Abstract
Muscle metabolic by-products stimulate thin fiber muscle afferent nerves and evoke reflex increases in blood pressure and sympathetic nerve activity. Previous studies reported that chemically sensitive transient receptor potential vanilloid type 1 (TRPV1) channels present on sensory muscle afferent neurons have an important impact on sympathetically mediated cardiovascular responses. The reflex-mediated reduction in blood flow to skeletal muscle leads to limited exercise capacity in patients with peripheral arterial occlusive disease. Thus, in this study, we tested the hypothesis that the expression of enhanced TRPV1 receptor and its responsiveness in primary afferent neurons innervating muscles initiate exaggerated reflex sympathetic responses after vascular insufficiency to the muscle. Muscle vascular insufficiency was induced by the femoral artery ligation in rats for 24 h. Our data show that 1) the ligation surgery leads to the upregulation of TRPV1 expression in the dorsal root ganglion; 2) the magnitude of the dorsal root ganglion neuron TRPV1 response induced by capsaicin is greater in vascular insufficiency (4.0 ± 0.31 nA, P < 0.05 vs. sham-operated control) than that in sham-operated control (2.9 ± 0.23 nA); and 3) renal sympathetic nerve activity and mean arterial pressure responses to capsaicin (0.5 μg/kg body wt) are also enhanced by vascular insufficiency (54 ± 11%, 9 ± 2 mmHg in sham-operated controls vs. 98 ± 13%, 33 ± 5 mmHg after vascular insufficiency, P < 0.05). In conclusion, sympathetic nerve responses to the activation of metabolite-sensitive TRPV1 receptors are augmented in rats with the femoral artery occlusion compared with sham-operated control animals, due to alterations in the expression of TRPV1 receptor and its responsiveness in sensory neurons.
Keywords: muscle afferent, claudication, peripheral vascular disease, sympathetic nervous system, exercise, transient receptor potential vanilloid type 1
metabolite-sensitive receptors on muscle afferent nerves are stimulated when interstitial metabolite concentrations increase in active muscle (12, 14). This increases sympathetic nervous activity (SNA) and arterial blood pressure via a reflex mechanism (23, 31, 39), and this is accentuated when muscle contraction is performed under ischemic conditions (12, 14). The augmented response of these “metaboreceptors” with ischemic contraction is attributed to the generation of a variety of chemicals by local muscle cells (26).
Peripheral arterial occlusive disease due to atherosclerosis includes the two major clinical presentations: intermittent claudication and critical limb ischemia. With intermittent claudication, arterial occlusive disease is manifested by insufficient blood flow during exercise. In patients with critical limb ischemia, blood flow is inadequate to meet the resting demands of the limb. Given the evidence that vascular insufficiency and ischemia alter the metabolic profile of active muscle in arterial occlusive disease (3, 22), we postulated in this study that metabolite-sensitive receptors on sensory nerves would be altered and that muscle afferent-mediated sympathetic activation would be exaggerated in rats after vascular insufficiency induced by ligation of the femoral artery.
Chemically sensitive transient receptor potential vanilloid type 1 (TRPV1) receptors on sensory nerves have recently been shown to play an important role in the muscle reflex-mediated sympathetic nerve activity (19, 20). It has been reported that TRPV1 receptors appear at the peripheral terminals and cell body of the sensory afferent neurons. The receptor activity and its characteristics in the sensory cell body have generally been used to reflect its effect in the nerve endings (30, 37, 38). Therefore, immunocytochemical methods were first employed to examine the expression of the TRPV1 receptors in the dorsal root ganglion (DRG) neurons of sham-operated control rats and rats with hindlimb vascular insufficiency in this study. We also used the whole cell patch-clamp method to characterize responsiveness of TRPV1 receptors in the DRG neurons of control rats and rats after the induction of vascular insufficiency. In addition, we further determined the reflex responses of TRPV1 receptors in mediating the SNA after capsaicin was injected into the arterial blood supply of the hindlimb muscles after insult of the vascular insufficiency.
The purposes of this study were to provide evidence for better understanding the mechanisms responsible for altered SNA after the femoral artery occlusion by evaluating characteristics of TRPV1 receptor and SNA responsiveness in whole animal preparation. We tested the main hypothesis that altered expression of TRPV1 receptor and its responsiveness in primary afferent neurons innervating insulted muscles initiate abnormal reflex sympathetic responses observed after muscle vascular insufficiency.
METHODS
All procedures outlined in this study were approved by the Institutional Animal Care Committee.
