Abstract
The prolonged production of reactive oxygen species due to ischemia-reperfusion (I/R) is a potential cause of the pathological remodeling that frequently precedes heart failure. We tested the ability of a potent dithiol antioxidant, bucillamine, to protect against the long-term consequences of I/R injury in a murine model of myocardial infarction. After transiently occluding the left anterior descending coronary artery for 30 min, saline or bucillamine (10 μg/g body wt) was injected intravenously as a bolus within the first 5 min of reperfusion. The antioxidant treatment continued with daily subcutaneous injections for 4 wk. There were no differences in infarct sizes between bucillamine- and saline-treated animals. After 4 wk of reperfusion, cardiac hypertrophy was decreased by bucillamine treatment (ventricular weight-to-body weight ratios: I/R + saline, 4.5 ± 0.2 mg/g vs. I/R + bucillamine, 4.2 ± 0.1 mg/g; means ± SE; P < 0.05). Additionally, the hearts of bucillamine-treated mice had improved contractile function (echocardiographic measurement of fractional shortening) relative to saline controls: I/R + saline, 32 ± 3%, versus I/R + bucillamine, 41 ± 4% (P < 0.05). Finally, I/R-induced injury in the saline-treated mice was accompanied by a fetal pattern of gene expression determined by ribonuclease protection assay that was consistent with pathological cardiac hypertrophy and remodeling [increased atrial natriuretic peptide, β-myosin heavy chain (MHC), skeletal α-actin; decreased sarco(endo)plasmic reticulum Ca2+ ATPase 2a, and α-MHC-to-β-MHC ratio]. These changes in gene expression were significantly attenuated by bucillamine. Therefore, treatment with a dithiol antioxidant for 4 wk after I/R preserved ventricular function and prevented the abnormal pattern of gene expression associated with pathological cardiac remodeling.
Keywords: echocardiography, fetal gene expression, heart, hypertrophy, ribonuclease protection assay
the pathological remodeling of the heart is a serious adverse outcome of acute myocardial infarction, despite the widespread application of reperfusion strategies. Ventricular remodeling, defined as hypertrophy of the myocytes, fibrosis of the noninfarcted myocardium, and changes in the geometry of the ventricle, presages the development of congestive heart failure (4, 29). Ventricular remodeling is also associated with a pattern of gene expression normally present in the fetal heart but absent in the adult heart. This abnormal pattern of gene expression generally accompanies decreased contractile function (22, 28). Changes in ventricular structure, function, and gene expression have been described in patients with heart failure (22, 35) and animal models of myocardial infarction (8, 47). Similar abnormalities in gene expression have been induced in myocardial cell cultures (2, 33).
Oxidative stress has been proposed as an important regulator of cardiac remodeling (24, 31). Cardiac ischemia-reperfusion (I/R) triggers a vigorous inflammatory response involving cytokine release, leukocyte activation, and generation of high levels of reactive oxygen species (3, 7, 19, 41). Leukocyte release of reactive oxygen species is very intense immediately after the onset of reperfusion (6), and there is evidence that antioxidant therapies given the first few hours of reperfusion can reduce infarct size (12, 13, 25). However, less robust increases in oxidative activity, generated from mitochondria or other sources, may persist for weeks or months (24, 40). A few studies in genetically altered murine models of permanent coronary artery occlusion or models of pressure overload have provided evidence that the long-term generation of oxidative stress may cause ventricular dysfunction (32, 39, 46). In addition, in vitro cell culture models support a role for oxidative signaling in the regulation of pathological remodeling at the cellular level (2, 9, 36). Nevertheless, in two recent human clinical studies, the administration of the antioxidant vitamin E was unsuccessful in preventing the development of heart failure (20, 23). Therefore, the importance of oxidative processes in remodeling or the subsequent development of heart failure has not been clearly established, particularly following early reperfusion of myocardial infarctions.
We investigated the long-term protective effects of bucillamine, a potent antioxidant dithiol donor (1, 11, 13, 43) on ventricular remodeling following myocardial I/R injury in genetically normal mice. We found that daily treatment with bucillamine, started during reperfusion and continued for 4 wk, attenuated ventricular dysfunction and reduced pathological patterns of myocardial gene expression without altering infarct size.
MATERIALS AND METHODS
Experimental animals.
All procedures were conducted in conformance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the University of Colorado at Denver and Health Sciences Center Institutional Animal Care and Use Committee. C57Bl/6J mice (11 to 12 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate for 1 wk before any experimental intervention.
Surgical generation of myocardial I/R in mice.
