscle contraction. RSNA was rectified and integrated (Spike 2), and 60-s values were used to compare the differences between baseline and the response to each maneuver. The integrated voltage found after hexamethonium injection was subtracted from the integrated RSNA values. In addition, the time course of the mean arterial pressure and RSNA responses to static contraction and tendon stretch were analyzed at 2 s following the onset of each maneuver, and also at 5-s time points until the maneuver ended. The tension-time index was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kilograms per second (28). Statistical comparisons were performed with either a one-way repeated-measures ANOVA or two-way repeated-measures ANOVA. If significant main effects were found with an ANOVA, post hoc tests were performed with the Tukey test between individual means. We used the Bonferroni post hoc tests to determine significant differences between time course means. The criterion for statistical significance was P < 0.05.
RESULTS
Static contraction.
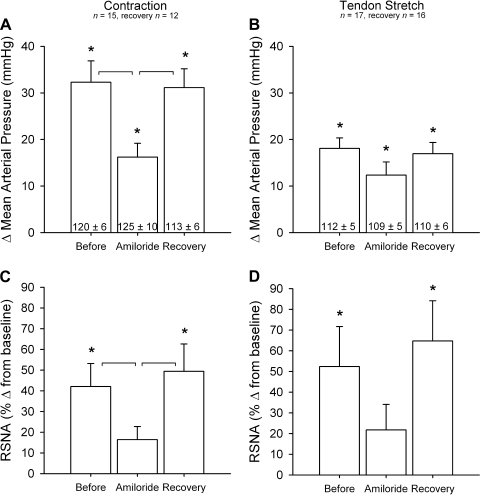
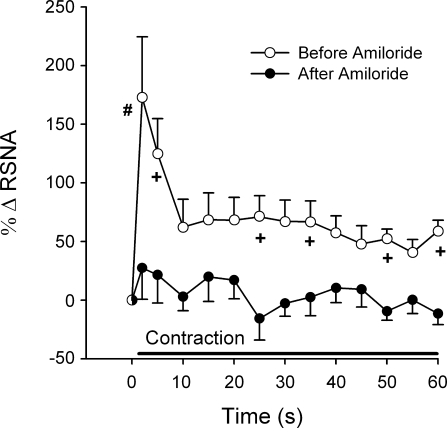
After a latent period of 30 min, amiloride attenuated the pressor and renal sympathetic nerve responses to static contraction (n = 15, n = 12 for recovery) (P < 0.05; Figs. 1–3). After a latent period of 60 min, the pressor and renal sympathetic nerve responses returned to their initial (i.e., before amiloride injection) values (Fig. 1; P > 0.95). We did not attempt to obtain recovery data for the first three cats tested in this experimental series. Mean arterial pressure increased 5 s after onset of contraction before the injection of amiloride (Fig. 2). Amiloride blunted the pressor response to contraction 5 s after the onset and continued the attenuation throughout the contraction (Fig. 2). RSNA increased within 2 s after onset of contraction before the injection of amiloride (Fig. 2). On average, amiloride did not blunt RSNA until 10 s after the onset of contraction, but then did so at almost every 5-s time point afterwards (Fig. 2). In five (2 male, 3 female) of the 15 cats tested, amiloride blunted the renal sympathetic nerve response to contraction within 5 s (Fig. 4). The cardioaccelerator response to static contraction was small and approached significance (P < 0.06; Table 1); amiloride appeared to have little effect on it (Table 1). The tension-time indexes before amiloride injection for static contraction were not different from those at 30 or 60 min after injection (P = 0.58; Table 2).
Fig. 1.
Effects of amiloride on pressor and renal sympathetic nerve activity (RSNA) responses to contraction (A and C) and tendon stretch (B and D) before amiloride, after amiloride, and during recovery from amiloride. Vertical axes represent the change (Δ) in baseline to peak mean arterial pressure (A and B) and RSNA (C and D). Values are means ± SE; n, no. of animals. Values inside open bars are baseline values. *Significant difference (P < 0.05) between baseline and peak. Horizontal brackets connect significantly different increases (P < 0.05) before amiloride, after amiloride, and during recovery from amiloride.