A rat model of the femoral artery occlusion has been well characterized (29, 44). We used this method to induce hindlimb muscle vascular insufficiency. Male Sprague-Dawley rats (5–7 wk old) were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). The bilateral femoral arteries were surgically exposed and isolated immediately distal to the inguinal ligament. A ligature (3-0 silk) was placed tightly around the femoral artery ∼3 mm distal to the inguinal ligament. It has been reported that this procedure reduces blood flow reserve capacity to ∼10–20% of normal but remains sufficient to meet resting blood flow requirements (29, 42–44). Sham-operated control animals underwent the same procedure as described, except that a suture was placed below the artery but was not tied. The rats were allowed to recover 12, 24, and 48 h before the experiments were started.
Immunocytochemistry.
Five control and five rats with 24 h of hindlimb vascular insufficiency were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen) and then were transcardially perfused with 200 ml of ice-cold saline containing 1,000 units heparin followed by 500 ml of 4% fresh, ice-cold paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4).
The L4–L6 DRGs were removed quickly and postfixed for 2 h in the same fixative solution in a refrigerator (4°C). The tissues were then transferred into 30% sucrose and stored overnight at 4°C. Sections (35 μm) were then cut. After being rinsed in PBS and 0.5% hydrogen peroxide, the sections were transferred into the mixture of PBS, Triton X-100, and normal goat serum (PBS-TX-NGS) for 60 min at room temperature. To label TRPV1 receptors, the sections were incubated with guinea pig anti-TRPV1 receptor (Neuromics Antibodies; 1:000 diluted in PBS-TX-NGS) for 48 h at 4°C as the process of the primary antibody. The primary antibody was enhanced with tyramide signal amplification. The sections were removed from the primary antibody and rinsed in PBS for two times for 10 min and PBS-TX-NGS for 20 min. At room temperature the sections were then incubated with goat anti-guinea pig IgG secondary antibody labeled with FITC (diluted 1:40; Neuromics Antibodies) for 1 h. The tissues were then rinsed in distilled water, mounted on slides from the PBS, and air-dried overnight at room temperature. Subsequently, the sections were dehydrated in ascending alcohol and xylene baths. Slides were then coverslipped. Sections were examined under a fluorescent microscope. The TRPV1 reaction product appeared as a green color in the DRG tissue.
The images of all DRG sections from each rat were taken using a digital camera (Nikon 880, 7M pixels). It has been reported that TRPV1 receptors are selectively expressed in thin fiber sensory neurons (21). Thus neurons with small and medium diameter were selected to count, and maximum optical densities and areas were determined using Adobe Photoshop (34). Background was determined by averaging the maximum optical density of five random areas. Cells with >1.75 times of maximum optical density of background were considered to be positive (11, 27).
Electrophysiology.
The 23 control rats and 25 rats with the femoral artery occlusion were anesthetized by inhalation of an isoflurane-oxygen mixture, and the following injections were performed 3–5 days before a ligation surgery of the femoral artery. The skin was incised and pulled away from underlying muscle tissue, and a fluorescent retrograde tracer, 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine percholate (DiI; 60 mg/ml; Molecular Probes, Eugene, OR), was injected into the white portion of the gastrocnemius muscle. The injection volume was 1 μl, and the injection was repeated three times at different locations. The injection needle was placed in the muscle for 5–10 min to prevent the leakage of the tracer. The skin overlying the muscle was then sutured to cover the incised area, and the rats were allowed to recover from the surgery.
The rats were anesthetized with isoflurane and decapitated 12, 24, and 48 h following the ligation of the femoral arteries. DRG cells at lumbar levels 4–6 were then removed and immediately placed into Dulbecco's modified Eagle's medium (DMEM; GIBCO, Carlsbad, CA). The DRGs were then incubated with 1 mg/ml collagenase IV (Sigma-Aldrich) and 0.5 mg/ml trypsin (Sigma-Aldrich) for 30 min at 34°C in a shaking water bath. Soybean (1.25 mg/ml; Sigma-Aldrich), a trypsin inhibitor, was then added to stop trypsin action. The cell suspension was centrifuged (500 rpm, 5 min) to remove the supernatant, replenished with DMEM, and plated onto a 35-mm culture dish containing poly-l-lysine (50 μg/ml; Sigma)-precoated coverslips and maintained for at least 60 min before electrophysiological recordings. Following the isolation of DRG neurons, the gastrocnemius muscle was dissected to confirm the locations of DiI. These data were included in this experiment if DiI was in the white portion of the gastrocnemius muscle.