Mice were anesthetized by an injection of 2% 2,2,2-tribromoethanol (0.66 mg/g ip; Aldrich Chemical, St. Louis, MO). The mice were then orally intubated with a 20-gauge angiocath and mechanically ventilated with 90% O2-10% room air at a tidal volume of 0.4 ml and a rate of 120 breaths/min (model CIV-101, Columbus Instruments, Columbus, OH). The heart was accessed via a parasternal thoracotomy at the fourth intercostal space and a 7-0 silk suture passed under the left anterior descending coronary artery (LAD) at the point where it emerged from under the left atrial flap. Myocardial ischemia was achieved by occluding the LAD against a 22-gauge J-shaped stainless steel probe and verified by visually noting the regional akinesis and blanching of the left ventricle. The chest was closed in layers, with the long end of the probe remaining outside the chest wall, allowing the animal to be removed from the ventilator. After 30 min of ischemia, reperfusion was initiated by carefully pulling the probe out from under the ligature and then removing it from the chest cavity. Other investigators have shown that a 30-min ischemic interval in wild-type mice results in infarction of ∼50% of the area at risk (45). Following the surgical procedure, the mouse was allowed to recover on a warmed surface, with supplemental oxygen delivered through a nose cone. Sham-operated animals underwent all procedures described, except the actual occlusion of the LAD. I/R was verified by three-lead electrocardiograms, which were obtained preoperatively, at the end of the ischemic interval and immediately after the initiation of reperfusion. Mice fully recovered from the surgical procedure were returned to standard animal housing conditions. Postsurgical pain was controlled with buprenorphine injections (2 μg/g sc, bid) for the first 48 hr following surgery and acetaminophen (2 mg/ml, ad libitum in the drinking water) for 7 days.
Bucillamine treatment.
Powdered bucillamine (>99% purity) was obtained from Keystone Biomedical (Los Angeles, CA). Stock solutions of bucillamine (5 mg/ml) were made in normal saline, pH adjusted to ∼7.4 with equimolar NaOH, and filter sterilized. Within 5 min of reperfusion being initiated, an intravenous bolus of bucillamine (10 μg/g) was administered via tail-vein injection. The mice were subsequently treated with daily injections of bucillamine (10 μg/g sc), rotating the injection sites. Control mice received saline injections.
Echocardiography.
Cardiac function was assessed in the University of Colorado at Denver Small Animal Hemodynamic Core Facility by two-dimensional transthoracic echocardiography (echo). The mice were sedated with intraperitoneal injections of fentanyl (34 ng/g) plus droperidol (1.7 μg/g) to maintain heart rates consistently above 550 beats/min. Echoes were obtained with an HP Sonos 5500 echocardiograph machine using a 15-MHz linear array intraoperative probe (Philips Ultrasound, Andover, MA). Parasternal short-axis views, long-axis views, and M-modes (at the level of the short axis) were routinely obtained. Echo images were obtained on the mice 4–6 days before surgical intervention (baseline) and then 4 wk following surgery just before death. All analyses were performed off-line by an individual blinded as to treatment status.
Infarct size.
Region at risk and infarct size were determined in mice that underwent I/R surgery as described in Surgical generation of myocardial I/R in mice, except that a slip knot was tied in the suture used to occlude the LAD. Forty-eight hours after reperfusion began, the mice were anesthetized and heparinized, and the hearts were excised. The slip knot was then pulled taut to reocclude the LAD. The aorta was cannulated and the heart perfused with 10 ml of cardioplegia solution containing (in mM) 140.0 NaCl, 15.0 KCl, 1.0 MgSO4, 1.0 Na2HPO4, 11.0 glucose, 15.0 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 10.0 EGTA, and 30.0 2,3-butanedione monoxime and 0.10% BSA and 10 U/ml heparin. Regions of the heart still receiving blood flow during LAD occlusion were identified by perfusion with 5 ml of 2% Evans blue. After the heart was removed from the cannula, the atria and right ventricle were trimmed away before transversely slicing the left ventricle into four sections. Infarcted myocardium was identified by incubating the heart slices with 1% triphenyltetrazolium chloride at 37°C for 15 min. Each slice was weighed and then imaged with a Nikon SMZ800 stereoscope equipped with a Cool-Snap CCD camera. Perfused (dark blue), nonperfused but noninfarcted (brick red), and infarcted (white) myocardial regions were quantitated by planimetry using ImagePro software. The region at risk and infarct size were determined using the following equations: Weight of region of interest = (A1 × Wt1) + (A2 × Wt2) + (A3 × Wt3) + (A4 × Wt4), where A is area of the region of interest determined by planimetry from each of the four heart sections and Wt is the weight of each section. Region of interest as percentage of the left ventricle = (Wt of region of interest/Wt of left ventricle) × 100%.
Tissue harvest.
At the end of the 28-day experimental interval, the mice were weighed before heart excision. The excised hearts were rinsed in cardioplegia solution containing (in mM) 140.0 NaCl, 5.4 KCl, 1.0 MgSO4, 1.0 Na2HPO4, 11.0 glucose, 15.0 BES, 1.0 EGTA, and 30.0 2,3-butanedione monoxime and 0.1% BSA (pH 7.4), and the atria and major vessels were removed. The combined ventricles were blotted dry, weighed, and stored in RNALater (Ambion, Austin, TX) at −20°C. The lung and liver were also excised, trimmed of vascular tissue, blotted dry, and weighed.