Fig. 3.
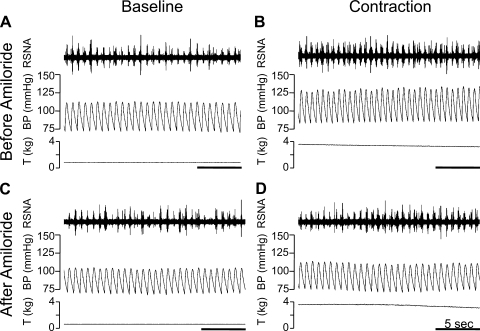
Amiloride (0.5 μg/kg) attenuates the renal sympathetic and pressor responses to static contraction. A: baseline RSNA and arterial blood pressure (BP) with the triceps surae muscles at rest (before amiloride). B: renal sympathetic and BP responses 20 s after start of static contraction (before amiloride). C: baseline RSNA and arterial BP with the triceps surae muscles at rest (after amiloride). D: renal sympathetic and BP responses 20 s after start of static contraction (after amiloride). Horizontal bar represents 5 s. T, tension developed by the triceps surae muscles.
Fig. 2.
Time course of the mean arterial pressure (A) and RSNA (B) responses to static contraction. The contraction lasted for 60 s, as indicated by the solid bar. ○, Mean responses before amiloride (0.5 μg/kg) was injected into the popliteal artery; •, mean responses after amiloride was injected. A: the first symbol represents baseline mean arterial pressure. The second symbol represents the average peak mean arterial pressure 2 s after onset of contraction. The third symbol represents the average peak mean arterial pressure 5 s after onset of contraction, and each symbol thereafter represents peak mean arterial pressure in 5-s increments. B: baseline RSNA, expressed as 0% change in RSNA, is represented by a half-filled circle. The first symbol following baseline RSNA represents change from baseline in RSNA 2 s after onset of contraction, the second symbol represents change from baseline in RSNA 5 s after onset of contraction, and each symbol thereafter represent change from baseline in RSNA in 5-s increments. Values are means ± SE; n = 15 animals. *P < 0.05 vs. baseline; +P < 0.05 vs. after amiloride at same time point.
Fig. 4.
Time course of the RSNA responses in 5 cats where amiloride blunted the RSNA increase within the first 5 s of static contraction. The contraction lasted for 60 s, as indicated by the solid bar. ○, Mean responses before amiloride (0.5 μg/kg) was injected into the popliteal artery; •, mean responses after amiloride was injected. Baseline RSNA, expressed as 0% change in RSNA, is represented by a half-filled circle. The first symbol following baseline RSNA represents change from baseline in RSNA 2 s after onset of contraction, the second symbol represents change from baseline in RSNA 5 s after onset of contraction, and each symbol thereafter represents change from baseline in RSNA in 5-s increments. Values are means ± SE; n = 5 animals. +P < 0.05 vs. after amiloride at same time point; #P = 0.05 vs. after amiloride at same time point.
Table 1.
Baseline heart rate and change in heart rate from baseline to peak during static contraction and tendon stretch
| Baseline Heart Rate | Change in Heart Rate | |
|---|---|---|
| Static contraction | ||
| Before amiloride | 131±9 | 6±2 |
| After amiloride | 131±12 | 3±1 |
| Recovery | 122±10 | 7±2 |
| Tendon stretch | ||
| Before amiloride | 130±9 | 3±1 |
| After amiloride | 130±9 | 3±1 |
| Recovery | 134±8 | 4±1 |
Values are means ± SE in beats/min; n = 15 (static contraction), 12 (static contraction recovery), 17 (tendon stretch), and 16 (tendon stretch recovery).
Table 2.
Tension-time indexes for static contraction and tendon stretch
| Static Contraction | Tendon Stretch | |
|---|---|---|
| Before amiloride | 122±9 | 155±15 |
| After amiloride | 116±11 | 164±17 |
| Recovery | 118±12 | 163±16 |
Values are means ± SE in kg/s; n = 15 (static contraction), 12 (static contraction recovery), 17 (tendon stretch), and 16 (tendon stretch recovery).