Whole cell recordings were made using fire-polished glass electrodes (2–5 MΩ resistance) pulled from glass capillaries (1.17 mm ID, 1.5 mm OD; Harvard Apparatus) on a model P-97 micropipette puller (Sutter Instruments). The recording chamber was continuously perfused (1 to 2 ml/min) with artificial cerebral spinal fluid containing (in mM) 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH adjusted 7.4; and osmolarity, 320 mosM). Electrodes were filled with a solution containing (in mM) 124 KCl, 13.2 NaCl, 2 MgCl2, 0.3 NaGTP, 1 EGTA, 10 HEPES, and 4 Mg-ATP (pH brought to 7.2; osmolarity to 300 mosM). All solutions were made fresh daily and filtered. DiI-labeled DRG neurons were visualized by using a combination of fluorescence illumination and differential interference contrast (×20–40) optics on a Nikon TE2000-inverted microscope. Under differential interference, contrast images of cells were displayed on a video monitor. A tight gigaohm seal was subsequently obtained in the selected neuron. The size of the cell soma was estimated by calculating the mean of the longest and shortest cross-sectional diameters with the aid of a calibrated eyepiece reticle.
For all chemical tests with capsaicin, solutions were applied locally and rapidly (2-s duration) to the neuron of interest using silica 28-gauge syringes of 0.25 mm ID (World Precision Instruments). The tip of each syringe was placed 100 μm from the cell soma using a manipulator. The gravity-fed solutions were controlled using manual switching of one-way stopcock valves. For capsaicin responsiveness experiments, 1 μM capsaicin was prepared fresh each day from a 1 mM capsaicin stock solution. The effect of capsaicin on DRG neurons of sham-operated control rats and rats with vascular insufficiency was examined by applying 1 μM of capsaicin. One neuron per coverslip was studied, and, after each recording, the chamber was washed with ethanol and water to eliminate any residual chemicals.
Signals were recorded with a MultiClamp 700B amplifier (Axon Instruments, Foster City, CA), digitized at 10 kHz with a DigiData 1322A, and filtered at 1 to 2 kHz, and saved in a personal computer-based computer using pClamp 10.1 software (Axon Instruments). Whole cell configuration was maintained at −60 mV. Seals ranged from 1.5 to 6.0 GΩ. An equilibration period of 5–10 min was allowed after whole cell access was established and the recording reached a steady state. The recording was then made to measure changes in inward currents evoked by chemical stimuli. Electrical access to the cell was monitored throughout each recording. The recording was abandoned if the monitored input resistance changed >10%. The magnitude of inward current was determined using Clampfit 10.1 (Axon Instruments). Neurons were considered to be capsaicin sensitive if an evoked inward current was >50 pA in peak amplitude.
Recording of renal SNA.
The eight sham-operated control rats and nine rats with 24 h of the femoral artery occlusion were anesthetized by inhalation of an isoflurane-oxygen mixture as described above. An endotracheal tube was inserted and attached to a ventilator (model AWS, Hallowell EMC). Polyethylene (PE-50) catheters were inserted into an external jugular vein and the carotid arteries for the purposes of drug administration and measurement of arterial blood pressure, respectively. A continuous infusion of physiological saline (0.1 ml/h) into the jugular vein was established by using a syringe pump (Medical Industries). This maintained fluid balance and basal blood pressure. The femoral arteries were carefully isolated in one hindlimb. An incision was made in the femoral artery. PE-10 catheters were inserted into the femoral arteries so drugs could be injected into the arterial blood supply of the hindlimb muscles of the leg, as previously described (19, 20). The skin covering the triceps surae muscle and femoral region was surgically separated from the muscle below to eliminate inputs from cutaneous afferents in the hindlimb.
The animals were artificially ventilated, and end-tidal CO2 was monitored by a respiratory gas monitor (Datex-Ohmeda, Madison, WI) and maintained within normal ranges, as previously described (19, 20). Body temperature was carefully maintained at 37.5–38.5°C by a heating pad and external heating lamps.
Arterial blood pressure was measured by connecting the carotid arterial catheter to a pressure transducer (model P23 ID, Statham). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. Heart rate (HR) was determined from the arterial pressure pulse. All measured variables were continuously recorded on an eight-channel chart recorder (model TA 4000, Gould, Valley View, OH) and stored on an iMac computer that used the PowerLab system (ADInstruments, Castle Hill, Australia).
Renal SNA (RSNA) was recorded as previously described (16, 17). Briefly, a bundle of the renal nerves was carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode to record neural activity were placed between the nerves and the film. These were embedded in a silicone gel. Once the gel was hardened, the silicone rubber was fixed to the surrounding tissue. The RSNA signal was amplified with an amplifier (P511, Grass Instruments) with a band-pass filter of 300 Hz in low-cut frequency and of 3 kHz in high-cut frequency and made audible.
