Abstract
Several sympathoexcitatory reflexes, such as the cardiac sympathetic afferent reflex (CSAR) and arterial chemoreflex, are significantly augmented and contribute to elevated sympathetic outflow in chronic heart failure (CHF). This study was undertaken to investigate the interaction between the CSAR and the chemoreflex in CHF and to further identify the involvement of angiotensin II type 1 receptors (AT1Rs) in the nucleus of the tractus solitarius (NTS) in this interaction. CHF was induced in rats by coronary ligation. Acute experiments were performed in anesthetized rats. The chemoreflex-induced increase in cardiovascular responses was significantly greater in CHF than in sham-operated rats after either chemical or electrical activation of the CSAR. The inhibition of the CSAR by epicardial lidocaine reduced the chemoreflex-induced effects in CHF rats but not in sham-operated rats. Bilateral NTS injection of the AT1R antagonist losartan (10 and 100 pmol) dose-dependently decreased basal sympathetic nerve activity in CHF but not in sham-operated rats. This procedure also abolished the CSAR-induced enhancement of the chemoreflex. The discharge and chemosensitivity of NTS chemosensitive neurons were significantly increased following the stimulation of the CSAR in sham-operated and CHF rats, whereas CSAR inhibition by epicardial lidocaine significantly attenuated chemosensitivity of NTS neurons in CHF but not in sham-operated rats. Finally, the protein expression of AT1R in the NTS was significantly higher in CHF than in sham-operated rats. These results demonstrate that the enhanced cardiac sympathetic afferent input contributes to an excitatory effect of chemoreflex function in CHF, which is mediated by an NTS-AT1R-dependent mechanism.
Keywords: sympathoexcitatory reflexes, sympathetic activity, angiotensin II type 1 receptor, microinjection, extracellular recording, nucleus of tractus solitarius
the elevated sympathetic outflow in the chronic heart failure (CHF) has long been known to be closely associated with the accelerated progression and the poor prognosis of this syndrome (6, 9). The sympathetic hyperactivity is closely related to abnormalities in cardiovascular reflexes in CHF. Sympathoinhibitory cardiovascular reflexes such as the arterial baroreceptor reflex are significantly suppressed in CHF (21, 38). On the other hand, the sympathoexcitatory reflexes including the arterial chemoreceptor reflex and the cardiac sympathetic afferent reflex (CSAR) are exaggerated in CHF (34, 39). Although functional alterations of the above-mentioned reflexes have been independently used to illustrate the sympathoexcitation observed in CHF, the interaction among these reflexes in both the normal and CHF conditions, especially on their contributions to the sympathoexcitatory state in CHF, has been not extensively studied.
In CHF, a variety of substances, such as bradykinin, that are released by the myocardium during ischemia can effectively stimulate the cardiac sympathetic afferents and subsequently increase sympathetic outflow (24, 25). It is of interest that the stimulation of cardiac sympathetic afferents inhibits baroreflex function (13, 15), suggesting a close relationship between the CSAR and the other cardiovascular reflexes. More recently, we demonstrated that, in normal rats, the activation of the CSAR produces a significant enhancement of chemoreflex function (12). In a recent study it was shown that acute myocardial ischemia (MI) enhanced the carotid chemoreflex after administration of capsaicin to the ventricular surface (28). However, the exact mechanism and significance of this agonistic interaction between the CSAR and the chemoreflex, especially the benefit of blockade of increased cardiac sympathetic afferent tone, have not been investigated in the CHF state.
The central angiotensin II (ANG II) system has been widely reported to be increased in CHF (11, 45). Antagonism of ANG II reduces sympathetic outflow and effectively normalizes cardiovascular reflexes in CHF (7, 14, 19). The blockade of ANG II type 1 receptors (AT1Rs) in the brain attenuates the increased sympathetic activity and restores the blunting of baroreflex induced by activation of the CSAR, suggesting that the ANG II system in the central nervous system may be a major mechanism responsible for CSAR-induced cardiovascular effects (13, 15). The nucleus of the solitary tract (NTS) receives sensory input from a vast array of peripheral receptors located in viscera, somatic, and cardiorespiratory organs and is a critical medullary nucleus for modulating cardiovascular reflexes (3, 33). The NTS is known to be the central termination site of the peripheral chemoreceptors (5, 23, 29). On the other hand, the ascending fibers from cardiac sympathetic afferents entering through the stellate ganglia and the spinal cord also terminate in the NTS (4, 17). Therefore, the NTS receives convergent inputs from the peripheral chemoreceptors and cardiac sympathetic afferents. It is reasonable to speculate, therefore, that the interaction of these two reflexes may occur at the level of the NTS. In addition, AT1R in the NTS have been widely reported to participate in the control of sympathetic outflow and cardiovascular reflexes (16, 20, 22, 26). Therefore, the major purpose of this study was to determine the interaction between cardiac sympathetic afferent input and the arterial chemoreflex, to especially assess the beneficial effect of blockade of tonic cardiac sympathetic afferent input on the enhanced chemoreflex in rats with CHF and to further investigate whether the ANG II mechanism is involved in the processing of this interaction at the level of NTS.
METHODS
Animal preparation.
Male Sprague-Dawley rats weighing between 180 and 200 g were used in these experiments. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
CHF model.
CHF was produced by coronary artery ligation as previously described (13, 42). The rat was ventilated at a rate of 60 breaths/min with 3% isoflurane during the surgical procedure. A left thoracotomy was performed through the fifth intercostal space, the pericardium was opened, the heart was exteriorized, and the left anterior descending coronary artery was ligated. Sham-operated rats were prepared in the same manner but did not undergo coronary artery ligation. All the rats survived from the sham surgery. However, ∼70% of rats survived from coronary artery ligation surgery.
In this study, the cardiac function in all experimental animals was measured by echocardiography 6 wk after coronary ligation or sham operation. In addition, at the end of each acute experiment, the Millar catheter (SPR 524; size, 3.5-Fr; Millar Instruments, Houston, TX) was advanced through the carotid artery into the left ventricle (LV) to determine LV end-diastolic pressure (LVEDP) and LV systolic pressure. The rats were then euthanized with an overdose of pentobarbital sodium. The hearts and lungs were removed, and the ratio of the infarct area to whole LV minus septum was measured.
General animal preparation.