Tendon stretch.
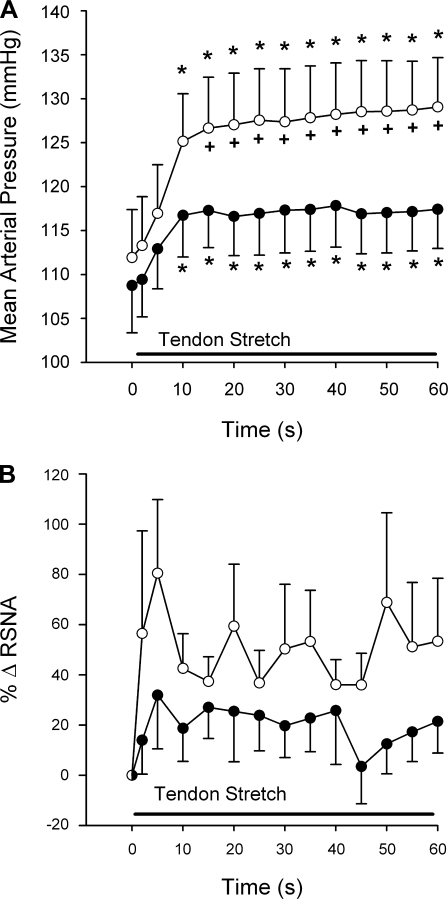
Amiloride had no significant effect on the pressor and renal sympathetic nerve responses to tendon stretch (Fig. 1). Nevertheless, amiloride abolished the renal nerve responses to stretch in 11 of the 17 cats tested but had little effect on the renal nerve response in the remaining 6 (2 male, 4 female). This finding resulted in the large variability for the time course means (Fig. 5) but still gave the appearance of an effect by amiloride. The tension-time indexes before amiloride injection for tendon stretch were not different from those at 30 or 60 min after injection (P = 0.52; Table 2).
Fig. 5.
Time course of the mean arterial pressure (A) and RSNA (B) responses to tendon stretch. The tendon stretch lasted for 60 s, as indicated by the solid bar. ○, Mean responses before amiloride (0.5 μg/kg) was injected into the popliteal artery; •, mean responses after amiloride was injected. A: the first symbol represents baseline mean arterial pressure. The second symbol represents the average peak mean arterial pressure 2 s after onset of stretch. The third symbol represents the average peak mean arterial pressure 5 s after onset of stretch, and each symbol thereafter represents peak mean arterial pressure in 5-s increments. B: baseline RSNA, expressed as 0% change in RSNA, is represented by a half-filled circle. The first symbol following baseline RSNA represents change from baseline in RSNA 2 s after onset of stretch, the second symbol represents change from baseline in RSNA 5 s after onset of stretch, and each symbol thereafter represents change from baseline RSNA in 5-s increments. Values are means ± SE; n = 17 animals. +P < 0.05 vs. after amiloride at same time point; *P < 0.05 vs. baseline.
Lactic acid.
In each of the 19 cats in which we either contracted or stretched the triceps surae muscles, we compared the pressor and renal sympathetic nerve responses to lactic acid, injected into the popliteal artery before amiloride with those to lactic acid ∼30 min after amiloride was injected into the popliteal artery. We found that, in each of the 19 cats tested, amiloride attenuated these responses (Fig. 6). In addition, intravenous injection of hexamethonium bromide (20 mg/kg) abolished the renal sympathetic discharge in each of the 19 cats.
Fig. 6.
Effects of amiloride on pressor and RSNA responses to lactic acid injection into the popliteal artery before and after amiloride. Vertical axes represent the change in baseline to peak mean arterial pressure (A) and RSNA (B). Values are means ± SE. Values inside open bars are baseline values. *Significant difference (P < 0.05) between baseline and peak. Horizontal brackets connect significantly different increases (P < 0.05) before and after amiloride.
