Abstract
How granzymes gain entry into the cytosol of target cells during killer cell attack has been the subject of several studies in the past, but the effective delivery mechanism during target cell encounter has not been clarified. Here we show that granzyme B (GzmB) mutants lacking binding to negatively charged, essentially heparan-sulfate-containing membrane receptors are poorly endocytosed yet are delivered to the cytosol with efficacy similar to that of WT GzmB. In a cell-based system GzmB-deficient natural killer cells provided perforin (pfn) by natural polarized secretion and synergized with externally added GzmB. Whereas receptor (heparan sulfate)-dependent endocytosis was dispensable, delivery of larger cargo like that of GzmB fusion proteins and GzmB–antibody complexes was restricted by their size. Our data support the model in which granzymes are primarily translocated through repairable membrane pores of finite size and not by the disruption of endocytosed vesicles. We conclude that structurally related translocators, i.e., perforin and cholesterol-dependent cytolysins, deliver deathly cargo across host cell membranes in a similar manner.
Keywords: apoptosis, NK cells, pore-forming protein, serine protease
Apoptosis-inducing granzymes are granule-associated serine proteases of cytotoxic lymphocytes (CTL) and natural killer (NK) cells that are delivered to the cytosolic compartment of target cells during killer cell attack (1–4). The only known vertebrate membrane translocator for these effector proteases is a pore-forming protein, known as perforin (pfn), which is coreleased upon target cell recognition. In the presence of calcium, pfn monomers bind to cellular membranes, assemble into multimeric, ring-like structures, and form transmembrane pores similarly to the structurally related terminal complement protein C9 (5) and the pore-forming toxins of some bacteria, e.g., streptolysin O (SLO) or perfringolysin O.
Previous ultrastructural studies revealed the dimensions of pfn pores with an inner diameter of 16 nm (6, 7). Electron micrographs of the membrane attack complex (C5b-9) and poly(C9) indicated a smaller inner diameter of 10 nm for complement pores (5, 8). Conversely, ghost membranes of SLO-lysed erythrocytes carried larger ring-like structures with an internal diameter of 22 nm (9). Since their cloning in the 1980s, membrane-interacting domains of pfn and the terminal complement proteins (C6, C7, C8α, C8β, and C9) have been known to share distant sequence homologies, but conservation of the core fold (MACPF domain) between these host defense proteins and cholesterol-dependent pore-forming bacterial toxins was only recently discovered by crystallographic analyses of MACPF domains from C8α (10) and a pfn-related bacterial protein (11).
Membrane-inserted pfn, SLO, or complement pores directly lyse erythrocytes by a colloid-osmotic process (6, 7). By contrast, most nucleated target cells attacked by cytotoxic lymphocytes do not die from necrosis. They withstand pfn lesions by membrane repair but eventually die from apoptosis initiated by granzymes that are translocated to the cytosol of target cells by the membrane-disturbing pfn (12, 13).
Two possible mechanisms for this vitally important translocation process have been proposed. According to the endosomolysis (vesicle burst) model, GzmB first binds to negatively charged, essentially heparan sulfate (HS)-containing cell surface receptors (14–16), which leads to its efficient endocytosis. GzmB then accumulates in endocytic vesicles in response to the sublytic effects of membrane-bound pfn and is finally released into the cytosol after vesicle disruption (12, 14, 16–19). According to this view, binding of granzymes to cell surface HS is essential for delivery, but the size of the deliverables would not matter. The implications of a pore-mediated delivery model, however, are quite distinct. First, this model predicts a size limit of deliverable macromolecules due to the molecular sieve effect of the membrane-inserted pores. Second, delivery of proteins can directly occur across surface membranes and does not rely on receptor-mediated uptake.
We previously demonstrated that delivery occurs independent of receptor-mediated endocytosis (15). When the surface density of HS on target cells was strongly reduced or GzmB binding sites were blocked (15), apoptosis of target cells by active GzmB or human NK cells was unchanged (15). Here we provide several lines of evidence in favor of the “pore delivery model.” GzmB mutants with little HS binding were poorly endocytosed but were effectively translocated into the cytosol of target cells when attacked by pfn-expressing but GzmB-deficient killer cells. The role of endosomolysis was further evaluated by applying GzmB derivatives and conjugates of larger size. The more the efficacy of transmembrane delivery was restricted, the larger these deliverables were. In aggregate our data prove the existence of transiently induced membrane pores of finite functional size that permit granzymes to invade the cytosol of target cells during killer cell attack.
Results
GzmB Variants Lacking Heparan Sulfate (HS) Binding.