Ribonuclease protection assay.
Tissue RNA was purified using TRIzol reagent (Invitrogen, Carlsbad, CA). Blinded analysis of myocardial mRNAs was performed by ribonuclease protection assay using a cassette of mouse cardiac riboprobes as described previously (14). Briefly, 10 μg aliquots of RNA were hybridized with [32P]-labeled anti-sense probes for 1) cardiac specific genes, including murine α- and β-myosin heavy chain (MHC), sarco(endo)plasmic reticulum Ca2+ ATPase 2a (SERCA2a), atrial natriuretic peptide (ANP), skeletal α-actin, and GAPDH (internal control) or 2) cytokine genes, including interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and GAPDH (BD Biosciences, San Jose, CA). Unhybridized RNA was digested with RNase, and the protected fragments were separated by polyacrylamide gel electrophoresis. The detection and quantitation of the individual protected fragments were accomplished by Phosphorimager densitometry (Molecular Dynamics, Sunnyvale, CA). The densitometry values for each mRNA species were normalized to the GAPDH mRNA signal from the same sample to correct for variations in RNA loading.
Experimental groups.
The mice to be analyzed after 4 wk of reperfusion were randomly assigned to one of four experimental groups: 1) a sham-operated group not exposed to I/R and injected daily for 4 wk with control saline solution (Sham + saline), 2) a group exposed to I/R and treated with daily injections of saline for 4 wk (I/R + saline), 3) a sham-operated group not exposed to I/R and injected daily with bucillamine for 4 wk (Sham + bucillamine), and 4) a group exposed to I/R and treated with daily injections of bucillamine for 4 wk (I/R + bucillamine).
Mice to be analyzed for infarct size after euthanasia at 48 h were randomly assigned to two groups: 1) a group assigned to I/R that received bucillamine as described above for 2 days of I/R (I/R + bucillamine) and 2) a group assigned to I/R that received saline as described above for 2 days of I/R (I/R + saline).
Data analysis.
All data were expressed as means ± SE. Statistical comparisons between treatment groups were performed by two-way ANOVA with Bonferroni's post test (GraphPad Software, San Diego, CA). A P < 0.05 was considered statistically significant.
RESULTS
Infarct size.
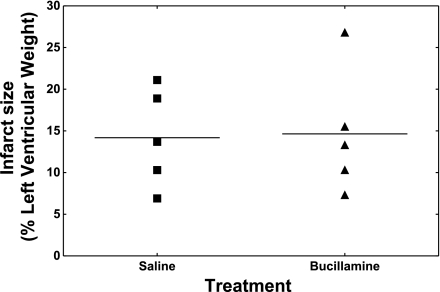
Mice receiving bucillamine or saline (control) were euthanized after 48 h of reperfusion for the determination of infarct size. Individual values of infarct size as a percentage of the left ventricle weight are plotted in Fig. 1. The area-at-risk measurements were 37 ± 5% (saline) versus 36 ± 6% (bucillamine). The infarct size measurements were 14 ± 6% (saline) versus 15 ± 8% (bucillamine). There were no significant differences or trends between the two groups.
Fig. 1.
Infarct sizes in bucillamine-treated and control mice. Infarct size as a percentage of the left ventricle was measured after 48 h of reperfusion as described in materials and methods. Individual values for each mouse are represented by circles, and the mean value for each group is designated by a horizontal line.
The 4-wk experimental model.
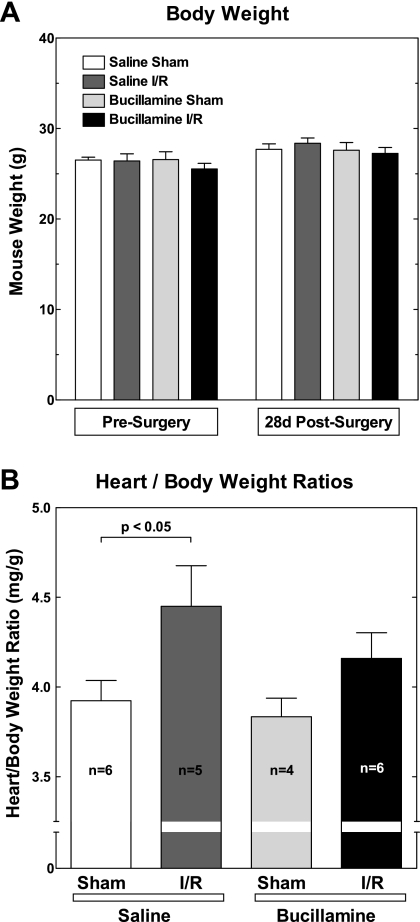
There were no apparent adverse reactions to bucillamine during the 4-wk treatment period in any of the treated mice. The gain in body weight was similar and did not differ statistically among the experimental groups (Fig. 2A). All mice exposed to I/R had visible infarcts postmortem.