Decerebration was performed as previously described (19, 20, 32). A transverse section was made anterior to the superior colliculus and extending ventrally to the mamillary bodies. The brain rostral to the section was then removed. This approach afforded the opportunity to examine the effect of the arterial injection of capsaicin on blood pressure without considering the confounding effects of anesthesia. Once the decerebration was complete, anesthesia was removed from the inhaled mixture. A recovery period of 60 min after decerebration was employed to allow sufficient time for the elimination of the effects of anesthesia gas from the preparation.
The purpose of this protocol was to determine the sympathetic responses to activation of TRPV1 receptors in sham-operated control rats (n = 8) and rats after vascular insufficiency (n = 9). Capsaicin (obtained from Sigma) was dissolved in 1% Tween 80-1% ethanol-98% saline to make a stock solution of 250 μg/ml (19, 20). On the day of the experiment, capsaicin was diluted in saline to make the concentrations of 0.5 and 1.0 μg/kg body wt. Capsaicin (0.1–0.15 ml) was then injected into the blood supply of the triceps surae muscle. The capsaicin concentrations employed were based on the results of previous studies (19, 20). The duration of the injections was 1 min. At least 20 min were allowed between injections.
RSNA signals were transformed into absolute values, integrated over 1-s intervals, and subtracted by the 1-s integrated background noise. The absolute values of the RSNA varied between rats. To quantify the sympathetic responses to experimental interventions, basal values were obtained by taking the mean value for the 30 s immediately before each intervention and by ascribing the mean value of 100%, and relative changes from baseline during and after intervention were then evaluated. The peak response of each variable was determined by the peak change from the control value.
Statistics.
The immunocytochemical data, amplitude of capsaicin-evoked currents, and measured variables of RSNA, MAP, and HR were analyzed using a one-way repeated-measure analysis of variance. As appropriate, Tukey post hoc tests were used. Values are presented as means ± SE. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SPSS for Windows version 15.0 (SPSS, Chicago, IL).
RESULTS
To test our hypothesis, we first examined the expression of TRPV1 receptors in DRG neurons of sham-operated control rats and rats with the femoral artery occlusion. We next examined capsaicin-induced currents in the DRG neurons innervating muscles. We further examined the effects of vascular insufficiency insult on TRPV1-mediated RSNA response by injecting capsaicin into the arterial blood supply of hindlimb muscles.
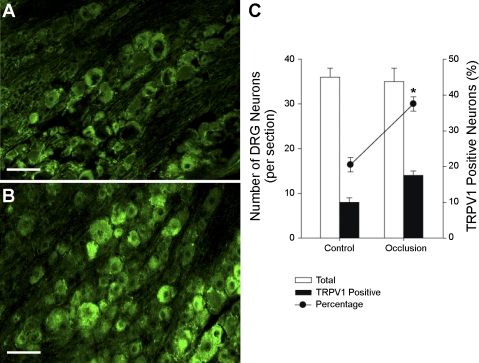
Previous studies have shown that TRPV1 receptors are selectively expressed in thin fiber sensory neurons (21). Thus, in this study, the small and medium diameters of DRG neurons were examined, and neurons labeled with TRPV1 immunostaining were counted for comparison between sham-operated control rats and rats with the femoral artery occlusion. Figure 1, A and B, shows the TRPV1 receptor expression in the DRG neurons of both experimental groups. As reported previously, the immunoreactivity of TRPV1 receptor proteins was highly localized in the DRG cell body (35). Furthermore, when compared with that in control rats (Fig. 1A), a higher density of TRPV1 immunostaining DRG neurons was seen in rats with vascular insufficiency (Fig. 1B). Figure 1C demonstrates that a greater percentage of TRPV1 immunostaining-positive neurons in the DRG was seen in insulted rats compared with sham-operated control rats. TRPV1-positive neurons were 22 ± 2% in five sham-operated control rats and 39 ± 2% (P < 0.05 vs. control) in five rats with the femoral artery occlusion.
Fig. 1.
Transient receptor potential vanilloid type 1 (TRPV1) receptor expression in the dorsal root ganglion (DRG) (L4–L6) neurons. Photographs show TRPV1 immunoreactivity in the DRG from a control rat (A) and a rat with 24 h arterial occlusion (B). The receptors were seen in small and medium diameter of cells and rarely in large diameter of cells. Scale bar = 35 μm. The percentage of TRPV1-positive neurons was significantly higher in rats with arterial occlusion (n = 5) than that in sham-operated control rats (n = 5) (C). *P < 0.05 compared with control group.