To explore the relevance of HS binding for the delivery of GzmB, we aimed at engineering GzmB variants that no longer bound to HS. The linear GzmB sequence contains the signature for two putative HS binding sites that are located on its surface [supporting information (SI) Fig. S1; HS-Box 1 and HS-Box 2]. The internal site, at position 112–117 (AKRTRA, chymotrypsinogen numbering), overlaps with a potential nuclear localization signal (20). For this reason, we modified only the other site at the C terminus by replacing this α-helical segment with the acidic C-terminal peptide DSVLA derived from human complement factor D (mutant GzmBFacD; Fig. 1A). This sequence motif is not positively charged and should have little immunogenic potential because complement factor D occurs at relatively high levels in human plasma. In a previous publication we reported on differential competition between GzmB and several other cationic serine proteases in HS binding (15). Using these experimental findings and the topological information on conserved basic residues in strongly HS-interacting serine proteases, additional HS binding sites were considered. The region around K127 and K131 is known to function as a heparin binding site in α-thrombin (21, 22) (see also Fig. S2). We therefore modified it as well by replacing both lysines with aspartate residues (GzmBKD). Finally, we combined both modifications to yield the double mutant GzmBKD-FacD (Fig. 1A). Fig. S3A displays the location of the basic residues exchanged in GzmBKD-FacD. All GzmB variants retained full enzymatic activity toward the tetrapeptide substrate Ac-IEPD-pNA (Fig. S3B).
Fig. 1.
Strongly diminished heparan sulfate binding and endocytosis of GzmB mutants. (A) Schematic representation of WT-GzmB and the three variants that were expressed and produced in active form (SI Materials and Methods). Sequences around the mutated positions are shown in single-letter code. The lysine residues that were replaced by aspartates are displayed in blue with chymotrypsinogen numbering above the bar. (B) GzmB variants were incubated with HL-60 cells at 4°C and detected by cell surface staining with the PE-labeled anti-GzmB antibody GB11-PE and analyzed by flow cytometry (SI Materials and Methods). (C) HL-60 cells were preincubated with unlabeled competitor proteins, and then GzmB-bio (10 μg/ml) was added at 4°C. Binding of GzmB-Bio was analyzed by flow cytometry using SA-PE (SI Materials and Methods). Shown are normalized data pooled from several independent experiments with 100% representing geometrical mean fluorescence intensity (MFI) without competitor proteins [GzmB, GzmBFacD, and GzmBKD-FacD, n = 3; GzmBKD, n = 1; GzmB-CD8, n = 2 (all ± SD)]. (D) Confocal imaging of HL-60 cells with endocytosed GzmB-Alexa633 or GzmBKD-FacD-Alexa633 after 1 h of incubation at 37°C (SI Materials and Methods). Blue, Hoechst 33342; red, Alexa Fluor 633-labeled granzymes. (Scale bar: 20 μm.) (E) Quantification of endocytosed granzymes 1 h after incubation at 37°C by intracellular FACS staining with GB11-PE. (Left and Center) Histograms of stainings at the indicated granzyme concentrations. (Right) Summary of two independent, pooled experiments (background subtracted).
Reduced HS Binding of GzmB Variants.
After incubation at 4°C binding of the new GzmB variants to the surface of HL-60 cells was assessed by flow cytometry using the PE-labeled monoclonal anti-GzmB antibody GB11 (Fig. 1B). GB11 recognized WT GzmB and all of its variants equally well because the activity of GzmB and GzmBKD-FacD was completely removed from the supernatant after immunoprecipitation with the GB11 antibody (Fig. S3C). Whereas WT-GzmB bound strongly to HL-60 cells, the single-site mutation in GzmBKD already caused a strong reduction in cell surface binding (Fig. 1B). However, we found that by mutating the C-terminal HS binding site in GzmBFacD binding was completely abolished. As expected, the combined mutant GzmBKD-FacD also lacked any detectable attachment. To monitor distinct qualitative differences in binding, unlabeled GzmB variants at various concentrations were used to compete for the binding of biotinylated WT-GzmB (GzmB-Bio) to HL-60 cells (Fig. 1C). As a positive control, we preincubated HL-60 cells with WT-GzmB. Low concentrations of 1.56 μg/ml and 3.12 μg/ml already prevented the binding of GzmB-Bio drastically. By contrast, the three mutants GzmBKD, GzmBFacD, and GzmBKD-FacD (in this order) showed a progressive loss of their ability to compete with GzmB-Bio. Whereas GzmBKD nearly completely inhibited the binding of GzmB-Bio at concentrations above 12.5 μg/ml, GzmBFacD reduced its binding by barely 50% at 2-fold-higher concentrations of 25 μg/ml. The weakest competition for membrane binding was observed with the double mutant GzmBKD-FacD, which accounted for a mere 15% reduction at 25 μg/ml. These findings indicate that the C-terminal site is of major importance in HS binding whereas the internal site seems to play a subsidiary role. To answer the question as to whether the overall net charge or particular surface regions of the GzmB variants played key roles in promoting the binding to HS, we made use of a previously described (23) GzmB variant, GzmB-CD8, which carried a C-terminal, highly acidic elongation of eight aspartate residues. This variant competed as effectively as WT-GzmB for HS binding, indicating that HS chains interacted with specifically structured regions of the natively folded GzmB.