Fig. 2.
Bucillamine protects against cardiac hypertrophy following ischemia-reperfusion (I/R) injury. A: body mass. Each mouse was weighed before surgery, weekly during the treatment interval, and just before death. Group averages are shown for the weights just before surgery (presurgery) and at the end of the experiment interval [28-day (28d) postsurgery]. B: cardiac mass (n, number of mice/group). Twenty-eight days after surgical induction of I/R injury, hearts were excised and trimmed of atria and major blood vessels. The ventricles were blotted dry before weighing. Ventricular weights were normalized to body weights for each mouse (heart weight-to-body weight ratio, in mg/g).
Twenty-one of the 22 mice originally receiving injections survived the 4-wk treatment interval. One mouse that had undergone I/R injury and had received saline injections died from cardiac rupture 5 days after surgery. The final animal numbers per group were as follows: Sham + saline (n = 6), I/R + saline (n = 5), Sham + bucillamine (n = 4), and I/R + bucillamine (n = 6).
There were no statistical differences in lung weights or liver weights between the different groups (data not shown).
Cardiac mass.
Figure 2B shows the cardiac mass, as defined by the heart weight-to-body weight ratio, in the four groups of mice. There was a significant increase in cardiac mass (13%, P < 0.05) in the I/R + saline group compared with the Sham + saline group 4 wk after I/R exposure. Bucillamine had no effect on cardiac mass in the sham-operated mice. The increase in heart weight-to-body weight ratio in response to I/R was attenuated in the I/R + bucillamine group and was statistically indistinguishable from the Sham + bucillamine group. Thus bucillamine provided a long-term protective effect against the cardiac hypertrophic response to injury following I/R injury.
Cardiac function.
Figure 3 shows M-mode echo tracings from individual mice representative of each experimental group. The echo measurement data compiled for each experimental group are shown in Table 1. I/R injury caused a statistically significant increase in the left ventricular end-systolic diameter in the I/R + saline group compared with the Sham + saline group. The end-diastolic diameter tended to increase in the I/R + saline group compared with the Sham + saline group, but the results did not reach statistical significance. There were no differences in ventricular dimensions between the saline- and bucillamine-treated sham-operated mice. Neither end-diastolic diameter nor end-systolic diameter was statistically different between the I/R + bucillamine group and either of the two Sham groups. No significant differences were seen in septal or posterior wall thicknesses in any group, although there was a trend toward an increase in posterior wall thickness in the I/R + saline group compared with the Sham + saline group.
Fig. 3.
Representative echocardiographic M-mode tracings. Echocardiograms were obtained as detailed in materials and methods. M-mode echoes representative for each experimental group are shown.
Table 1.
Cardiac dimensions and functional parameters
| Treatment Groups | n | LVDd, mm | LVDs, mm | IVSd, mm | PWd, mm | FS, % | HR, beats/min |
|---|---|---|---|---|---|---|---|
| Presurgery | 21 | 2.9±0.1 | 1.3±0.1 | 0.96±0.02 | 0.94±0.03 | 56±2 | 639±9 |
| 28-day postsurgery | |||||||
| Saline | |||||||
| Sham | 6 | 3.2±0.2 | 1.5±0.2 | 1.02±0.04 | 0.87±0.07 | 55±3 | 677±4 |
| I/R | 5 | 3.8±0.3† | 2.5±0.3*† | 0.95±0.03 | 0.99±0.06 | 32±3*† | 679±8 |
| Bucillamine | |||||||
| Sham | 4 | 2.9±0.2 | 1.4±0.1 | 1.04±0.05 | 0.95±0.06 | 51±2 | 668±20 |
| I/R | 6 | 3.3±0.2 | 2.0±0.2 | 1.02±0.06 | 0.87±0.04 | 41±4 | 668±7 |
Values are means ± SE for each animal group; n, number of animals in each group. Left ventricular dimensions were obtained from short-axis two-dimensional-guided M-mode echoes. Measurements were obtained over 3 separate contractile cycles and then averaged to obtain mean values for each animal before obtaining the group means. Left ventricular internal diameter (LVD) was measured at end diastole (d) and end systole (s). Interventricular septal (IVS) and posterior wall (PW) thicknesses were measured at end diastole (d). Heart rate (HR) was calculated from diastole-to-diastole intervals. Percent fractional shortening (FS) for each animal was calculated as FS = [(LVDd − LVDs)/LVDd] × 100%.
P < 0.01, ischemia-reperfusion (I/R) + saline vs. Sham + saline;
P < 0.05, I/R + saline vs. Sham + bucillamine.