The general animal preparation, chemoreflex/CSAR activation, recording of renal sympathetic nerve activity (RSNA), NTS microinjection, and neuronal recording are similar to that described in our previous studies (13, 15, 40, 41). In brief, 6 to 8 wk after coronary ligation, under anesthesia with urethane (800 mg/kg ip) and α-chloralose (40 mg/kg ip), the trachea was cannulated through a midline cervical incision to facilitate mechanical respiration. The ventilation parameters were adjusted to maintain arterial partial pressure of O2 at ∼100 mmHg and arterial partial pressure of CO2 below 40 mmHg. The right common carotid artery was catheterized with a Millar transducer (SPR 524) for measurement of arterial pressure. Heart rate (HR) was derived from the arterial pressure pulse using the cardiotachometer function of the PowerLab (model 16S; ADInstruments, Colorado Springs, CO). A cannula was introduced retrogradely into the right external carotid artery so that its tip was at the origin of the artery supplying the carotid bodies. A femoral vein was cannulated for the administration of α-chloralose (10 mg/kg, supplemental anesthesia), and pancuronium bromide (1 mg/kg, paralysis) was for neuromuscular blockade. In rats for epicardial application of lidocaine, each vagus was then identified, tied, and sectioned. Body temperature was kept at 37°C.
Recording of RSNA.
The left kidney, renal artery, and nerves were exposed through a left retroperitoneal flank incision. The distal terminal of the renal nerve was cut to avoid afferent activity. The renal sympathetic nerves were placed on a pair of platinum-iridium recording electrodes and then were covered with a fast-setting silicone (Kwik-Sil; World Precision Instruments). The amplified discharge was monitored and imported to the PowerLab system with other parameters. With the use of the unit conversion of PowerLab Chart system, the baseline RSNA was taken as 100% from absolute value after noise level was subtracted. Background noise levels were determined by an intravenous injection of hexamethonium (30 mg/kg).
Evaluation of chemoreflex and the CSAR.
The chemoreflex was activated by right intracarotid artery bolus injections of potassium cyanide (KCN) (1 and 10 μg in 100 μl normal saline) (2). The apex of the change in mean arterial pressure (MAP) and RSNA were used as the chemoreflex response values. Because repeated injections (short interval) of KCN may produce tachyphylaxis, the interval of KCN injections was at least 30 min. For the activation or inhibition of cardiac sympathetic afferents, the chest was opened through the fourth intercostal space. The pericardium was removed to expose the LV. Filter paper (3 × 3 mm) containing capsaicin (0.4 μg in 2 μl) or lidocaine (2%, 10 μl) was applied to the epicardial surface of the anterior surface of the LV. Capsaicin was first dissolved in ethanol solution and then diluted to the final concentration with normal saline. In some experiments, we also electrically stimulated the cardiac sympathetic afferent nerves. The left stellate ganglion was identified, and a branch innervating the heart was tied and cut as distal as possible. A pair of stainless steel stimulation electrodes was placed on the central end of the nerve. The stimulus (7 V, 1 ms, 20 Hz) was delivered with a square wave stimulator (Grass S88, Astro-Med; W. Warwick, RI) and a stimulus isolation unit. The epicardial application of drugs (capsaicin or lidocaine) or electrical stimulation of cardiac sympathetic afferents was performed for about 2 min until the end of chemoreflex activation. KCN was injected during the epicardial application of drugs and electrical stimulation. About 1 min after the chemoreflex measurement by KCN injection, the piece of filter containing the drug (capsaicin or lidocaine) was removed and the heart surface was washed with warm saline (10 ml). Similarly, the electrical stimulation was continued for about 1 min after the KCN injection.
NTS microinjection.
The rats were placed in a stereotaxic instrument (Stoelting, Chicago, IL), and the dorsal surface of medulla was exposed by removing the atlanto-occipital membrane and a portion of the occipital bone. Microinjections were made from multibarrel micropipettes and performed by a four-channel pressure injector (PM2000B, World Precision Instruments). The coordinates of the NTS ranged from −0.4–0.4 mm rostral and 0–0.3 mm lateral to calamus scriptorius and 0.4–0.6 mm from the dorsal surface of the medulla. The NTS was functionally identified by a depressor response to injection of l-glutamate (2 nmol, Sigma-Aldrich, St. Louis, MO) described elsewhere (10, 22). It has been demonstrated that both the peripheral chemoreceptors and the cardiac sympathetic afferents mainly terminate in the commissural portion of the NTS, which plays a crucial role in mediating these sympathoexcitatory cardiovascular reflexes (18, 29, 37). The injections were made over a 10-s period, and a 50-nl injection volume was measured by observing the movement of the fluid meniscus alone a reticule in a microscope. The AT1R antagonist losartan (0.2 and 2 mM, a gift from Merck, Whitehouse Station, NJ) and l-glutamate (40 mM) were dissolved in artificial cerebrospinal fluid. The time interval between bilateral injections was within 2 min. One barrel of a multibarrel pipette was filled by 2% Pontamine sky blue for marking the injection site. At the end of experiments, the brains were removed from the skulls, placed in 10% formalin, and sectioned to verify the microinjection site according to the atlas of Paxinos and Watson (27).
Electrophysiology.
Single-unit extracellular recording was restricted within the right commissural NTS and obtained using a single micropipette filled with 0.5 M sodium acetate dissolved in 2% Pontamine sky blue. The spontaneous action potentials were amplified using a high-impedance preamplifier (Dagan; band pass, 100–3,000 Hz) and fed into a window discriminator (Mentor N-750, Spike Analyzer), which generates a standard pulse for each spike. The potentials were visualized on an oscilloscope (model 121N, Tektronix, Beaverton, OR). The pulse output of the discriminator was then fed into a rate/interval monitor (FHC, Bowdoinham, ME), the analog output of which is proportional to the number of spikes per second. Chemosensitive neurons were identified by a 30% response to the intracarotid arterial bolus injection of KCN (10 μg), but also its discharge did not respond to elevated arterial pressure (at least 40 mmHg) by aorta occlusion. To confirm that the recording neuron is single-unit activity, an overlay of the action potential trajectories was performed before and after treatments. After a neuron of interest was recorded, a small amount of 2% Pontamine sky blue was iontophoresed to mark the recording location for histological analysis. The recording sites were identified and plotted on standardized sections according to the atlas (27).
Western blot analysis.
