Abstract
A-kinase anchoring proteins (AKAPs) influence the spatial and temporal regulation of cAMP signaling events. Anchoring of PKA in proximity to certain adenylyl cyclase (AC) isoforms is thought to enhance the phosphorylation dependent termination of cAMP synthesis. Using a combination of immunoprecipitation and enzymological approaches, we show that the plasma membrane targeted anchoring protein AKAP9/Yotiao displays unique specificity for interaction and the regulation of a variety of AC isoforms. Yotiao inhibits AC 2 and 3, but has no effect on AC 1 or 9, serving purely as a scaffold for these latter isoforms. Thus, Yotiao represents an inhibitor of AC2. The N terminus of AC2 (AC2-NT), which binds directly to amino acids 808–957 of Yotiao, mediates this interaction. Additionally, AC2-NT and Yotiao (808–957) are able to effectively inhibit the association of AC2 with Yotiao and, thus, reverse the inhibition of AC2 by Yotiao in membranes. Finally, disruption of Yotiao–AC interactions gives rise to a 40% increase in brain AC activity, indicating that this anchoring protein functions to directly regulate cAMP production in the brain.
Keywords: cyclic AMP, protein kinase A, AKAP9
AKinase Anchoring Proteins, or AKAPs, are defined by their ability to bind the regulatory subunit of protein kinase A (PKA) (ref. 1; reviewed in ref. 2). This family of proteins functions to target PKA and other cAMP effector proteins to specific regions of the cell allowing for increased signaling specificity. Several members of this diverse protein family bind other receptors, channels, or enzymes to form tightly regulated signaling modules, facilitating PKA interactions with its downstream effectors. In addition, these signaling modules often constitute feedback loops between kinases and phosphatases or recruit phosphodiesterases to terminate the cAMP signal (3).
Our labs previously identified a complex containing AKAP79/150 and type 5 adenylyl cyclase (AC), that facilitates the spatiotemporal organization of cAMP signaling (4). AKAP79 inhibits AC5 activity, while providing a mechanism for feedback inhibition via PKA phosphorylation of anchored AC5. However, with ≈30 gene families of AKAPs identified thus far, and nine mammalian isoforms of AC, it begs the question, does AKAP scaffolding of AC and PKA represent a general paradigm for cAMP signaling? The AKAP Yotiao represents an ideal test case to begin to address this question. Yotiao is a splice variant of the AKAP9 gene and is present on the plasma membrane (5, 6). In addition to PKA, Yotiao binds protein phosphatase 1 (PP1), NMDA receptors, the heart potassium channel subunit KCNQ1, and the IP3R1 (5, 7–9). Interestingly, AKAP anchored PKA phosphorylation of the NMDA receptor, KCNQ1, and IP3R1 is necessary for each effector's normal function (8–11). For example, disruption of the KCNQ1 association with Yotiao gives rise to long QT syndrome, a type of heart arrhythmia that can be fatal (12). The requirement for a tight coupling between PKA and KCNQ1, and other effectors suggests that cAMP production must also be tightly regulated, perhaps as even part of this complex.
We now show that Yotiao is associated with brain and heart adenylyl cyclases. Yotiao displays specificity among AC isoforms, interacting with AC 1, 2, 3, and 9 but not AC 4, 5, or 6. The interaction of Yotiao with AC2 is direct, involving the N terminus of AC2 and residues 808–957 of Yotiao. Finally, disruption of the Yotiao and AC2 complex results in a significant increase in brain AC activity; this is evidence of direct regulation of brain AC activity by an AKAP.
Results
Adenylyl Cyclase Activity Associates with Yotiao in Rat Brain and Heart.