DISCUSSION
The purpose of our experiments was to determine whether ASICs and ENaCs on thin-fiber muscle afferents played a role in evoking the mechanical component of the exercise pressor reflex. We blocked ASICs and ENaCs on these afferents by injecting amiloride into the arterial circulation of the muscles being contracted. The efficacy of the blockade was confirmed by showing that the pressor and renal sympathetic responses to popliteal arterial injection of lactic acid were attenuated by about one-half. On average, we found no evidence that RSNA was attenuated during the first 5 s of static contraction, a finding that suggests that ASICs and ENaCs played little role in discharging group III mechanoreceptors during static contraction.
The effect of amiloride on the renal sympathetic response to tendon stretch was highly variable and, therefore, was difficult to interpret. Nevertheless, its effect on the renal sympathetic response to stretch contrasted markedly with the effect of amiloride on the renal sympathetic responses to static contraction (compare Figs. 2B and 5B). In addition, amiloride had small and nonsignificant effects on the peak pressor response to tendon stretch, a finding that replicates that previously reported (11). However, when the pressor response is broken down into 5-s averages, amiloride did in fact attenuate the pressor response after 15 s through to the end of the tendon stretch (see Fig. 5).
A major limitation of our experiments is that we do not know the specific receptor protein(s) blocked by amiloride, a compound that antagonizes the α-, β-, and γ-proteins comprising ENaCs as well as the six proteins comprising the ASICs. In vitro, amiloride blocks ENaCs subunits at a lower concentration than it blocks the ASICs subunits (18). Also in vitro, amiloride in high concentrations blocks calcium channels as well as the sodium/calcium exchanger (22). We do not know the concentration of amiloride that existed at the endings of the group III and IV afferents in the triceps surae muscles in our experiments and, therefore, cannot distinguish between effects attributable to blockade of ENaCs from those attributable to blockade of ASICs. Nevertheless, based on information obtained from our laboratory's previous experiments (11), we can state that the dose of amiloride injected (i.e., 0.5 μg/kg) has been found to have no effect on transient receptor potential vanilloid type 1 channels on group III and IV afferents, as well as no effect on the discharge properties of group I and II spindle afferents. Therefore, the probability that amiloride impaired impulse conduction by blocking voltage-gated sodium channels is low in our experiments (1). The lack of effect of amiloride on transient receptor potential vanilloid type 1 channels in our experiments might explain why this compound only attenuated one-half of the pressor response to lactic acid injection.
Perhaps our most important finding was that amiloride had no effect on the first 5 s of the renal sympathetic response to static contraction, whereas it greatly attenuated the first 5 s of this response to tendon stretch in 11 of the 17 cats studied. This finding seems consistent with our laboratory's previous finding that static contraction stimulated a different, although overlapping, population of group III mechanoreceptors than did tendon stretch (10). Specifically, it may be that the group III mechanoreceptors responsive to stretch possessed ENaCs and ASICs, whereas those responsive to contraction did not. Alternatively, the two groups of mechanoreceptors may have different configurations of these receptor proteins. We can only speculate as to why amiloride blocked the early (i.e., the first 5 s) renal nerve response to tendon stretch in 11 of the 17 cats tested but had no effect on the renal nerve response to stretch in the remaining 6. This speculation would have to include the thought that group III mechanoreceptors on the 11 amiloride-sensitive cats had a different ENaC and ASIC receptor configuration than did the group III mechanoreceptors on the 6 amiloride-insensitive cats. If this were the case, it may be due to genetic polymorphisms for ENaC and ASIC receptors among cats.
On average, ASICs and ENaCs did not appear to contribute to the stimulation of group III mechanoreceptors by static contraction. However, we did find in 5 of the 15 cats studied that RSNA was attenuated at 2 and 5 s after onset of contraction, suggesting that ASICs and ENaCs did play a role in stimulating group III mechanoreceptors in this subpopulation. The reasoning for the divergent findings between cats could be that ASICs and ENaCs on group III mechanoreceptors have genetic polymorphisms.