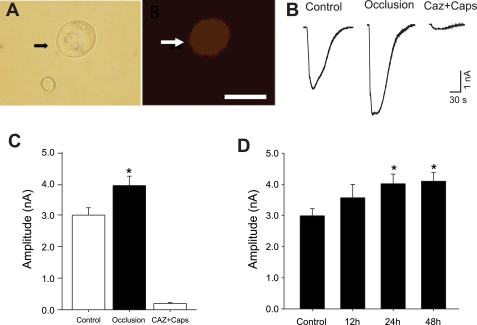
Figure 2, A and B, shows typical images of a DRG neuron innervating hindlimb muscle that was identified by fluorescent tracers, DiI, and original traces of DRG neuron responses to 1 μM capsaicin in sham-operated controls and in rats exposed to 24 h of hindlimb vascular insufficiency. When compared with that in sham-operated control rats, the arterial occlusion insult induced greater peak inward current amplitudes (Fig. 2C; 2.9 ± 0.23 nA in 43 neurons of control vs. 4.0 ± 0.31 nA in 43 neurons of arterial occlusion, P < 0.05). We also examined the effects of the capsaicin receptor blocker, capsazepine, on evoked currents in this experiment. Capsaicin-induced currents in DRG neurons were attenuated by prior exposure of 50 μM of capsazepine (Fig. 2, B and C). We also examined the time course of DRG responses after chronic vascular insufficiency was evoked (Fig. 2D). There was a significant difference in the amplitudes of the capsaicin-induced currents in control and 24 and 48 h after vascular insufficiency.
Fig. 2.
Capsaicin (Caps)-induced currents in DRG neurons recorded using whole cell patch-clamp methods. A, left: DRG neuron (indicated by the arrow) viewed with differential interference contrast optics. A, right: same microscopic field viewed with fluorescence illumination shows that the neuron was labeled with 1,1-dioctadecyl-3,3,3,3-tetramethy lindocarbocyanine percholate (DiI) that was injected into the hindlimb muscle 5 days before the experiment. The DiI-positive neuron is indicated by the arrow. Scale bar = 30 μm. B: original traces of DRG neuron response to 1 μM capsaicin in sham-operated control and 24 h arterial occlusion. C: average data show that 24 h of arterial occlusion insult induced a larger peak amplitude of inward currents compared with sham-operated control. Capsaicin-induced currents in DRG neurons were attenuated by a prior blocking of TRPV1 receptors with capsazepine (Caz). D: a time-course of DRG response shows that there was significant difference in amplitudes of capsaicin-induced currents in control and 24 and 48 h after chronic vascular insufficiency. There was no difference in the evoked currents between 24 and 48 h. *P < 0.05 vs. sham-operated control.
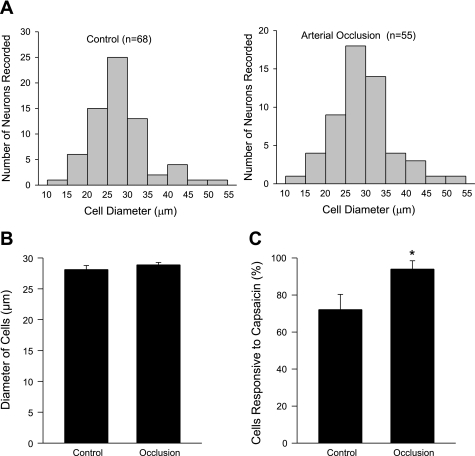
The distribution of the size of all DRG neurons recorded in control and 24-h arterial occlusion are presented in Fig. 3A. A similar size distribution was seen in control and the insulted animals. All capsaicin-sensitive DRG neurons were small and medium size. There was no difference in the average size of recorded DRG cells responding to capsaicin in both experimental groups (Fig. 3B). However, the percentage of the capsaicin-sensitive DRG neurons was greater after a 24-h arterial occlusion (93 ± 5%) than that in the control (72 ± 8%) (Fig. 3C).
Fig. 3.
A: distribution of the size of all DRG neurons recorded in sham-operated control and femoral artery occlusion. B: average size of recorded DRG cells responding to capsaicin. No difference was seen in the average size of recorded DRG cells in both experimental groups. C: percentage of the DRG neurons innervating muscle responsive to capsaicin. A larger percentage of the DRG neurons were seen in 24 h arterial occlusion than in control in response to capsaicin. *P < 0.05 compared with a sham-operated control group.