Because HS-bound GzmB is rapidly endocytosed by target cells (14–16), we anticipated that the GzmBKD-FacD double mutant escaped the HS-mediated endocytosis pathway. To this end we compared the endocytosis of Alexa Fluor 633-labeled WT-GzmB and GzmBKD-FacD by confocal microscopy imaging (Fig. 1D). After 1 h of incubation at 37°C, WT-GzmB was endocytosed by HL-60 cells and showed the typical punctate fluorescence pattern in the cytosol. After the same time period very small amounts of fluorescent GzmBKD-FacD, close to background levels, were observed inside HL-60 cells. To quantify the amounts of WT-GzmB and GzmBKD-FacD that were internalized by both HS-bound and free bulk flow endocytosis, we fixed the cells and assessed the endosomal, cell-associated content by intracellular FACS staining using the anti-GzmB antibody GB11-PE (Fig. 1E). At a concentration of 200 nM (5 μg/ml) the cell-associated signal for WT GzmB was eight times higher than for the double mutant GzmBKD-FacD. As more of it was taken up by bulk flow endocytosis at high concentrations (1,000 nM), signal intensities for GzmBKD-FacD increased, but to a much smaller extent than WT-GzmB. These experiments clearly demonstrated that endocytosis of GzmB in our cell culture system was largely dependent on cell surface binding.
GzmB Mutants Escape the Inhibitory Effects of Heparin.
Heparin, which is closely related to HS, was recently found to block GzmB-dependent killing using pfn as a translocator (14). This inhibition was interpreted as competition between soluble heparin and membrane-associated HS for GzmB. Similarly, we found that target cells exposed to GzmB and streptolysin O (SLO) were strongly protected by soluble heparin (19 kDa) at concentrations in the equimolar range. GzmB mutants killed target cells as efficiently as WT-GzmB (Fig. 2A). Inhibition of cell death induction by heparin, however, was correlated to the HS-binding property of different mutants. WT-GzmB was most efficiently suppressed (by 60%), whereas GzmBKD-FacD was not affected by heparin concentrations of 10 and 50 μg/ml. Evidently, translocation of GzmB by SLO did not depend on HS binding. Moreover, assembly and translocation by SLO were not affected by soluble heparin. The inhibitory effect of heparin on GzmB at concentrations between 1 and 25 μg/ml (Fig. 2B) was indeed caused by direct enzymatic inhibition. Heparin at 5 μg/ml inhibited WT-GzmB (200 nM) by 75% and GzmBKD by 47%, but GzmBFacD and GzmBKD-FacD by only 3% and 9%, respectively. Diminished cell surface binding and endocytosis, as well as the resistance to the inhibitory effects of heparin, are evidently linked to the same few basic residues of GzmB.
Fig. 2.
Decreased inhibition of GzmB-mutants by heparin. (A) Heparin inhibits GzmB-mediated cell death. GzmB mutants (10 μg/ml) and heparin were added to HL-60 cells. Streptolysin O (SLO), at sublytic concentration, was applied to deliver granzymes. Two hours later, cell death and apoptosis were monitored by annexin V-FITC (AV)/propidium iodide (PI) staining. Dead (AV+/PI+) and apoptotic (AV+/PI−) cells were summed up. The columns and error bars indicate the mean of two independent experiments (n = 2 ± SD). (B) Apoptotic effects are abrogated by heparin because of direct inhibition of enzymatic activity. Granzymes (200 nM) and heparin were preincubated, and the activity was measured against Ac-IEPD-pNA. Reduction of the slope (initial increase in OD405) was calculated as percentage (n = 2 ± SD) (SI Materials and Methods).
Delivery of GzmB Variants Using Different Translocators.
SLO is a well characterized, pore-forming protein of bacterial origin that delivers molecules in an endocytosis-independent manner (24). The inner diameter of these ring-like pores is 22 nm (9, 24, 25). We compared the apoptotic potential of the different GzmB variants in titration experiments using SLO as a delivery reagent. All variants efficiently induced apoptosis of HL-60 cells in a concentration-dependent fashion, demonstrating that the mutations do not affect the caspase-activating potential of the GzmB variants (Fig. 3A). Next, we tested the GzmB variants together with purified human pfn and found that all variants were able to kill HL-60 cells. When compared with WT-GzmB, GzmBKD-FacD, however, killed with somewhat reduced efficiency (Fig. 3B), an observation that is in agreement with a previous study (14).