Our data indicate that amiloride attenuated the exercise pressor reflex by acting on metabolically sensitive thin-fiber muscle afferents (16, 17), most of which conduct impulses in the group IV (i.e., C-fiber) range. These afferents usually respond to static contraction with an onset latency of at least 5 s, and their responses are increased by occlusion of the blood supply to the working muscles (16, 17). Mechanical stimuli, such as tendon stretch and nonnoxious probing of their receptive fields, usually have little effect on their impulse activity (16, 17). In addition, group IV afferents respond to lactic acid injection into the arterial supply of the muscles (32), an effect that is attenuated by amiloride (11). Similarly, blockade of presumptive ASICs and ENaCs with amiloride attenuates the exercise pressor reflex (11).
The role played by ENaCs and ASICs in transducing mechanical stimuli is controversial. For example, in mice, some investigators believe that the ASIC2 protein is essential for cutaneous and visceral mechanoreception (29, 30), whereas others disagree (3, 35). ENaCs may also play a role in transducing mechanical stimuli in both sensory nerves and vascular smooth muscle cells. Specifically, the γ-subunit of ENaCs has been thought to play a role in carotid sinus baroreceptor transduction of arterial pressure (4). In addition, both ASIC2 (7) and the β- and γ-subunits of ENaCs (14) are believed to play a role in evoking myogenic vasoconstriction.
We are tempted to suggest that amiloride attenuated the metabolic component of the exercise pressor reflex by antagonizing ASIC3 on group IV muscle afferents. ASIC3 are found, for the most part, on dorsal root ganglion cells (38), but not in the central nervous system. In addition, ASIC3 are opened by pH values ranging between 7.4 and 7.0 (12, 13), proton concentrations that are found within the muscle interstitium during exercise. Furthermore, ASIC3 is more responsive to lactic acid than it is to hydrochloric acid of the same pH, because lactate ions chelate extracellular calcium (13). These findings, however, do not allow us to exclude the possibility that amiloride attenuated the metabolic component of the exercise pressor reflex by antagonizing ENaCs on group IV muscle afferents.
In conclusion, our finding that ASICs and ENaCs on thin-fiber muscle afferents played little role in activating group III mechanoreceptors during static contraction, but seemed to play an important role in activating group IV metaboreceptors during this maneuver, enables us to draw some interesting parallels with findings reported by investigators working with humans. Specifically, Middlekauff et al. (24) concluded that, in healthy humans, reduction in lactic acid production by infusion of dichloroacetate had little effect on the reflex pressor and muscle sympathetic nerve responses evoked by stimulation of presumptive group III mechanoreceptors. Similarly, Ettinger et al. (5) concluded that reductions in lactic acid production with dichloroacetate attenuated the pressor and muscle sympathetic nerve responses to stimulation of presumptive group IV metaboreceptors. Finally, Victor and colleagues (6, 31), using both healthy subjects and patients with McArdle's disease, concluded that lactic acid production by exercising muscles played an important role in causing the reflex pressor and muscle sympathetic nerve responses evoked by stimulation of presumptive group IV metaboreceptors. Considered together, our laboratory's present and past findings (11), as well as those in humans, provide support for the hypothesis that ASICs and ENaCs do not play a role in discharging group III mechanoreceptors during static contraction.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR 051503.
Acknowledgments
We thank Jennifer Probst for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Carr MJ, Gover TD, Weinreich D, Undem BJ. Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues. Br J Pharmacol 133: 1255–1262, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556: 691–710, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol 261: H1653–H1658, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol 548: 983–993, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci 21: 2678–2686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–2323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous beta and gamma ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447.
- 16.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fiber mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol 292: H866–H873, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kleyman TR, Cragoe EJ Jr. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988. [DOI] [PubMed] [Google Scholar]
- 23.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci 25: 9893–9901, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Gonzalez JF Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-86, 1981. [PubMed] [Google Scholar]
- 29.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's Disease). J Clin Invest 85: 1444–1449, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Rowell L, O'Leary D. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol 558: 659–669, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59: 645–654, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann N Y Acad Sci 868: 67–76, 1999. [DOI] [PubMed] [Google Scholar]