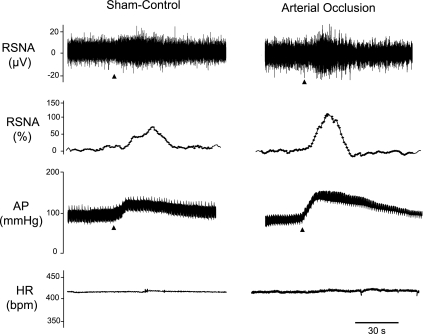
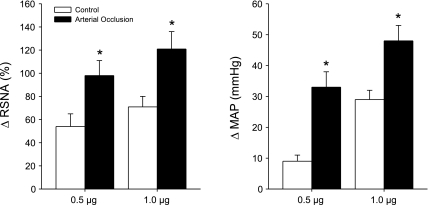
Baseline values for MAP and HR before arterial injections of saline and capsaicin are presented in Table 1. There were no significant differences in basal MAP and HR before injections. In control experiments, the vehicle solution (0.2 ml) was injected into the arterial line in three rats. This did not significantly alter RSNA and blood pressure. The changes in MAP and RSNA were <5 mmHg and <5%, respectively. Figures 4 and 5 show that capsaicin enhances RSNA and blood pressure responses in rats exposed to 24 h of muscle vascular insufficiency. In control rats, 0.5 μg/kg body wt of capsaicin increased MAP by 9 ± 2 mmHg and RSNA by 54 ± 11%. After 24 h of arterial occlusion, the peak pressor and RSNA responses were 33 ± 5 mmHg and 98 ± 13% (P < 0.05 vs. sham-operated control). The MAP and RSNA responses induced by 1.0 μg/kg of capsaicin were 29 ± 3 mmHg and 71 ± 9% in control and 48 ± 5 mmHg and 121 ± 15% after arterial occlusion (P < 0.05 vs. sham-operated control).
Table 1.
Basal and reflexive MAP and HR responses after arterial injection of capsaicin
| MAP, mmHg |
HR, beats/min | |||
|---|---|---|---|---|
| Baseline | Peak | Baseline | Peak | |
| 0.5 μg | ||||
| Sham-operated control | 84±2 | 93±2 | 410±18 | 412±19 |
| Arterial occlusion | 97±8 | 130±11* | 405±21 | 409±21 |
| 1.0 μg | ||||
| Sham-operated control | 92±7 | 121±8* | 413±23 | 414±23 |
| Arterial occlusion | 95±5 | 143±8* | 415±15 | 418±15 |
Values are means ± SE; n = 8 animals in control and 9 animals in femoral artery occlusion. MAP, mean arterial pressure; HR, heart rate. There is no significant difference among basal values.
P < 0.05 vs. baseline.
Fig. 4.
Effects of capsaicin on the renal sympathetic nervous activity (RSNA), arterial blood pressure (AP), and heart rate (HR; bpm, beats/min). Typical recordings are shown of the changes in RSNA and the relative changes in the RSNA, AP, and HR in control and in 24 h arterial occlusion after arterial administration of 1 μg/kg body wt of capsaicin. Arrows indicate a start of injections.
Fig. 5.
Effects of capsaicin on the RSNA and mean arterial pressure (MAP). Average data show that both 0.5 and 1.0 μg/kg body wt of capsaicin induced significant increases in the RSNA and MAP in rats with the femoral artery occlusion. Values are means ± SE. *P < 0.05 vs. control. Number of animals equals 8 in control and 9 in vascular insufficiency insult.
DISCUSSION
Exercise performance is markedly impaired in patients with peripheral arterial occlusive disease (7). A number of factors contribute to this limitation, including reductions in large vessel blood flow and oxygen delivery as well as metabolic abnormalities in skeletal muscle (3). The relative contribution of these factors and their roles in the pathophysiology of the reduced exercise capacity is poorly understood.
Two distinct clinical syndromes, intermittent claudication and critical limb ischemia, are noted when peripheral arterial occlusive disease impedes limb flow. Patients with intermittent claudication have normal or slightly diminished lower extremity blood flow at rest but an inability to adequately increase blood flow with exercise. Thus the femoral artery ligation model of rat that exhibits impaired limb blood flow reserve capacity with exercise but normal flow at rest is widely used for the study of exercise-induced ischemia associated with arterial occlusive disease (40).
In this article, two groups were studied: 1) sham-operated controls; and 2) rats in whom the femoral artery was ligated. The sham-operated control rats underwent the same procedures as the ligation animals except that a suture was placed below the artery and was not tied. After 24 h of the ligation surgery and the sham operation, a catheter was inserted into the femoral artery in both groups of rats. This procedure allowed capsaicin to be injected into the arterial blood supply of the hindlimb muscles to examine the effects of capsaicin on the reflex sympathetic nerve responses. Note that both experimental groups underwent an acute reduction of flow reserve to the hindlimb due to the injecting cannula.