Fig. 3.
Apoptotic potential of GzmB mutants. GzmB mutants were delivered to the cytosol of HL-60 cells either by SLO (A) or by soluble pfn (B). (A) SLO transfers each mutant with similar efficacy (n = 2 ± SEM). (B) The pfn-induced transfer of GzmB variants is slightly impaired compared with WT-GzmB (n = 3 ± SEM).
Complementation of GzmB-Deficient Killer Cells with GzmB Variants.
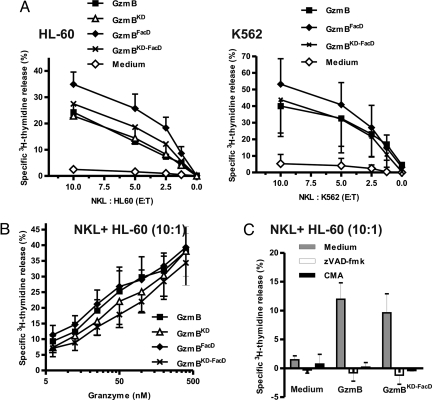
Because NK cell-mediated killing did not require HS on target cells (15), we have recently questioned the relevance of endocytosis of membrane-bound GzmB during target cell attack. Observations with purified pfn may have been misleading, and hence we aimed to evaluate different GzmB variants during NK cell attack. NKL is a NK cell line that expresses pfn (Fig. S4A) and GzmA (26) and is able to kill target cells in a pfn-dependent fashion (Fig. S4C). NKL cells, however, express only trace amounts of GzmB (26) as shown here by Western blotting (Fig. S4A) and by enzymatic activity measurements of cell lysates (Fig. S4B). Consequently NKL cells were unable to induce rapid, caspase-dependent apoptosis in target cells, as verified by measuring the release of [3H]thymidine-labeled DNA from target cells (Fig. 4A, Medium). This deficiency of endogenous GzmB, however, could be balanced by the addition of recombinant GzmB to the cell culture medium. Delivered to the target cell cytosol by endogenous, naturally secreted pfn, recombinant GzmB strongly induced apoptosis of the two different target cell lines, namely K562 and HL-60 (Fig. 4A). Importantly, WT-GzmB and the mutant GzmB variants, GzmBFacD and GzmBKD-FacD, were equally effective in complementing the insufficient quantities of endogenous GzmB at different effector-to-target ratios. Because the HS-dependent uptake of GzmB might solely play a role at low doses of GzmB, we titrated WT-GzmB and GzmB variants to very low concentrations (Fig. 4B). Here we found that GzmBKD and GzmBKD-FacD were only slightly less efficient whereas the low HS-binding mutant GzmBFacD was as potent as WT-GzmB. To ascertain that endogenous pfn was implicated in the delivery of GzmB during killer cell attack, we used concanamycin A (CMA) to prevent the acidification of NK cell granules and storage of pfn during biosynthesis (27). Under these experimental conditions, complementation by GzmB did not have any impact on target cells. In line with this, the effect of externally added GzmB was completely blocked by the depletion of calcium with EGTA during killer cell attack (data not shown). Furthermore, the pan-caspase inhibitor Z-VAD-FMK completely prevented caspase activation and apoptosis in target cells, indicating that the GzmB variants, added to the medium, reached the cytosol and triggered a caspase-dependent cell death program (Fig. 4C). Finally, adding the granzymes in the absence of NKL cells did not have any direct effect on target cells (data not shown).
Fig. 4.
Externally added GzmB and GzmB mutants complement NKL cells lacking GzmB. (A) Granzymes added externally to the medium correct the GzmB deficiency of NKL cells. K562 cells (Left) or HL-60 cells (Right) were coincubated with NKL cells. Apoptosis induced by NKL cells and monitored via [3H]thymidine release was very weak. Addition of GzmB or GzmB mutants (400 nM with K562 and 200 nM with HL-60 cells) strongly induced apoptosis even at very low effector-to-target (E:T) ratios. n = 2 ± SEM (Left), and n = 2–3 ± SEM (Right). (B) Dose-dependent apoptosis induction by GzmB and GzmB mutants. Granzymes were titrated over a wide concentration range for a given E:T ratio of 10:1 (NKL:HL60). n = 2 ± SEM. (C) Complementation effects are caspase- and pfn-dependent. Treatment with the pan-caspase inhibitor Z-VAD-FMK or concanamycin A (CMA) totally abolished [3H]thymidine release induced by granzymes (400 nM) and NK cells at an E:T ratio of 10:1. (n = 2 ± SEM).