Five sham-operated and seven CHF rats were anesthetized, and the cardiac functions were measured within 30 min. The intact rats were euthanized, and the brains were removed for Western blot analysis. The brains were cut into 100-μm coronal sections with a cryostat (Leica, Heidelberg, Germany) at −18°C, and eight consecutive sections at the level of the NTS were collected. The NTS was punched and homogenized in radioimmunoprecipitation assay buffer. The protein concentration was measured using a protein assay kit (Pierce Chemical, Rockford, IL). The proteins were loaded onto a 10% SDS-PAGE gel along with protein standards (Bio-Rad, Hercules, CA) in a separate lane for electrophoresis and then transferred to polyvinylidene difluoride membrane. The membrane was probed with rabbit polyclonal antibody against AT1R (∼1:500–1:1,000 dilutions, Santa Cruz, CA) and secondary antibody of goat anti-rabbit IgG (1:5,000 dilutions, Pierce Chemical, Rockford, IL). The protein signals were detected by enhanced chemiluminescence reagent (Pierce Chemical) and analyzed using UVP BioImaging Systems (Upland, CA). The levels of target proteins were normalized with GAPDH as a loading control (1:1,000 dilutions, Santa Cruz, CA).
Statistical analysis.
All values are expressed as means ± SE. The changes in integrated RSNA and neuronal activity after treatments were evaluated as percent changes from control level because of the variability in baseline RSNA in each animal and the discharge in each neuron. The chemosensitivity of NTS neurons was evaluated by the KCN-induced percent change from baseline discharge and normalized to 100% of the control level. The differences of hemodynamic parameters and the AT1R protein ratio to control between the sham-operated and CHF groups were evaluated by Student's t-test for unpaired observations. A one- or two-way repeated-measures ANOVA followed with the Newman-Keuls test for post hoc analysis was used when multiple comparisons were made. These statistical analyses were performed with SigmaStat software (version 3.5). A value of P < 0.05 was considered statistically significant.
RESULTS
Evaluation of body weight, organ weight, and baseline hemodynamics.
Echocardiographic and hemodynamic measurements of sham-operated and CHF rats (before vagotomy) are summarized in Table 1. The heart weight and lung weight-to-body weight ratios were significantly higher in CHF rats than that in sham-operated rats, suggesting cardiac hypertrophy and substantial pulmonary congestion in the CHF state. Moreover, in rats with CHF, a gross examination revealed a dense scar in the anterior ventricular wall. The mean infarct area was 46.1 ± 4.1% of the LV area. No infarcts were identified in sham-operated rats. Pleural fluid and ascites were also found in the CHF rats but none in the sham-operated rats. There were no statistically significant differences in baseline MAP and HR between the sham-operated and CHF rats. However, the LVEDP was significantly increased in CHF rats compared with sham-operated rats, documenting a failing LV. In addition, HR was increased immediately after vagotomy and gradually returned to the control level within about 3 h as shown previously, and the following experiments were then carried out (13).
Table 1.
Body weight, heart weight, lung weight, IS, and hemodynamics parameters in rats subjected to either coronary ligation or sham operation
| Sham | CHF | |
|---|---|---|
| n | 48 | 53 |
| Body weight, g | 396±19 | 375±13 |
| Heart weight, g | 1.8±0.2 | 2.7±0.3* |
| Heart weight/body weight, mg/g | 4.6±0.3 | 7.2±0.4* |
| Lung weight/body weight, mg/g | 5.1±0.4 | 9.5±0.7* |
| IS, %LV area | 0 | 46.1±4.1* |
| MAP, mmHg | 91±2 | 88±3 |
| HR, beats/min | 342±10 | 332±12 |
| LVEDP, mmHg | 0.4±0.3 | 12.3±2.1* |
| LVESD, mm | 4.1±0.2 | 7.5±0.3* |
| LVEDD, mm | 7.9±0.2 | 10.3±0.3* |
| LVESV, μl | 74±9 | 308±34* |
| LVEDV, μl | 333±24 | 611±45* |
| Fractional shortening, % | 48.4±1.3 | 27.3±1.6* |
| Ejection fraction, % | 78.0±1.2 | 50.3±2.5* |
Values are means ± SE; n, number of rats. CHF, chronic heart failure; IS, infarct size; MAP, mean arterial pressure; HR, heart rate; LVEDP, left ventricular (LV) end-diastolic pressure; LVESD, LV end-systolic diameter; LVEDD, LV end-diastolic diameter; LVESV, LV end-systolic volume; LVEDV, LV end-diastolic volume.
P < 0.05 compared with sham (unpaired t-test).
Effects of CSAR on chemoreflex sensitivity.
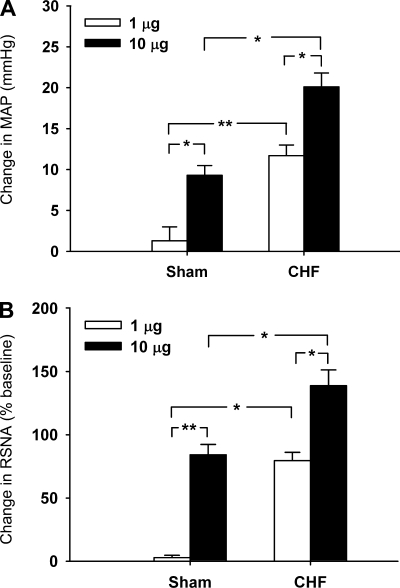
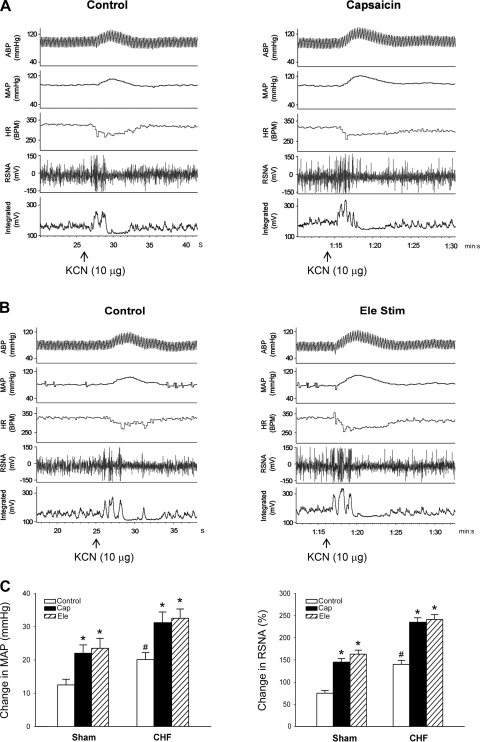
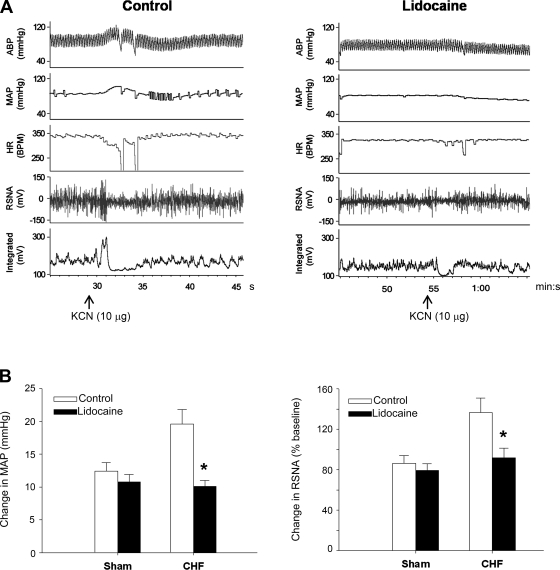