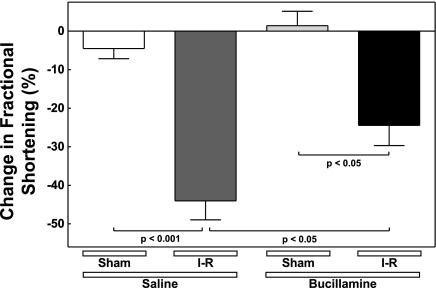
Following sham operations, contractile function, measured as fractional shortening (FS), remained constant over the 4-wk interval in both the saline- and bucillamine-treated mice (Table 1). However, I/R injury resulted in a large and highly significant (67%, P < 0.01) decrease in FS in the I/R + saline mice compared with the Sham + saline mice (Fig. 4). The decrease in FS caused by I/R was significantly attenuated in the I/R + bucillamine group (P < 0.05 compared with the I/R + saline group). Therefore, long-term treatment with bucillamine preserves cardiac contractile function following I/R injury.
Fig. 4.
Bucillamine protects against the loss of cardiac contractile function following I/R injury. Fractional shortening (FS) was measured as described in Table 1. The change in FS over the experimental interval in each individual animal was calculated as (FSpre − FS28d)/FSpre × 100%, where FSpre is FS before surgery and FS28d is FS 28 days after surgery. The individual values were averaged to determine the means ± SE for each experimental group.
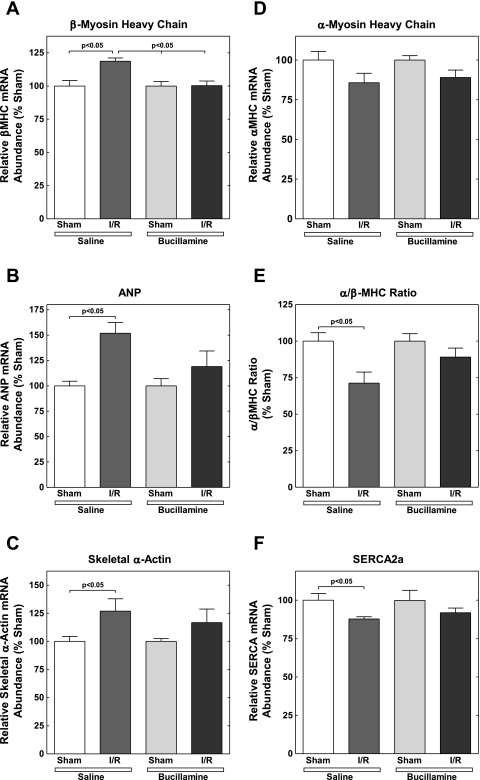
Fetal gene expression.
Expression of fetal isoforms of several genes has been observed in pathological cardiac hypertrophy (8, 22, 33, 35, 47). For example, the expression of the natriuretic peptides, ANP and brain natriuretic peptide, the skeletal isoform of α-actin, and the β-isoform of MHC are increased. In contrast, the expression of the SERCA2a gene decreases in pathological hypertrophy. The expression of the α-isoform of MHC is also decreased, resulting in a further decrease in the α-MHC-to-β-MHC ratio. We compared the myocardial expression of these genes in saline- and myocardial-treated mice 4 wk after I/R injury.
Figure 5 shows the effects of I/R injury, without or with bucillamine treatment, on the expression of the fetal isoforms of specific cardiac genes. The data were expressed as the abundance of each mRNA species relative to the sham-operated controls. I/R injury resulted in a significant increase in β-MHC expression in the saline-treated mice. This increase was prevented by long-term bucillamine treatment (Fig. 5A). I/R injury in the saline-treated mice also resulted in increases in ANP and skeletal α-actin expression (Fig. 5, B and C). Again, bucillamine prevented the increases in the expression of these genes.
Fig. 5.
Bucillamine attenuates the expression of the pathological fetal gene program following I/R injury. Ventricular tissue was analyzed by ribonuclease protection assay for expression of pathological marker gene mRNAs as described in materials and methods. Individual values for each mRNA species were normalized to the mean sham value (saline or bucillamine, as appropriate) for the same mRNA. Each panel indicated the individual mRNA species measured, as follows: β-myosin heavy chain (β-MHC; A); atrial natriuretic peptide (ANP; B); skeletal α-actin (C); α-MHC (D); α-MHC-to-β-MHC ratio (E); and sarco(endo)plasmic reticulum Ca2+ ATPase 2a (SERCA2a; F).
As shown in Fig 5D, I/R tended to decrease the expression of α-MHC in both the saline- and bucillamine-treated mice, although these results did not reach statistical significance. However, I/R caused a statistically significant decrease in the α-MHC-to-β-MHC ratio in the saline-treated mice, which was prevented by bucillamine treatment (Fig. 5E). Finally, SERCA2a expression significantly decreased following I/R injury in the saline-treated mice, and this decrease was attenuated by bucillamine (Fig. 5F).