Taken together, these results, along with our previous data, clearly support the view that the membrane interactions of GzmB on target cells are irrelevant and dispensable for the delivery of GzmB by killer cells. Exploiting the natural granzyme delivery process instead of soluble pfn, we obtained clear evidence against the necessity of endocytosis and endosomolysis.
Mechanism of pfn-Mediated GzmB Delivery.
If effector molecules pass through membrane pores during killer cell attack, entry and efficiency of delivery should be size-dependent. We therefore varied the physical size of GzmB in two distinct ways. First, the extracellular domain of the human myelin oligodendrocyte glycoprotein (MOG) was covalently attached to the C terminus of human GzmB, thereby increasing its molecular mass by 15 kDa. This GzmB-MOG fusion protein was expressed and refolded exactly as recombinant GzmB and retained full enzymatic activity (data not shown). When delivered with the help of SLO into HL-60 cells, it showed the same apoptosis-inducing effects as GzmB under identical conditions (Fig. 5A). Apparently, the large transmembrane pore formed by SLO did not discriminate between proteins of 25 kDa (GzmB) and 40 kDa (GzmB-MOG). Likewise, the delivery of GzmB-MOG induced by soluble pfn was only marginally impaired (Fig. 5B). In the NKL complementation assay (Fig. 5C) the larger GzmB-MOG fusion was also capable of reaching the cytosol, although with somewhat lower efficacy than GzmB (Fig. 5B).
Fig. 5.
Size dependence of GzmB entry. (A) Comparisons between the GzmB-MOG fusion and WT-GzmB show almost similar apoptotic activities in HL-60 cells after permeabilization with SLO (n = 2 ± SEM). (B) pfn-induced delivery of GzmB-MOG is moderately reduced (n = 3 ± SEM). (C) Delivery of GzmB-MOG by NKL-derived pfn was lower than that of WT-GzmB (E:T, 10:1) (n = 3 ± SEM). *, P < 0.05. (D) The mAb to MOG (MAB2439, 266 nM) is a rat IgG2b and forms a stable complex with GzmB-MOG (100 nM), which is pulled down by protein G-coupled Sepharose. The residual activity in the supernatant (ΔOD per hour) is depicted by columns, and the activity in the pellets after 1 h of incubation in substrate is shown by symbols. (E) pfn-mediated delivery is size-restricted. Granzymes at 200 nM, free or precomplexed with anti-MOG or its isotype (both 400 nM), were delivered to HL-60 cells by sublytic amounts of purified pfn (n = 2 ± SEM). (F) NKL cell-mediated delivery of exogenously added GzmB-MOG is specifically blocked by anti-MOG mAb. Same experimental setup as in E except that NKL cells were used instead of pfn. Release of [3H]thymidine by apoptotic HL-60 cells at an E:T of 10:1 was measured (n = 2 ± SEM). N.D., not determined; **, highly significant at the P < 0.0001 level; ns, not significant with P > 0.05.
To increase the size of GzmB-based effectors further, an anti-MOG-directed complex between a monoclonal antibody (MAB2439) and the GzmB-MOG fusion was formed. To verify the completeness of complexation, MAB2439 was shown to capture all GzmB-MOG, when a 2.7-fold molar excess of the MOG-specific mAb was added to the culture medium (Fig. 5D). The activity of GzmB-MOG, but not that of GzmB alone, was completely removed from the supernatant and was recovered in the protein G-Sepharose pellet (Fig. 5D). pfn-mediated delivery of GzmB-MOG into HL-60 cells (Fig. 5E) was specifically blocked by MAB2439 (anti-MOG) but not by an unrelated rat IgG2b (rIgG2b). As expected, MAB2439 did not change the delivery of GzmB, excluding the possibility that the mAb interfered with the function of pfn itself. Finally, we added the anti-MOG mAb to NKL and HL-60 target cells (Fig. 5F). The anti-MOG mAb specifically diminished the delivery of GzmB-MOG but not that of GzmB into HL-60 cells as measured by [3H]thymidine release. Both sets of experiments clearly revealed that transmembrane delivery by pfn was size-dependent. Similar results were also obtained with a GzmB-specific mAb (GB11) (Fig. S5) when pfn or NKL cells were used.