The right intracarotid artery bolus injection of KCN (1 and 10 μg) dose-dependently produced a pressor response and an increase in RSNA in both intact sham-operated and CHF rats. These responses to KCN were significantly (P < 0.05) greater in CHF than in sham-operated rats (Fig. 1), confirming an enhanced peripheral chemoreflex function in the CHF state. Similar to our previous studies (12, 13, 41), the increase in MAP, HR, and RSNA response to chemical or electrical stimulation of the CSAR rats were greater (P < 0.05) in CHF than in sham-operated rats (Table 2), suggesting a magnified CSAR in the CHF state. During CSAR activation, the chemoreflex response to KCN injection was measured again. The interval between the KCN injections before and after CSAR activation was at least 30 min. It was found that the stimulation of cardiac sympathetic afferents significantly (P < 0.05) increased the response of MAP and RSNA to KCN (Fig. 2). In vagotomized rats, the epicardial application of lidocaine significantly (P < 0.05) decreased the baseline RSNA in CHF but not sham-operated rats, suggesting tonic excitation of cardiac sympathetic afferent input on RSNA in the CHF state. The response of lidocaine reached a peak within about 30 s and reached a stable level, which also was somewhat lower than the control level. In vagotomized CHF but not sham-operated rats, the epicardial application of lidocaine induced a significant (P < 0.05) reduction in the pressor and RSNA responses to KCN (Fig. 3).
Fig. 1.
Changes in mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in response to chemoreflex activation with potassium cyanide (KCN; 1 and 10 μg) injected into right carotid artery in intact sham-operated (baseline MAP, 93.1 ± 3.2 mmHg, n = 9) and chronic heart failure (CHF) (baseline MAP, 89.8 ± 3.6 mmHg, n = 10) rats. *P < 0.05 and **P < 0.01 (2-way ANOVA).
Table 2.
Peak changes in cardiovascular parameters during chemical (capsaicin, 0.4 μg) and electrical stimulation of cardiac sympathetic afferent in intact sham-operated and CHF rats
| Changes in Sham |
Changes in CHF | |||||||
|---|---|---|---|---|---|---|---|---|
| n | MAP, mmHg | HR, beats/min | RSNA, % | n | MAP, mmHg | HR, beats/min | RSNA, % | |
| Epicardial capsaicin | 9 | 12.5+1.6 | 27.5+4.6 | 52.4+6.7 | 10 | 21.2+2.9* | 52.5+4.4* | 94.3+7.5* |
| Electrical stimulation | 9 | 14.3+1.5 | 31.5+5.4 | 43.4+3.3 | 10 | 22.2+2.4* | 50.5+4.8* | 88.3+5.2* |
Values are means ± SE; n, number of rats. RSNA, renal sympathetic nerve activity.
P < 0.05 compared with sham (2-way ANOVA).
Fig. 2.
Effects of cardiac sympathetic afferent stimulation on the chemoreflex response. A and B: representative recordings of MAP, heart rate (HR), and RSNA in response to carotid arterial injection of KCN (10 μg) during epicardial application of saline (control), capsaicin (Cap, 0.4 μg), or electrical stimulation (Ele Stim) of cardiac sympathetic afferent in CHF rats. ABP, arterial blood pressure. C: changes in MAP and RSNA in response to KCN injection during stimulation of cardiac sympathetic afferent in intact sham-operated (baseline MAP, 93.1 ± 3.2 mmHg, n = 9) and CHF (baseline MAP, 89.8 ± 3.6 mmHg, n = 10) rats. *P < 0.05 vs. control; #P < 0.05 vs. sham (2-way ANOVA).
Fig. 3.
Effects of epicardial application of lidocaine on the KCN-induced change in MAP and RSNA in vagotomized sham-operated and CHF rats. A: representative recordings of MAP, HR, and RSNA in response to carotid artery bolus injection of KCN (10 μg) and after epicardial application of normal saline (control, left) and lidocaine (2% in 20 μl, right) in CHF rats. B: decreased change in MAP (left) and RSNA (right) responses to KCN by lidocaine in sham-operated (baseline MAP, 91.4 ± 3.1 mmHg, n = 6) and CHF (baseline MAP, 87.4 ± 2.7 mmHg, n = 8) rats. *P < 0.05 vs. control (2-way ANOVA).
Effects of AT1R blockade in the NTS.
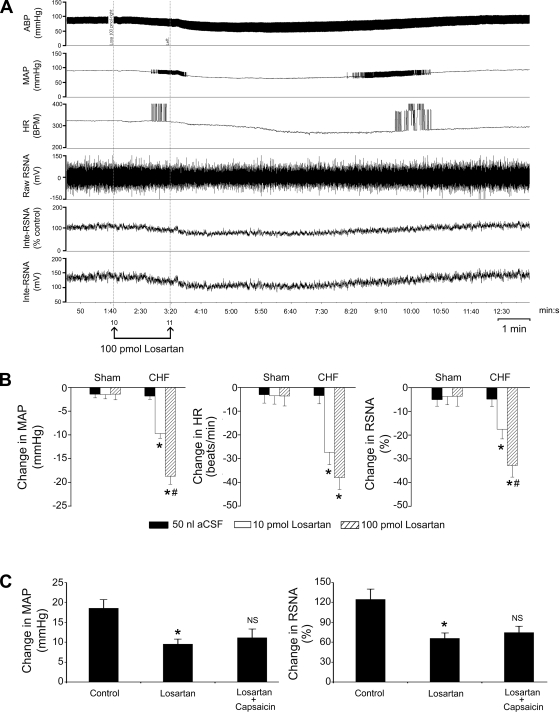
In 15 CHF and 12 sham-operated rats, we detected the effects of bilateral injection of the AT1R antagonist losartan (10 and 100 pmol) into the NTS on the interaction between the chemoreflex and the CSAR. NTS injection of losartan dose-dependently (P < 0.05) reduced the basal MAP, HR, and RSNA in intact CHF rats but not in intact sham-operated rats (Fig. 4, A and B). The responses of losartan in the NTS usually lasted for about 6–10 min. In CHF rats, 10 min after NTS injection of losartan (100 pmol), the increases in MAP and RSNA evoked by intra-arterial injection of KCN (10 μg) or epicardial capsaicin (0.4 μg) were significantly (P < 0.05) attenuated. Importantly, the data demonstrated that NTS losartan completely abolished the capsaicin-induced increase in chemoreflex response in CHF rats (Fig. 4C). In addition, we also found that in sham-operated rats, NTS losartan completely abolished the above interaction, which is similar to our previous data from normal rats (12).