The results of our present study show that the femoral artery ligation upregulates TRPV1 receptors in the primary sensory neurons and that the responsiveness of the receptor is augmented. In turn, TRPV1-associated muscle metabolites enhance the reflex sympathetic responses after insult of chronic femoral artery occlusion. Thus alterations in TRPV1 are likely to contribute to enhanced sympathetically mediated vasoconstriction leading to reduced muscle blood flow and limited exercise capacity under circumstances of muscle vascular insufficiency associated with peripheral arterial occlusive disease.
TRPV1 receptor appears preferentially on metabolite sensitive groups III and IV sensory neurons (21). These receptors are located on afferents in a variety of tissues and mediate the effect of the vanilloid compound capsaicin (4). Capsaicin injected into the pulmonary circulation activates C fibers that play a role in evoking a pulmonary chemoreflex (5, 28). The epicardial application of capsaicin stimulates cardiac TRPV1 receptors, evoking a sympathoexcitatory reflex (45). The competitive capsaicin antagonist capsazepine has been shown to reduce capsaicin-induced activation of the cloned nonselective cation channel TRPV1 (4). Capsazepine also abolishes capsaicin-induced C fiber activity both in vitro and in vivo (9, 18). Although the endogenous TRPV1 ligand has not been determined, both the metabolic by-products accompanying the inflammatory process (lactic acid, H+) and inflammatory mediators themselves (histamine, seratonin, prostaglandin E2) have been identified as potential endogenous ligands for the C fiber “capsaicin” receptor. Hydrogen ions (H+) in general and lactic acid in particular have been shown to activate C fiber afferents similar to the effect seen with capsaicin (2, 10, 33). In vitro studies have demonstrated that H+ inhibits the binding of the capsaicin analog resiniferatoxin to vanilloid receptors, a finding attributed to competition for the same binding site (36).
The activation of thin fiber muscle afferent nerves increases blood pressure and HR via a reflex muscle response (13, 14). When capsaicin is injected into the arterial supply of the dog hindlimb, blood pressure rises by 20%, an effect abolished by sectioning afferent nerves (6). The muscle pressor response is likely to be due to the stimulation of both groups III and IV fibers since capsaicin stimulates 71% of group IV and 26% of group III dog hindlimb muscle afferent fibers (13). In a recent study, we observed that when capsaicin is injected into the arterial supply of the hindlimb muscles of rats, blood pressure increases and the effect is mediated via the engagement of TRPV1 receptors on sensory afferents (19). This is consistent with our current findings. Moreover, we have observed that TRPV1-mediated SNA and pressor responses are exaggerated after the femoral artery ligation, suggesting that vascular insufficiency sensitizes TRPV1 receptors, which is evidenced by showing that 1) arterial occlusion leads to the upregulation of TRPV1 expression in the DRG neurons and 2) the amplitude of capsaicin-evoked currents of the DRG neuron is greater after the arterial occlusion.
The precise mechanism by which TRPV1 receptors are upregulated and their responses are augmented after muscle vascular insufficiency insult needs to be determined. It has been reported that levels of femoral venous lactate, a muscle metabolic by-product, is increased after the femoral artery ligation (22). The responsiveness to capsaicin can be sensitized by acidosis (41), and this may be a stimulus for increased TRPV1 seen in this study. Furthermore, an important report has shown that NGF levels are elevated in hindlimb muscles of rats 24 h after the femoral artery ligation (8). NGF can increase TRPV1 expression in the DRG neurons (1). Thus NGF may play an important role in TRPV1-mediated response after the arterial occlusion insult. Taken together, the data suggest that vascular insufficiency may alter the metabolic milieu of the muscle, and thus sensory neurons with nerve endings in the muscle respond differently to a given level of metabolic stimulation.
Recent data have shown that exercise training has benefits for patients with peripheral arterial occlusive disease (24). Exercise training can improve SNA and alleviate levels of norepinephrine leading to vasoconstriction (15, 25). A decrease in chronic sympathetic tone produced during exercise may also contribute to a reduced progression of peripheral arterial occlusive disease, thereby reducing overall susceptibility to inflammation and coagulation predisposing to further injury. Thus we speculate that TRPV1-mediated SNA may play an important role in regulating exercise capacity in patients with peripheral arterial occlusive disease.