In SI Materials and Methods we describe a series of control experiments in more detail, showing that binding of antibodies to the GzmB-MOG fusion does not abolish caspase activation, the binding of GzmB-MOG to the cell surface, or its route of internalization (Fig. S6). Our experiments clearly indicate that endocytosis via HS is dispensable for granzyme delivery by NK cells and that delivery is size-restricted.
Discussion
The use of functionally active recombinant GzmB derivates as a deliverable effector protease has allowed us to explore the mechanism of outside-in delivery during target cell attack. Two sets of experiments performed with low heparin-binding variants of GzmB and with larger GzmB-MOG fusions and GzmB-MOG antibody complexes provided evidence for the view that GzmB enters the cytosolic compartment of target cells via membrane pores and not by disruption of GzmB-containing endocytic vesicles. To change the natural trafficking of GzmB while retaining natural delivery and trafficking of pfn, we added to the extracellular fluid tailor-made GzmB variants that were poorly endocytosed by target cells. Nevertheless, these GzmB variants were able to complement pfn-expressing NKL cells that lacked endogenous GzmB.
In most previous studies, like in some of our initial experiments, target cells were exposed to sublytic amounts of purified soluble pfn. Using sublytic pfn concentrations, we indeed observed a partial impaired delivery of the low heparin-binding mutant GzmBKD-FacD (Fig. 3B) in accordance with a previous study (14).
At first glance these data appeared to support the view of delivery via endocytosis. We, however, interpret this finding differently. Externally added, soluble pfn has access to the entire cellular surface. When perforated membrane patches are endocytosed without concomitant uptake of the GzmBKD-FacD mutant, then, in contrast to WT GzmB, GzmBKD-FacD concentrations at the internalized pore openings decline on average and the efficacy of delivery drops. Under these conditions segregating pores from deliverables most likely impairs the delivery process.
To circumvent the experimental limitations of soluble pfn in fluid phase experiments, we adopted a different experimental approach that mimicked the in vivo conditions of killer cell attack. In contrast to the attack of target cells by purified soluble pfn, complementation of NK cells by externally added GzmB variants simulated the natural, localized delivery mode with vectorial secretion of pfn onto a small membrane area. The GzmBKD-FacD mutant that was poorly endocytosed induced caspase-dependent apoptosis with efficacy similar to that of WT-GzmB. Hence, our NKL complementation studies clearly pointed to a pore-based mechanism of GzmB delivery.
The biological effects we observed with different GzmB variants depended on secretion of pfn and on caspase activation and were not a downstream action of soluble GzmB on already necrotic cells. Cells killed by high concentrations of pfn cannot be converted by simultaneous addition of GzmB from “necrotic cells” into “apoptotic cells” (12). This notion is well supported by our own observations at higher effector-to-target ratios (data not shown). Under these conditions, DNA fragmentation was not detected and the complementation effects of GzmB declined.
Compatible with our observations is a channel- or pore-mediated delivery mechanism that does not require endocytic uptake and disrupture of GzmB-containing vesicles. pfn concentrations at the immunological synapse between target and killer cells are probably higher at least transiently (19) than the sublytic concentrations used in vitro. Hence, a local cluster of transient membrane pores may well form, and granzymes may directly reach the cytosol across protein channels of sufficient size and density (1, 28). Necrosis of target cells, nevertheless, is prevented by the rapid repair of these circumscribed membrane lesions (12, 28), and apoptosis is induced by caspase activation.
To obtain further functional evidence for a pore-based delivery mechanism, we explored the sieving property of transmembrane channels in target cells during killer cell attack. To measure the outside-in transit of protein deliverables, we used GzmB as a functional indicator and increased its size by fusing it to the extracellular domain of MOG. Using SLO or purified pfn, the GzmB-MOG fusion was delivered nearly as well as GzmB. Translocation of both effector molecules was also induced by the local pfn release from NKL cells, but GzmB-MOG was delivered with lower efficacy (Fig. 5C), suggesting that granule deposits around the pore openings compete for diffusion of GzmB-MOG through membrane channels more than for that of the smaller GzmB.
Stokes radii of effector molecules that are small enough to be delivered give an upper estimate for the pore dimensions. The stokes radii of globular protein domains can be calculated from their atomic structure with low deviation errors (29). Because the stokes radii for MOG and GzmB are 2.1 and 2.5 nm, respectively (Table S1), the two-domain structure of the fusion protein (39.1 kDa) is expected to be significantly larger but is still not large enough to abrogate pore-mediated delivery. Other fusion partners with larger dimensions either were degraded by GzmB (maltose-binding protein) or did not fold into a catalytically active two-domain effector molecule. Moreover, fusion partners like maltose-binding protein and GST do not occupy much larger space, even if they formed dimers after refolding (Table S1).