Fig. 4.
Effects of bilateral injection of losartan into the nucleus of the tractus solitarius (NTS) on basal cardiovascular activity and the KCN-induced change in MAP and RSNA in intact sham-operated and CHF rats. A: representative recordings of MAP, HR, and RSNA in response to NTS injection of losartan (100 pmol) in an intact CHF rat. B: change in MAP (left), HR (middle), and RSNA (right) after losartan (10 and 100 pmol) or vehicle [artificial cerebrospinal fluid (aCSF), 50 nl] injected into the NTS in sham-operated (baseline MAP and HR, 89.6 ± 3.8 mmHg and 342 ± 12.6 beats/min, n = 7) and CHF (baseline MAP and HR, 87.3 ± 3.5 mmHg and 336 ± 14.5 beats/min, n = 12) rats. *P < 0.05 vs. aCSF; #P < 0.05 vs. 10 pmol losartan (2-way ANOVA). C: changes in MAP (left) and RSNA (right) evoked by KCN injection after treatment with NTS injection of losartan (100 pmol) and epicardial application of capsaicin (0.4 μg) in CHF rats (baseline MAP and HR, 88.5 ± 3.3 mmHg and 331 ± 17.2 beats/min, n = 7). *P < 0.05 vs. control; NS, no significance vs. losartan (1-way ANOVA).
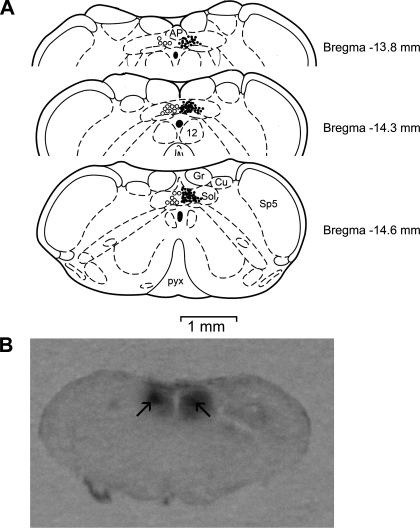
The injection sites in these experiments were verified to correctly locate within the commissural NTS and are shown in Fig. 5 (left opened circles).
Fig. 5.
Histological analysis for microinjection and recording sites in the lower brain stem. A: distributions of the microinjection (○, left) or neuronal recording sites (•, right) plotted on standard coronal sections according to the atlas of Paxinos and Watson (Ref. 27). AP, area postrema; Cu, cuneate nucleus; Gr, gracile nucleus; pyx, pyramidal tract; Sol, nucleus of tract solitary; Sp5, spinal trigemina nucleus; 12, nucleus of hypoglossal nerve. B: the arrow-pointed spots in the raw picture marked the microinjection sites in a 50-μm-thick section of brain stem.
Effects of CSAR on NTS chemosensitive neuronal discharge.
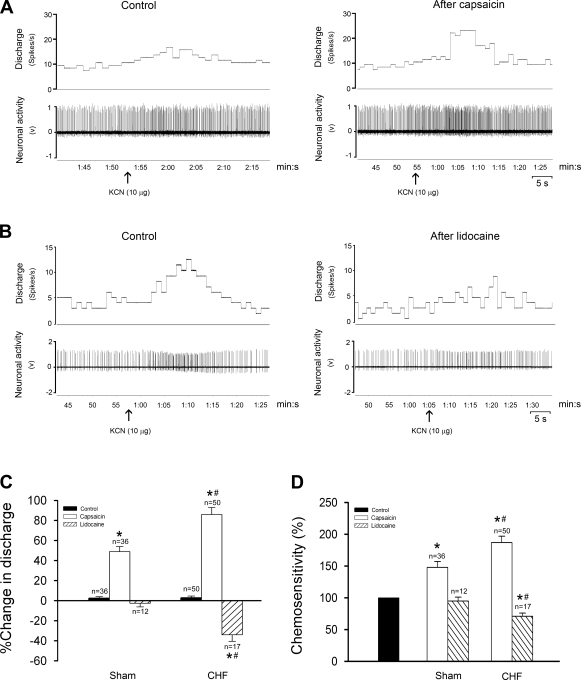
Figure 6 shows the effects of CSAR activation or inhibition on NTS chemosensitive neurons. A total of 119 chemosensitive neurons in the NTS were recorded from 18 CHF and 16 sham-operated rats. The baseline discharge (CHF, 7.5 ± 0.4 spikes/s, n = 62; and sham, 4.1 ± 0.3 spikes/s, n = 57) and chemosensitivity (KCN-induced percent change from baseline level: CHF, 84.5 ± 5.7%; and sham, 57.2 ± 5.4%) of the NTS chemosensitive units were significantly (P < 0.05) higher in CHF than in sham-operated rats. Of 57 chemosensitive neurons in intact sham-operated rats tested for epicardial application of capsaicin, 36 (63%) were excited and 21 did not respond. In intact CHF rats, 50/62 (84%) units were significantly excited during epicardial capsaicin and 12 did not respond. The excitation of capsaicin on neuronal activity usually persisted for about 60–90 s. Furthermore, epicardial capsaicin increased the chemosensitivity of neurons, and the degree of the capsaicin-induced increase in neuronal chemosensitivity was significantly higher in intact CHF than in intact sham-operated rats. Only in vagotomized CHF rats, epicardial lidocaine reduced the baseline discharge as well as the chemosensitivity of NTS capsaicin-sensitive neurons (Fig. 6C). The overlay of action potential trajectories confirmed that the recording neuron was the same before and after treatment (data not shown), suggesting that the recording is a single-unit, but not multiunit, activity. The recording sites were distributed within the commissural NTS (Fig. 6, right filled circles).