In conclusion, our data demonstrate that SNA responses to activation of metabolite-sensitive TRPV1 receptors are augmented in rats after the femoral artery ligation compared with a control group. These results suggest that alterations in TRPV1 receptors on muscle afferent nerves influence the processing of sensory information in diseases with hindlimb vascular insufficiency such as peripheral arterial occlusive disease and in turn, may alter the magnitude of SNS activity during exercise in patients. Thus the evidence of our study provides strong support for the proposition that skeletal muscle metabolic changes in vascular insufficiency contribute to the exercise intolerance. This is likely due to TRPV1 upregulation-enhanced sympathetic vasoconstriction. This notion has implications for the design of novel intervention strategies to improve physiological functions in patients with arterial occlusive disease and suggests a new line of experiments into such disease at the molecular, biochemical, and physiological levels.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-075533 (to J. Li), R01-HL-078866 (to J. Li), and R01-HL-060800 (to L. I. Sinoway) and American Heart Association Established Investigator Award 0840130N (to J. Li).
Acknowledgments
We express gratitude to Jennie Stoner for outstanding secretarial skills.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett 399: 51–56, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci 17: 509–512, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Brass EP, Hiatt WR, Green S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion. Vasc Med 9: 293–301, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Coleridge HM, Coleridge JC, Schultz HD. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther 42: 1–63, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Crayton SC, Mitchell JH, Payne FC 3rd. Reflex cardiovascular response during injection of capsaicin into skeletal muscle. Am J Physiol Heart Circ Physiol 240: H315–H319, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 31: S1–S296, 2000. [PubMed] [Google Scholar]
- 8.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106: 2257–2262, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience 67: 741–752, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Hong JL, Kwong K, Lee LY. Stimulation of pulmonary C fibres by lactic acid in rats: contributions of H+ and lactate ions. J Physiol 500: 319–329, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res 147: 511–519, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447.
- 13.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Kiilavuori K, Naveri H, Leinonen H, Harkonen M. The effect of physical training on hormonal status and exertional hormonal response in patients with chronic congestive heart failure. Eur Heart J 20: 456–464, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Koba S, Gao Z, Xing J, Sinoway LI, Li J. Sympathetic responses to exercise in myocardial infarction rats: a role of central command. Am J Physiol Heart Circ Physiol 291: H2735–H2742, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Koba S, Xing J, Sinoway LI, Li J. Differential sympathetic outflow elicited by active muscle in rats. Am J Physiol Heart Circ Physiol 293: H2335–H2443, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lee LY, Morton RF, Lundberg JM. Pulmonary chemoreflexes elicited by intravenous injection of lactic acid in anesthetized rats. J Appl Physiol 81: 2349–2357, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Maile M, Sinoway A, Sinoway L. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Sinoway A, Gao Z, Maile M, Pu M, Sinoway L. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Ma QP Vanilloid receptor homologue, VRL1, is expressed by both A- and C-fiber sensory neurons. Neuroreport 12: 3693–3695, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Mack CA, Magovern CJ, Budenbender KT, Patel SR, Schwarz EA, Zanzonico P, Ferris B, Sanborn T, Isom P, Ferris B, Sanborn T, Isom OW, Crystal RG, Rosengart TK. Salvage angiogenesis induced by adenovirus-mediated gene transfer of vascular endothelial growth factor protects against ischemic vascular occlusion. J Vasc Surg 27: 699–709, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Liu K, Ferrucci L, Criqui MH, Greenland P, Guralnik JM, Tian L, Schneider JR, Pearce WH, Tan J, Martin GJ. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med 144: 10–20, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanism, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 27.Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci 20: 474–482, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Paintal AS Vagal sensory receptors and their reflex effects. Physiol Rev 53: 159–227, 1973. [DOI] [PubMed] [Google Scholar]
- 29.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS, Ramkumar V. Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci 24: 3663–3671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol 66: 429–436, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl GL, Longhurst JC. Ischemically sensitive visceral afferents: importance of H+ derived from lactic acid and hypercapnia. Am J Physiol Heart Circ Physiol 262: H748–H753, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci 19: 6497–6505, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci 24: 9521–9530, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szallasi A, Blumberg PM, Lundberg JM. Proton inhibition of [3H]resiniferatoxin binding to vanilloid (capsaicin) receptors in rat spinal cord. Eur J Pharmacol 289: 181–187, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Tsuzuki K, Ase A, Seguela P, Nakatsuka T, Wang CY, She JX, Gu JG. TNP-ATP-resistant P2X ionic current on the central terminals and somata of rat primary sensory neurons. J Neurophysiol 89: 3235–3242, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 126: 429–436, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victor RG, Bertocci L, Pryor S, Nunnally R. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Xing J, Sinoway L, Li J. Differential responses of sensory neurons innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol 586: 3245–3252, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol 278: H85–H93, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Yang HT, Ogilvie RW, Terjung RL. Peripheral adaptations in trained aged rats with femoral artery stenosis. Circ Res 74: 235–243, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551: 515–523, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]