Thus, 4 wk after I/R injury, the pathological pattern of gene expression (increased β-MHC, ANP, and skeletal α-actin; and decreased α-MHC, α-MHC-to-β-MHC ratio, and SERCA2a mRNA) appeared in saline-treated mice but was attenuated by bucillamine.
Cytokine gene expression.
Since redox activity early in ischemia may stimulate cytokine activation, we tested whether the protection of cardiac function by bucillamine was mediated by prolonged alterations in myocardial cytokine gene expression. No statistically significance or trend toward differences in the cardiac expression of IL-1β, IL-6, or TNF-α was seen among the experimental groups (data not shown).
DISCUSSION
It is well established that early reperfusion of the infarcted myocardium results in the generation of high levels of oxidative activity. Considerable attention has been paid to possible reduction in infarct size by the acute administration of antioxidants or other anti-inflammatory measures during the early stages of reperfusion (12, 13, 25). However, there has also been evidence of the prolonged elevation of oxidative activity for weeks or months following I/R (10, 24, 40), and little is known about its importance in the long-term recovery from acute myocardial infarction. This study demonstrates that a thiol donor antioxidant administered daily for 1 mo after I/R markedly attenuates ventricular remodeling in genetically normal mice. In previous studies of transgenic animals, either antioxidant capacity was impaired or there was exaggerated oxidative activity before, during, and after the initiation of ischemia.
Bucillamine [N-(2-mercapto-2-methylpropionyl)-l-cysteine] is a member of a group of low molecular weight, cysteine-derived thiol donors that includes N-acetylcysteine (NAC) and N-2-mercaptopropionyl glycine (MPG). These compounds readily enter cells through the cysteine transport pathway and exert their antioxidant effect by maintaining the endogenous glutaredoxin and thioredoxin systems in a reduced state by transfer of thiol groups (1, 43). Bucillamine contains two donatable thiol groups, making it a considerably more potent antioxidant than NAC or MPG, which each contain only one thiol group (11, 13, 43). Based on the proven effectiveness of bucillamine in counteracting oxidative stress (1, 11, 13, 26, 38, 43), we tested whether bucillamine would reduce adverse remodeling following I/R. In our investigator blinded and sham-surgery controlled study, a long-term treatment with bucillamine attenuated the increase in cardiac mass, loss of contractile function, and pathological gene expression observed in saline-treated mice 4 wk after I/R injury.
A few previous studies have suggested the potential of antioxidant therapy to prevent long-term remodeling processes due to oxidative stress. Yamamoto et al. (46) reported that transgenic mice overexpressing a dominant negative form of thioredoxin, an endogenous antioxidant, developed cardiac hypertrophy in the absence of exogenous stress. This hypertrophy was prevented by 4 wk of treatment with MPG. Dimethylthiourea, which has antioxidant effects but also blocks sodium/calcium exchange by an unrelated mechanism (48), improved ventricular function when given after coronary ligation in mice (15). Treatment with the antioxidant flavonoid, 7-monohydroxyethylrutoside, before ischemia partially preserved cardiac contractile responses after I/R in mice (5), although these authors did not examine cardiac function beyond 2 wk. The knockout of the myeloperoxidase gene in mice protects against the loss of cardiac function 24 days after I/R, without altering infarct size (42). Adenoviral transfer of heme oxygenase-1 to rat myocardium prevented I/R-induced cardiac fibrosis and ventricular remodeling for up to 3 mo (18). Our study extends these results to show that a sustained delivery of a thiol donor antioxidant after the onset of reperfusion in normal mice attenuates long-term I/R-induced cardiac hypertrophy, loss of cardiac contractile function, and pathological patterns of gene expression. Oxidative activity immediately at the onset of reperfusion may primarily affect cytokine release and acute inflammatory responses (27), whereas later oxidative activity may have a greater influence on genes that control myocyte function and size (2, 9). Thus the benefits of antioxidant therapy following I/R may extend beyond the acute injury phase and continue during the chronic phase of myocardial remodeling.
Although oxidative stress is a determinant of infarct size in the acute response to I/R injury (12, 13, 25), there is not necessarily a consistent correlation between infarct size and the subsequent development of ventricular remodeling. As alluded to above, Vasilyev et al. (42) found that mice deficient in myeloperoxidase (an enzyme that catalyzes the generation of reactive oxygen species in leukocytes) had improved cardiac function 24 days after I/R compared with wild-type mice, despite equivalent infarct sizes. Additionally, in an ischemia model without reperfusion, Shiomi et al. (32) demonstrated that an overexpression of glutathione peroxidase in transgenic mice prevented ventricular remodeling and heart failure, independently of infarct size. Our data are in agreement with these published studies showing an improvement in cardiac function following antioxidant treatment, despite equivalent infarct sizes in treated and control animals. Thus it is likely that the development of ventricular remodeling is not solely related to the amount of damage sustained during or immediately following ischemia but, rather, is strongly influenced by the evolving response to injury in the surviving myocardium.