Fig. 6.
Effects of cardiac sympathetic afferent input on the activity of NTS chemosensitive neurons. A and B: representative recordings of neuronal discharge in response to right carotid artery bolus injection of KCN (10 μg) after epicardial application of normal saline (control), capsaicin (A, recording from an intact CHF rat) or lidocaine (B, recording from a vagotomized CHF rat). C and D: percent change of baseline discharge (C) and chemosensitivity (D, control level taken as 100%) of NTS chemosensitive neurons NTS in response to epicardial capsaicin or lidocaine in sham-operated and CHF rats. *P < 0.05 vs. control; #P < 0.05 vs. sham (2-way ANOVA).
Western blot analysis of AT1R expression in the NTS.
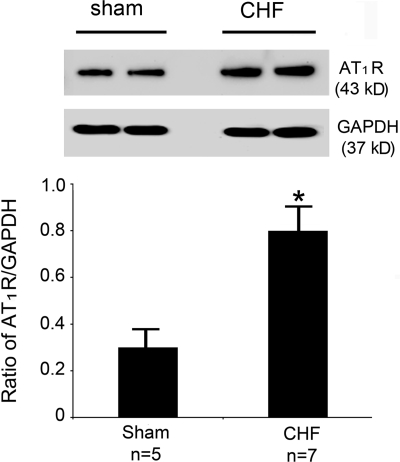
As shown in Fig. 7, the expression level of the NTS AT1R protein was significantly increased in CHF compared with sham-operated rats, suggesting that the expression of AT1R in the NTS is upregulated in the CHF state.
Fig. 7.
ANG II type 1 receptor (AT1R) protein expression in the NTS. Top: representative Western blot showing the NTS AT1R protein expression in intact sham-operated and CHF rats. Bottom: ratio of AT1R to GAPDH expression in the NTS. *P < 0.01 vs. sham (unpaired t-test).
DISCUSSION
The main goal of this study was to determine the central mechanism responsible for mediating the interaction between the chemoreflex and the CSAR in the CHF state. The major new findings are as follows. First, the activation of the CSAR significantly increases chemoreflex sensitivity and the activity of NTS chemosensitive neurons in CHF. Importantly, the blockade of tonic CSAR reduced the chemoreflex sensitivity in CHF. Second, the AT1Rs in the NTS are upregulated, and the blockade of these receptors effectively normalizes the exaggerated chemoreflex or CSAR in CHF. Third, the NTS AT1R blockade abolishes the CSAR-induced increase in chemoreflex response. These data strongly suggest that the augmented cardiac sympathetic afferent input is an important factor contributing to increased chemoreflex function in CHF. This effect is dependent on an NTS AT1R mechanism.
It has been demonstrated that CHF is characterized by elevated sympathetic outflow and abnormal cardiovascular reflexes, which are associated with increased ANG II (8, 11, 43, 44). Abnormalities in cardiovascular reflexes contribute to sympathetic hyperactivity in CHF(43–45). In CHF, the inhibition of the CSAR has a beneficial effect on normalizing the sympathetic hyperactivity and baroreflex impairment (13). Our recent study (12) has demonstrated that, in normal rats, CSAR activation significantly enhances the chemoreflex-induced increase in MAP and RSNA, suggesting a close relationship between the CSAR and the chemoreflex. However, it is unclear whether the augmented CSAR contributes to exaggerated chemoreflex function in the CHF state. Therefore, the purpose of the current study was to assess the effects of CSAR activation on chemoreflex responses in CHF rats and further determine the possible mechanism responsible for this interaction. To avoid interference from cardiopulmonary vagal afferents during epicardial application of lidocaine, these experiments were carried out in vagotomized rats. We found that the chemical stimulation of cardiac sympathetic afferents enhanced chemoreflex sensitivity. This conclusion was also confirmed by electrical stimulation of cardiac sympathetic afferents. In the present study, we did not further separate the stimulation patterns including frequency or intensity to selectively activate the specific fiber. We think that all fibers, including A and C fibers, in cardiac sympathetic afferents were activated under the present stimulation intensity (7 V). In addition to capsaicin-chemosensitive fibers, the other mechanical stimulations such as mechanical probing and cardiac distension are able to effectively increase the activity of cardiac sympathetic afferent input. Because this work was focused on CSAR activation induced by chemical stimulation, the effect of capsaicin-sensitive fiber stimulation on the chemoreflex response was mainly observed before and after the blockade of the NTS AT1R. Importantly, the tonic inhibition of CSAR by epicardial lidocaine produced a significant decrease in chemoreflex responses in CHF but not in sham-operated rats. These results confirmed that the stimulation of the CSAR augmented the responses to chemoreflex activation, suggesting the important significance of augmented CSAR on the contribution to the exaggerated chemoreflex function in CHF. In addition, we did not completely exclude the possibility that the very lipid-soluble substance lidocaine reaches the central nervous system to produce the central cardiovascular effects. However, the quantities of lidocaine within the brain may be very low because of the short time for lidocaine application and the long transmission of sympathetic afferents from the heart surface to the brain through ganglions and the spinal cord. Taken together with previous studies (13, 15) showing the interaction between the arterial baroreflex and the CSAR, we suggest that a reduction in the augmented CSAR in CHF has a potentially beneficial effect on normalizing elevated sympathetic outflow as well as on the blunted baroreflex and the exaggerated chemoreflex. This notion raises a new possible strategy for ameliorating the cardiovascular alternations in the CHF state.