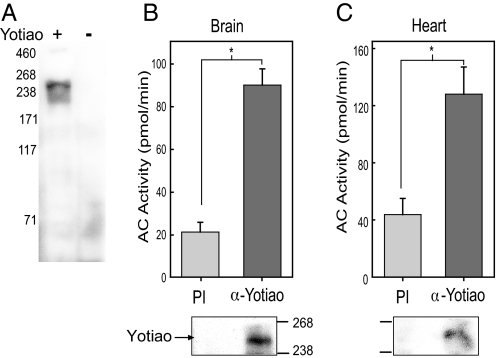
To probe endogenous Yotiao, a polyclonal antibody, which recognizes only a single protein in HEK293 cells expressing Yotiao, was generated against Yotiao (Fig. 1A). By using this antibody, Yotiao can be immunoprecipitated from rat brain or heart extracts. The associated AC activity present in these immunoprecipitants was measured upon stimulation with exogenously added Gαs-GTPγS and forskolin (referred to as an IP-AC assay). In both brain and heart, significantly more AC activity was associated with anti-Yotiao serum compared with preimmune serum (Fig. 1B and C). Western blot analysis confirmed expression of Yotiao in these tissues after immunoprecipitation, suggesting that AC exists in a complex with Yotiao in both brain and heart.
Fig. 1.
AC associates with Yotiao in brain and heart. (A) Western blot of HEK293 cell extracts expressing +/− Yotiao using Yotiao anti-serum. (B and C) Yotiao was immunoprecipitated from (B) rat brain or (C) rat heart extracts by using Yotiao anti-serum or preimmune serum. Immunoprecipitants were stimulated with 50 nM Gαs-GTPγS (Gαs) and 100 μM forskolin to measure associated AC activity. Significantly more AC activity associates with α-Yotiao in brain (P < 0.001) and heart (P < 0.01) compared with preimmune serum.
Yotiao Specifically Associates with AC 1, 2, 3, and 9.
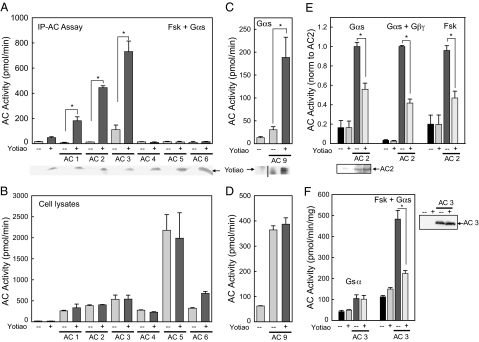
Various AC isoforms were screened for association with Yotiao in an over-expression system. HEK293 cells were transiently transfected with V5-tagged Yotiao, or individual AC isoforms ± V5-Yotiao. Yotiao was immunoprecipitated from cell lysates by using anti-V5 agarose gel (Fig. 2A), followed by measurement of associated AC activity upon stimulation with Gαs and forskolin. AC9 is insensitive to forskolin, so a higher concentration of Gαs was used in these samples (Fig. 2C). AC activity in the starting cell lysates confirmed that each AC isoform was expressed over background AC activity (Fig. 2B and D). As shown in Figs. 2A and C, AC 1, 2, 3, and 9 specifically associate with Yotiao, whereas types 4, 5, and 6 do not.
Fig. 2.
AC types 1, 2, 3, and 9 associate with Yotiao. (A) Immunoprecipitations of V5-tagged Yotiao from transfected HEK293 cells were assayed for AC activity. Samples transfected with Yotiao plus AC 1 (P < 0.05), 2 (P < 0.01), or 3 (P < 0.05) had significant AC activity compared with the AC isoform alone. Yotiao expression was confirmed by Western blot analysis. (B) Cell lysates (5% of starting material used in A) were assayed for AC activity to confirm expression of each AC isoform over vector alone. (C) Cells transfected with AC 9 and Yotiao were immunoprecipitated with α-Yotiao serum and then stimulated for associated AC activity with 400 nM Gαs (P < 0.05). (D) AC activity in transfected cell lysates used in C. (E) Membranes from HEK293 cells transfected with vector, or AC2 +/− Yotiao, were stimulated with either 50 nM Gαs (330 pmol/min/mg; P < 0.001), 50 nM Gαs and 30 nM Gβγ (1,606 pmol/min/mg; P < 0.001), or 100 μM forskolin (131 pmol/min/mg; P < 0.001). Western blot analysis of AC2 expression levels is shown below. (F) Membranes expressing AC3 were inhibited by Yotiao when stimulated with 50 nM Gαs and 100 μM forskolin (P < 0.01) but not Gαs alone. Western blot analysis confirmed that AC3 expression was not altered by Yotiao.