The analysis of gene expression in heart tissue from human patients has identified a pattern of abnormal myocardial gene expression that occurs with cardiac remodeling (22, 35). Similar changes are observed in rodent models (8, 47), although the magnitude of the responses of individual genes differs among species. Although it is not necessarily the case that all the changes in genetic expression have direct functional consequences, decreases in SERCA2a expression and the α-MHC-to-β-MHC ratio are associated with the loss of myocyte contractility (22, 28). Furthermore, sustained decreases in SERCA2a protein levels are thought to contribute to the loss of cardiac function during the progression to heart failure (28). Therefore, the attenuation of I/R-induced decreases in SERCA and α-MHC-to-β-MHC ratio by antioxidant therapy, as seen in this study, has potential usefulness for the prevention of heart failure. Indeed, in human clinical studies, the improvement of cardiac function through β-adrenergic blockade (21) or left ventricular assist devices (44) correlated with a reversal of the pathological pattern of gene expression.
A bucillamine-induced attenuation of the expression of the pathological pattern of gene expression may occur through the prevention of abnormal oxidative signaling. Oxidative stress induces pathological gene expression in isolated cardiac myocytes (2). In addition, SERCA2a mRNA levels are reduced by oxidative stress in the acute phase of I/R injury (37). Thus antioxidant therapy may modulate abnormal signaling pathways that alter myocardial gene transcription following I/R injury. Finally, the prevention of adverse remodeling by bucillamine could indirectly result in a more physiological pattern of gene expression due to a decreased stress environment in the heart.
Despite evidence in animal models that increased levels of oxidant stress are associated with cardiac remodeling and development of heart failure, vitamin E, a lipid-soluble antioxidant, did not reduce the incidence of heart failure either in patients with established atherosclerotic disease (20) or with recent myocardial infarction (23). Vitamin E largely exerts its antioxidant effects by preventing lipid peroxidation. However, lipid peroxidation is only one of the mechanisms by which adverse effects of oxidative stress may occur. The prevention of acute lipid peroxidation alone may not prevent left ventricular dysfunction induced by I/R (16). In contrast, there is abundant evidence that hydrogen peroxide, a highly diffusible reactive oxygen species, functions as a signaling agent in low concentrations (30, 34) and may stimulate hypertrophy or apoptosis in cardiac myocytes (17). Bucillamine and other synthetic thiol donors, through their ability to rapidly restore thiol groups to endogenous oxidized glutathione or thioredoxins, are extremely efficient in limiting intracellular hydrogen peroxide accumulation. In this manner thiol donors probably minimize hydrogen peroxide-mediated redox signaling (1, 43).
In conclusion, we have demonstrated that a chronic administration of a potent dithiol antioxidant, bucillamine, protects against long-term pathological ventricular remodeling subsequent to I/R injury. Bucillamine reduced hypertrophy, improved contractile function, and prevented pathological expression of cardiac-specific genes. These results are compatible with the hypothesis that, following a myocardial infarction treated with reperfusion, a prolonged redox modulation of cell signaling can exert adverse effects on ventricular shape and function that are associated with abnormal myocardial gene expression.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-55291 (to L. D. Horwitz) and HL-66399 and HL-79160 (to C. S. Long).