The only reasonable solution we found were experiments with intact monoclonal antibodies binding to MOG or GzmB directly. The GzmB (GB11)- and MOG (MAB2439)-directed antibodies were of different origin and isotype (murine IgG1 and rat IgG2b, respectively) with Stokes radii of 5.2 nm (Table S1). Cytosolic transfer of GzmB and GzmB-MOG antibody complexes was clearly reduced when pfn or NKL was used. Importantly, there was only a minor loss of caspase activation after antibody complexation in cell lysates (Figs. S5C and S6A). Our findings are complementary and coherent with respect to older studies showing that purified terminal complement proteins, C9, or pfn at high concentrations can assemble into pores with a maximum functional pore radius of 5.0–5.5 nm (30, 31). We are, therefore, convinced that transmembrane pores with a maximal cutoff size are formed during killer cell attack and permit the passage of death-inducing granzymes.
The discovery that GzmB variants lacking affinity to HS are effective apoptosis-inducing effectors widens the perspective on GzmB-based therapeutics. Reducing the binding to endothelial HS and extracellular matrix components improves not only bioavailability, but also unwanted side effects. GzmB was recently shown to induce anoikis of adherent cell lines (32), but mutants with low HS affinity had lost this undesirable side effect (33). With regard to the design of GzmB-based immunoconjugates (23, 34, 35), deficiency in HS binding is an essential prerequisite for in vivo applications.
Materials and Methods
Materials.
Chemicals were purchased from Merck, Sigma–Aldrich, and Roth. Cell culture media and additives were from Invitrogen, and 96-well plates were from NUNC.
Cell Culture.
HL-60, K562, and NK-92 cells were maintained as described (15, 23). NKL cells were grown in RPMI medium 1640 containing 2 mM GlutaMAX I, 15% FBS (Biochrom), 100 units/ml penicillin/streptomycin, and 100 units/ml IL-2.
Apoptosis Assay.
HL-60 cells were washed twice in RB medium (SI Materials and Methods), and the assays were performed in RB medium in V-shaped 96-well PS plates at a cell concentration of 5 × 104 cells in a volume of 50 μl. Granzymes and sublytic amounts of preactivated (23) SLO (50–75 ng/ml; Sigma–Aldrich) were added. Cells were analyzed after 2 h of incubation at 37°C and 5% CO2. Staining with AV-FITC and PI and FACS analysis were performed as described (23). Human pfn was used at 200 ng/ml (Alexis-Axxora). When blocking potential Fc receptors, HL-60 cells were preincubated for 10 min at room temperature with rat and human IgG (Jackson ImmunoResearch).
DNA Fragmentation Assay ([3H]Thymidine Release).
[3H]Thymidine release as a measure of target cell apoptosis was quantified as described (36). Briefly, 5,000 labeled target cells were coincubated with NKL cells at the indicated E:T ratios in 50 μl of OB medium (SI Materials and Methods) containing IL-2 (300 units/ml) in 96-well PS plates for 3.5 h. Granzymes were added to the medium. To increase the size of effectors, GzmB and GzmB-MOG fusion protein were preincubated with a MOG-specific monoclonal antibody (MAB2439; R & D Systems) or its isotype control for 10 min. Harvest of cytosolic extracts and calculation of specific [3H]thymidine release were done as described (36). Z-VAD-fmk (Bachem) was used at 100 μM.
Statistics.
To determine significance of data from pooled experiments the unpaired two-tailed t test was used.
Supplementary Material
Acknowledgments.
We thank H. Reimann for excellent technical assistance, G. Salvesen and S. Riedl for recombinant caspase, and H. Wekerle for his continuous interest in the project. We thank C. Essmann and B. Garvalov for their critical comments. The financial support of the German Research Council (SFB 571 and Grant JE194–2) is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801724105/DCSupplemental.