Yotiao Inhibits AC 2 and 3 in Crude Membranes.
To determine the effect of Yotiao on AC activity, HEK293 cells were transfected with AC 1, 2, 3, or 9 plus Yotiao, and membranes were isolated to measure AC activity. Yotiao had no effect on the basal activity of any isoform (data not shown). Membranes containing AC2 were inhibited in the presence of Yotiao when stimulated with activated Gαs (45% inhibition), Gαs and Gβγ (60%), forskolin alone (55%), or Gαs and forskolin (30%) (Fig. 2E, and data not shown). Yotiao also inhibited AC2 activity in whole cell assays in a dose-dependent manner (data not shown). AC3 activity was inhibited by ∼40% when stimulated with Gαs and forskolin but not Gαs alone (Fig. 2F). Western blot analysis for AC 2 or 3 plus Yotiao confirmed that the anchoring protein did not alter the expression of AC. In contrast, AC 1 and 9 activities were unaffected by Yotiao expression upon stimulation with calmodulin, Gαs and calmodulin (AC1), or Gαs alone (AC9) [supporting information (SI) Fig. S1].
Yotiao Binds the N Terminus of AC2.
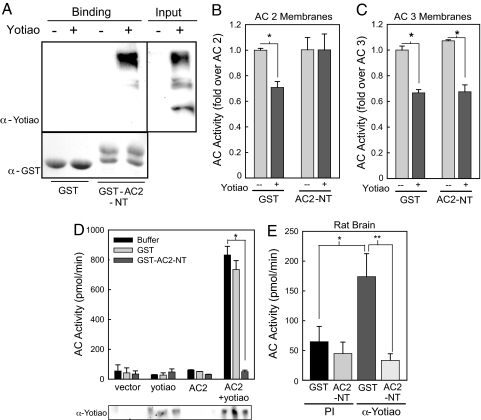
AC has three soluble domains that could possibly interact with Yotiao: the N terminus, C1, and C2 domain (C1 and C2 form the catalytic core). The C1 and C2 domains have relatively high homology between isoforms, whereas the N-termini are unique. Based on the isoform selectivity, the N terminus of AC2 fused to GST (GST–AC2-NT) was tested for binding. HEK293 cell lysate expressing vector or Yotiao was incubated with GST or GST–AC2-NT. Western blot analysis of GST-pull down assays revealed that Yotiao binds GST–AC2-NT but not GST alone (Fig. 3A).
Fig. 3.
AC2 N terminus (AC2-NT) binds Yotiao and reverses Yotiao inhibition of AC2. (A) Extracts from cells expressing vector or Yotiao were incubated with GST or GST–AC2-NT in a GST pull-down assay. Western blot analysis indicates an interaction of Yotiao with GST–AC2-NT (Upper). GST protein input is shown (Lower). (B and C) Membranes from transfected HEK293 cells were incubated with either GST or GST–AC2-NT (5 μM) for 15 min on ice before stimulation with 50 nM Gαs and 30 nM Gβγ for AC2 (B) or 50 nM Gαs and 100 μM forskolin for AC3 (C). Addition of AC2-NT reverses inhibition of AC2 activity by Yotiao (P < 0.001), but not that of AC3 (P < 0.001). (D) Transfected HEK293 cell lysates were incubated with GST or GST–AC2-NT (5 μM) before immunoprecipitation with α-Yotiao serum. Immunoprecipitants were assayed for associated AC activity with Gαs and Gβγ (P < 0.01). (E) Rat brain extracts were incubated with GST or GST–AC2-NT before immunoprecipitation and assay of AC activity with Gαs and forskolin (*, GST, P < 0.05; **, AC2-NT, P < 0.01). GST–AC2-NT blocked AC association with Yotiao.