Acknowledgments
We gratefully acknowledge Nancy Sherman and Ursula Jiron for daily mouse care, Dr. Ping Yue for performing the echo measurements, and Dr. R. Dale Brown for insightful discussions and critical review of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA 99: 8915–8920, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33: 131–139, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarten G, Knuefermann P, Kalra D, Gao F, Taffet GE, Michael L, Blackshear PJ, Carballo E, Sivasubramanian N, Mann DL. Load-dependent and -independent regulation of proinflammatory cytokine and cytokine receptor gene expression in the adult mammalian heart. Circulation 105: 2192–2197, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN Structural changes in cardiovascular disease. Am J Cardiol 76: 34E–37E, 1995. [PubMed] [Google Scholar]
- 5.De Celle T, Heeringa P, Strzelecka AE, Bast A, Smits JF, Janssen BJ. Sustained protective effects of 7-monohydroxyethylrutoside in an in vivo model of cardiac ischemia-reperfusion. Eur J Pharmacol 494: 205–212, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer WJ, Michael LH, West MS, Smith CW, Rothlein R, Rossen RD, Anderson DC, Entman ML. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation 84: 400–411, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Gidh-Jain M, Huang B, Jain P, Gick G, El Sherif N. Alterations in cardiac gene expression during ventricular remodeling following experimental myocardial infarction. J Mol Cell Cardiol 30: 627–637, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-κB activation in TNF-α-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol 34: 233–240, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Hill MF, Singal PK. Right and left myocardial responses during heart failure subsequent to myocardial infarction. Circulation 96: 2414–2420, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol 165: 2703–2711, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz LD, Fennessey PV, Shikes RH, Kong Y. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation 89: 1792–1801, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz LD, Sherman NA. Bucillamine prevents myocardial reperfusion injury. J Cardiovasc Pharmacol 38: 859–867, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, Camacho SA, Bristow MR, Long CS, Simpson PC. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res 89: 591–598, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Kong Y, Lesnefsky EJ, Ye J, Horwitz LD. Prevention of lipid peroxidation does not prevent oxidant-induced myocardial contractile dysfunction. Am J Physiol Heart Circ Physiol 267: H2371–H2377, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 35: 615–621, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J 20: 207–216, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Long CS The role of interleukin-1 in the failing heart. Heart Fail Rev 6: 81–94, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338–1347, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med 346: 1357–1365, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. Changes in gene expression in the intact human heart. Downregulation of α-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 100: 2315–2324, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F. Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 7: 347–350, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Marczin N, El-Habashi N, Bundy RE, Yacoub M. Antioxidants in myocardial ischemia-reperfusion injury: therapeutic potential and basic mechanisms. Arch Biochem Biophys 420: 222–236, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Mitsos SE, Askew TE, Fantone JC, Kunkel SL, Abrams GD, Schork A, Lucchesi BR. Protective effects of N-2-mercaptopropionyl glycine against myocardial reperfusion injury after neutrophil depletion in the dog: evidence for the role of intracellular-derived free radicals. Circulation 73: 1077–1086, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Nelson SK, Bose S, Rizeq M, McCord JM. Oxidative stress in organ preservation: a multifaceted approach to cardioplegia. Biomed Pharmacother 59: 149–157, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nossuli TO, Frangogiannis NG, Kneufermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNF-α-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol 281: H2549–H2558, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J Mol Cell Cardiol 33: 1053–1063, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Rhee SG Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 34: 379–388, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 109: 544–549, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Simpson PC, Long CS, Waspe LE, Henrich CJ, Ordahl CP. Transcription of early developmental isogenes in cardiac myocyte hypertrophy. J Mol Cell Cardiol 21, Suppl 5: 79–89, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human α-myosin heavy chain promoter. J Biol Chem 278: 31233–31239, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol 37: 676–685, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol Heart Circ Physiol 277: H584–H594, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji F, Miyake Y, Aono H, Kawashima Y, Mita S. Effects of bucillamine and N-acetyl-l-cysteine on cytokine production and collagen-induced arthritis. Clin Exp Immunol 115: 26–31, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsujimoto I, Hikoso S, Yamaguchi O, Kashiwase K, Nakai A, Takeda T, Watanabe T, Taniike M, Matsumura Y, Nishida K, Hori M, Kogo M, Otsu K. The antioxidant edaravone attenuates pressure overload-induced left ventricular hypertrophy. Hypertension 45: 921–926, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Tsutamoto T, Wada A, Matsumoto T, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Yamamoto T, Horie H, Sugimoto Y, Kinoshita M. Relationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol 37: 2086–2092, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Valgimigli M, Merli E, Malagutti P, Soukhomovskaia O, Cicchitelli G, Antelli A, Canistro D, Francolini G, Macri G, Mastrorilli F, Paolini M, Ferrari R. Hydroxyl radical generation, levels of tumor necrosis factor-alpha, and progression to heart failure after acute myocardial infarction. J Am Coll Cardiol 43: 2000–2008, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 112: 2812–2820, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, Horwitz LD, Brechun N, Diaz-Sanchez D, Nel AE. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol 168: 2560–2567, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Wohlschlaeger J, Schmitz KJ, Schmid C, Schmid KW, Keul P, Takeda A, Weis S, Levkau B, Baba HA. Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc Res 68: 376–386, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Huo Y, Toufektsian MC, Ramos SI, Ma Y, Tejani AD, French BA, Yang Z. Activated platelets contribute importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 290: H692–H699, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest 112: 1395–1406, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue P, Long CS, Austin R, Chang KC, Simpson PC, Massie BM. Post-infarction heart failure in the rat is associated with distinct alterations in cardiac myocyte molecular phenotype. J Mol Cell Cardiol 30: 1615–1630, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Ziegelstein RC, Zweier JL, Mellits ED, Younes A, Lakatta EG, Stern MD, Silverman HS. Dimethylthiourea, an oxygen radical scavenger, protects isolated cardiac myocytes from hypoxic injury by inhibition of Na+-Ca2+ exchange and not by its antioxidant effects. Circ Res 70: 804–811, 1992. [DOI] [PubMed] [Google Scholar]