Increased ANG II in the central nervous system has been widely demonstrated to be an important mechanism responsible for abnormalities in circulatory dysfunction in CHF (6, 11, 43, 45, 46). The AT1R antagonist losartan abolishes the CSAR-induced blunted baroreflex and augmented chemoreflex, suggesting that central ANG II may be a candidate for mediating the interaction between the CSAR and baro/chemoreflex (12, 13, 15). The commissural portion of NTS receives the convergent inputs from peripheral chemoreceptors and cardiac sympathetic afferents and plays an important role in mediating the central transmission of the CSAR and the chemoreflex (4, 5, 17, 18, 23). The commissural NTS has been demonstrated to contribute to alternations in cardiovascular and chemoreflex function in pathophysiological states (30). Moreover, AT1Rs have been demonstrated to be expressed within the NTS and mediate cardiovascular reflexes (23, 26, 32, 36). Therefore, we further determined the NTS-AT1R mechanism responsible for the interaction between the CSAR and the chemoreflex. First, we demonstrated that the AT1R protein expression in the NTS was significantly higher in CHF than in sham-operated rats, suggesting the upregulation of AT1R in the NTS in the CHF state. It has been reported that angiotensin-converting enzyme mRNA expression within the NTS is higher in rats with a model of high-output heart failure (31). In rabbits with heart failure, a radioautographic study showed that AT1R density in the NTS is upregulated (45). Second, similar to previous studies (22, 41), bilateral NTS injection of losartan failed to modify basal cardiovascular function in the normal state. However, NTS losartan significantly decreased the basal MAP, HR, and RSNA in CHF, suggesting that upregulated AT1Rs in the NTS are involved in the generation of tonic cardiovascular activity in CHF. Therefore, we believe that increased ANG II and upregulated AT1Rs in the NTS contribute to the CHF-induced sympathoexcitation. It is reported that microinjection of ANG II into the NTS including dorsal medial and commissural portions produces an inhibitory response, whereas AT1R blockade in the NTS has no effect on basal cardiovascular activity in the normal state (16, 22). It is logical to assume that the blockade of upregulated AT1Rs in the NTS would produce an excitatory response in CHF. The exact reasons why the blockade of upregulated AT1Rs in the commissural NTS has an inhibitory effect on basal cardiovascular activity in CHF are not clear. The possibility is that AT1Rs in the different portions of the NTS contribute to the different cardiovascular reflex responses. It is well known that the baroreflex and chemoreflex are predominantly mediated by the dorsal medial and commissural portion of NTS, respectively. Similar to glutamate action in the NTS, the ANG II effect in the dorsal medial NTS seems to be a reflex-evoked action because the period of the effect of exogenous ANG II is somewhat short (usually <3 min). This effect of NTS ANG II may be mediated by the NTS-caudal and rostal ventrolateral medulla connection (inhibitory pathway). We noted in this study that the effect time of losartan in the commisural NTS was longer (>5 min) than the effect of exogenous ANG II. Therefore, we suggest that endogenous ANG II through AT1R is involved in maintaining the tonic sympathetic outflow in the commissural NTS. This relative long-term effect of the NTS losartan may be mediated by the direct NTS-rostal ventrolateral medulla connection (excitatory pathway). Third, the bilateral injection of losartan into the NTS significantly reduced basal CSAR and chemoreflex function and attenuated the CSAR-induced increase in chemoreflex responses in CHF. Elevated ANG II in the NTS may present a deleterious effect on cardiovascular reflexes, whereas AT1R blockade would appear to be beneficial (26). Previous evidence has shown that the microinjection of AT1R antagonist into the NTS does attenuate chemoreflex function (26). It is possible that the change in baseline chemoreflex by NTS losartan influences the evaluation of the relationship between the CSAR and the chemoreflex. However, the present data clearly showed that an epicardial application of capsaicin was not able to significantly enhance the chemoreflex function after pretreatment with losartan in the NTS, even if the basal chemoreflex was attenuated at that time. Our previous study in normal rats has demonstrated that AT1R in the NTS participates in the enhancement of chemoreflex induced by CSAR activation (12). However, there is no direct evidence showing the contribution of AT1R blockade in the NTS to restore the CHF-induced abnormal cardiovascular reflexes. A study in the rabbit with acute MI raises this possibility. Rosario et al. (28) reported that the enhancement of chemoreflex function evoked by acute MI or epicardial application of capsaicin was partially reversed by losartan microinjection into the NTS. It is reasonable to speculate that antagonism of ANG II action within the NTS would present a beneficial effect on the CHF-induced abnormalities in sympathetic tone and cardiovascular reflexes. Besides the AT1R mechanism, we also agree that other mechanisms may mediate the interaction between the CSAR and the chemoreflex. For example, an excitatory amino acid (EAA) mechanism probably is another candidate for this interaction. It has been demonstrated that EAA is a major neurotransmitter for transmission of peripheral afferents of excitatory reflexes at the level of NTS (1, 18, 29, 33). Both the chemoreflex and the CSAR are significantly altered by the blockade of EAA receptors in the commissural NTS (18, 37). It is reported that the carotid chemoreceptor responses are abolished by an injection of both N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists into the commissural NTS (37). On the other hand, Li et al. (18) reported that the blockade of non-NMDA receptors within the commissural NTS eliminated the pressor and RSNA responses to epicardial application of bradykinin. Therefore, it is possible that the EAA release in the NTS triggered by the CSAR activation also acts on EAA receptors (both NMDA and non-NMDA) in the chemosensitive NTS neurons and subsequently enhances the chemoreflex response. In addition, it is not clear whether baroreflex function affects this interaction. It is reported that the stimulation of the CSAR attenuates baroreflex sensitivity in CHF (13). Because baroreceptor denervation was not performed in this work, we do not completely exclude the possibility that the decreased baroreflex sensitivity induced by the CSAR activation enhances the chemoreflex.
We further found that the chemosensitive neurons exhibit a high basal activity including discharge and chemosensitivity in CHF. CSAR activation produced a stronger excitatory effect on discharge and chemosensitivity of the NTS chemoreceptive neurons in CHF than that in sham-operated rats. It appears that more chemosensitive NTS neurons are excited by capsaicin in CHF (84%) than in sham-operated rats (63%). It is not clear whether neurons with different firing rates have different responses to chemoreceptor stimuli. Therefore, the percent change of neuron discharge in response to chemoreceptor stimuli was at least 30% for its sensitivity. We speculate that some neurons with lower chemosensitivity (<30% change in response to KCN) in the normal state increase their chemosensitivity (>30%) under some mechanisms during heart failure development. Furthermore, in CHF but not sham-operated rats, the tonic blockade of CSAR by lidocaine dramatically reduced the activity of the NTS chemoreceptive neurons. It is suggested that the excitatory interaction between these two reflexes occurs at the level of the NTS neurons. These results raise a notion that the enhancement in activity of chemosensitive NTS neurons evoked by the augmented cardiac sympathetic afferent input is a possible important mechanism responsive to increased sympathetic outflow in CHF. It has been demonstrated that the neurons in the commissural NTS can produce the excitatory effect on the vasomotor neurons in the rostral ventrolateral medulla via a direct excitatory projection. The rostral ventrolateral medulla is a key region and common pathway for generation of peripheral sympathetic tonic outflow in the brain.