References
- 1.Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol. 2003;15:522–527. doi: 10.1016/s0952-7915(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman J. Cell death and immunity: The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 3.Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19:339–347. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 5.Young JD, Cohn ZA, Podack ER. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: Structural, immunological, and functional similarities. Science. 1986;233:184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]
- 6.Podack ER, Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. Nature. 1983;302:442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- 7.Masson D, Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985;260:9069–9072. [PubMed] [Google Scholar]
- 8.Discipio RG, Hugli TE. The architecture of complement component C9 and poly(C9) J Biol Chem. 1985;260:14802–14809. [PubMed] [Google Scholar]
- 9.Sekiya K, et al. Ultrastructural analysis of the membrane insertion of domain 3 of streptolysin O. Microbes Infect. 2007;9:1341–1350. doi: 10.1016/j.micinf.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Hadders MA, Beringer DX, Gros P. Structure of C8alpha-MACPF reveals mechanism of membrane attack in complement immune defense. Science. 2007;317:1552–1554. doi: 10.1126/science.1147103. [DOI] [PubMed] [Google Scholar]
- 11.Rosado CJ, et al. A common fold mediates vertebrate defense and bacterial attack. Science. 2007;317:1548–1551. doi: 10.1126/science.1144706. [DOI] [PubMed] [Google Scholar]
- 12.Keefe D, et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 14.Bird CH, et al. Cationic sites on granzyme B contribute to cytotoxicity by promoting its uptake into target cells. Mol Cell Biol. 2005;25:7854–7867. doi: 10.1128/MCB.25.17.7854-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurschus FC, Bruno R, Fellows E, Falk CS, Jenne DE. Membrane receptors are not required to deliver granzyme B during killer cell attack. Blood. 2005;105:2049–2058. doi: 10.1182/blood-2004-06-2180. [DOI] [PubMed] [Google Scholar]
- 16.Raja SM, et al. A novel mechanism for protein delivery: Granzyme B undergoes electrostatic exchange from serglycin to target cells. J Biol Chem. 2005;280:20752–20761. doi: 10.1074/jbc.M501181200. [DOI] [PubMed] [Google Scholar]
- 17.Veugelers K, et al. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate. Mol Biol Cell. 2006;17:623–633. doi: 10.1091/mbc.E05-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, et al. Granzyme B binds to target cells mostly by charge and must be added at the same time as perforin to trigger apoptosis. J Immunol. 2005;174:5456–5461. doi: 10.4049/jimmunol.174.9.5456. [DOI] [PubMed] [Google Scholar]
- 19.Pipkin ME, Lieberman J. Delivering the kiss of death: Progress on understanding how perforin works. Curr Opin Immunol. 2007;19:301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blink EJ, et al. Interaction of the nuclear localizing cytolytic granule serine protease granzyme B with importin alpha or beta: Modulation by the serpin inhibitor PI-9. J Cell Biochem. 2005;95:598–610. doi: 10.1002/jcb.20415. [DOI] [PubMed] [Google Scholar]
- 21.Bode W, Turk D, Karshikov A. The refined 1.9-A x-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: Structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992;1:426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hink-Schauer C, et al. The 22-A crystal structure of human pro-granzyme K reveals a rigid zymogen with unusual features. J Biol Chem. 2002;277:50923–50933. doi: 10.1074/jbc.M207962200. [DOI] [PubMed] [Google Scholar]
- 23.Kurschus FC, et al. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]
- 24.Walev I, et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA. 2001;98:3185–3190. doi: 10.1073/pnas.051429498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Mahrus S, Craik CS. Selective chemical functional probes of granzymes A and B reveal granzyme B is a major effector of natural killer cell-mediated lysis of target cells. Chem Biol. 2005;12:567–577. doi: 10.1016/j.chembiol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka T, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 28.Griffiths GM. Endocytosing the death sentence. J Cell Biol. 2003;160:155–156. doi: 10.1083/jcb.200212143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia de la Torre J, Huertas ML, Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Criado M, Lindstrom JM, Anderson CG, Dennert G. Cytotoxic granules from killer cells: Specificity of granules and insertion of channels of defined size into target membranes. J Immunol. 1985;135:4245–4251. [PubMed] [Google Scholar]
- 31.Sauer H, Pratsch L, Tschopp J, Bhakdi S, Peters R. Functional size of complement and perforin pores compared by confocal laser scanning microscopy and fluorescence microphotolysis. Biochim Biophys Acta. 1991;1063:137–146. doi: 10.1016/0005-2736(91)90363-d. [DOI] [PubMed] [Google Scholar]
- 32.Buzza MS, et al. Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem. 2005;280:23549–23558. doi: 10.1074/jbc.M412001200. [DOI] [PubMed] [Google Scholar]
- 33.Buzza MS, Bird PI. Extracellular granzymes: Current perspectives. Biol Chem. 2006;387:827–837. doi: 10.1515/BC.2006.106. [DOI] [PubMed] [Google Scholar]
- 34.Dalken B, Giesubel U, Knauer SK, Wels WS. Targeted induction of apoptosis by chimeric granzyme B fusion proteins carrying antibody and growth factor domains for cell recognition. Cell Death Differ. 2006;13:576–585. doi: 10.1038/sj.cdd.4401773. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. Targeted delivery of human pro-apoptotic enzymes to tumor cells: In vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther. 2003;2:1341–1350. [PubMed] [Google Scholar]
- 36.Dressel R, et al. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J Biol Chem. 2004;279:20200–20210. doi: 10.1074/jbc.M313108200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.