Although the present study indicates the importance of the NTS AT1R mechanism in mediating the interaction between CSAR and chemoreflex in CHF, there are still several limitations. First, the current data about the central (within the NTS) interrupt with AT1R blockade and CSAR stimulation/inhibition with capsaicin/lidocaine were obtained from acute experiment. Obviously, either NTS pretreatment with AT1R blockade by chronic and conscious techniques such as gene transfer or chronic specific depletion of the enhanced cardiac sympathetic afferent input by resiniferatoxin (35) would be more useful to clarify the important significance of interrupting the interaction between the CSAR and the chemoreflex in normalization of the CHF-induced circulatory dysfunction. Second, previous studies suggest that NTS receives the convergent inputs from the cardiac sympathetic afferent and peripheral chemoreceptor. Our electrophysiological evidence further demonstrated that chemosensitive neurons were excited by stimulation of the cardiac sympathetic afferent. However, it is not clear whether both inputs terminate in one same neuron or through an indirect excitatory interneuron. We have reported that the stimulation of the cardiac sympathetic afferent input can inhibit the specific barosensitive neurons in the NTS, which probably is mediated by an inhibitory interneuron (41).
In conclusion, this study provides experimental evidence suggesting that the augmented cardiac sympathetic afferent input contributes to the exaggerated chemoreflex function in CHF, which is dependent on an AT1R mechanism in the NTS. The beneficial effects on lowering the elevated sympathetic outflow and attenuating the enhanced chemoreflex were associated with the inhibition of cardiac sympathetic afferent input or blockade of the AT1R in the NTS, suggesting that the normalization of enhanced cardiac sympathetic afferents is of potential clinical benefit in the circulatory dysfunctions under the CHF state.
GRANTS
This study was supported by grants from the American Heart Association Grant-in-Aid and National Heart, Lung, and Blood Institute Grants RO-1-HL-077691 and PO-1-HL-62222. W.-Z. Wang was supported by a postdoctoral fellowship from the American Heart Association, Heartland Affiliate (0720066Z).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol 284: R1340–R1353, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Barros RC, Bonagamba LG, Okamoto-Canesin R, de Oliveira M, Branco LG, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci 97: 110–115, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol 87: 259–266, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Cervero F Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev 74: 95–138, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol 268: R851–R858, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 269: R1189–R1196, 1995. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 266: R27–R39, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16: 1125–1134, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Fow JE, Averill DB, Barnes KL. Mechanisms of angiotensin-induced hypotension and bradycardia in the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol 267: H259–H266, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Francis GS The relationship of the sympathetic nervous system and the renin-angiotensin system in congestive heart failure. Am Heart J 118: 642–648, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J Appl Physiol 102: 37–43, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Zhu Z, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. Am J Physiol Heart Circ Physiol 286: H1706–H1711, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hegarty AA, Hayward LF, Felder RB. Influence of circulating angiotensin II and vasopressin on neurons of the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 270: R675–R681, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Kuo DC, Oravitz JJ, DeGroat WC. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res 321: 111–118, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Li DP, Averill DB, Pan HL. Differential roles for glutamate receptor subtypes within commissural NTS in cardiac-sympathetic reflex. Am J Physiol Regul Integr Comp Physiol 281: R935–R943, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res 71: 129–138, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Luoh HF, Chan SH. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Res 782: 73–82, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Seravalle G, Giannattasio C, Bossi M, Preti L, Cattaneo BM, Grassi G. Reflex cardiovascular control in congestive heart failure. Am J Cardiol 69: 17G–22G, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 275: R1611–R1619, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Mifflin SW Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 263: R368–R375, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Minisi AJ, Thames MD. Activation of cardiac sympathetic afferents during coronary occlusion. Evidence for reflex activation of sympathetic nervous system during transmural myocardial ischemia in the dog. Circulation 84: 357–367, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Nerdrum T, Baker DG, Coleridge HM, Coleridge JC. Interaction of bradykinin and prostaglandin E1 on cardiac pressor reflex and sympathetic afferents. Am J Physiol Regul Integr Comp Physiol 250: R815–R822, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Paton JF, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii—a microinjection study in the rat. J Physiol 521: 213–225, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (3rd). New York: Academic Press, 1998.
- 28.Rosario LB, Rocha I, Silva-Carvalho L. Effect of losartan microinjections into the NTS on the cardiovascular components of chemically evoked reflexes in a rabbit model of acute heart ischemia. Adv Exp Med Biol 536: 423–431, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Sapru HN Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. [PubMed] [Google Scholar]
- 30.Sato MA, Schoorlemmer GH, Menani JV, Lopes OU, Colombari E. Recovery of high blood pressure after chronic lesions of the commissural NTS in SHR. Hypertension 42: 713–718, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu H, Hirooka Y, Eshima K, Shihara M, Tagawa T, Takeshita A. Endogenous angiotensin II in the NTS contributes to sympathetic activation in rats with aortocaval shunt. Am J Physiol Regul Integr Comp Physiol 280: R1665–R1673, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Song K, Allen AM, Paxinos G, Mendelsohn FA. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol 316: 467–484, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Spyer KM Central nervous mechanisms contributing to cardiovascular control. J Physiol 474: 1–19, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol 86: 1264–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin. An ultrapotent neurotoxin of capsaicin-sensitive primary afferent neurons. Ann NY Acad Sci 632: 473–475, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsumi K, Saavedra JM. Quantitative autoradiography reveals different angiotensin II receptor subtypes in selected rat brain nuclei. J Neurochem 56: 348–351, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol 264: R41–R50, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor reflex in dogs with experimental heart failure. Circ Res 68: 1294–1301, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Schultz HD, Ma R. Cardiac sympathetic afferent sensitivity is enhanced in heart failure. Am J Physiol Heart Circ Physiol 277: H812–H817, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Wang WZ, Gao L, Pan YX, Zucker IH, Wang W. Differential effects of cardiac sympathetic afferent stimulation on neurons in the nucleus tractus solitarius. Neurosci Lett 409: 146–150, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang WZ, Gao L, Pan YX, Zucker IH, Wang W. AT1 receptors in the nucleus tractus solitarii mediate the interaction between the baroreflex and the cardiac sympathetic afferent reflex in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 292: R1137–R1145, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 287: H1828–H1835, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Zucker IH Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 48: 1005–1011, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Zucker IH, Pliquett RU. Novel mechanisms of sympatho-excitation in chronic heart failure. Heart Fail Monit 3: 2–7, 2002. [PubMed] [Google Scholar]
- 45.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84: 217–232, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940: 431–443, 2001. [PubMed] [Google Scholar]