AC2-NT Acts as a Competitive Inhibitor of Yotiao–AC2 Interactions.
The N terminus of AC2 was next tested for its ability to reverse the inhibition of AC2 by Yotiao. Purified GST or GST–AC2-NT was incubated with membranes containing AC2 or AC3 plus Yotiao. GST–AC2-NT reversed the inhibition of AC2 by Yotiao but not that of AC3 (Fig. 3B and C), indicating that AC 2 and 3 have unique binding sites on Yotiao. Thus, the remainder of the study will focus on the interaction of AC2 with Yotiao.
AC2-NT Completely Blocks AC2 Association with Yotiao in Cells and Rat Brain.
To confirm AC2-NT as the binding site, GST–AC2-NT was used as a competitive inhibitor in IP-AC assays. Yotiao was immunoprecipitated from transfected HEK293 cells in the presence of buffer, GST, or GST–AC2-NT, and then the immunoprecipitants were assayed for AC activity. GST–AC2-NT fully competed away the associated AC2 from Yotiao, whereas GST-alone had no effect (Fig. 3D). Western blot analysis confirmed the expression of Yotiao (Fig. 3D). This experiment was repeated by using rat brain extract with similar results; GST–AC2-NT blocked associated AC activity with Yotiao in brain (Fig. 3E).
AC2 Binds Yotiao at Amino Acids 808–957.
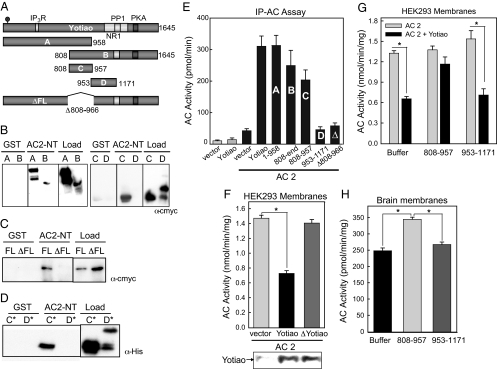
GST-pull down assays were used to map the AC2 binding site on Yotiao by using HEK293 cells expressing various truncations of Yotiao, as depicted in Fig. 4A. Amino Acids 1–958, 808–end, and 808–957 all bound GST–AC2-NT, whereas 953–1171 did not (Fig. 4B). When amino acids 808–966 were deleted from full length Yotiao (ΔFL), binding to AC2-NT was abolished (Fig. 4C), confirming that 808–957 represents the only binding site for AC2. To determine whether the binding of Yotiao and AC2 is direct, purified fragments of Yotiao were incubated with GST or GST–AC2-NT. The Yotiao fragment 808–957 bound directly to GST–AC2-NT, whereas no binding was observed with amino acid 953–1171 (Fig. 4D). This region of Yotiao is responsible also for the interactions observed with full-length AC2, as measured by an IP-AC assay with the cMYC-tagged constructs depicted in Fig. 4A. In accordance with the binding assay results, amino acid 808–957 are responsible for the interaction with AC2, as any portions of Yotiao excluding this domain did not pull down AC2 activity (Fig. 4E). Finally, although Yotiao inhibited the activity of AC2 by ≈50%, ΔFL Yotiao had no effect (Fig. 4F).
Fig. 4.
Amino acids 808–957 of Yotiao are required for AC2 binding and inhibition, and compete for AC2–Yotiao interactions. (A) Diagram of Yotiao fragments, each containing cMYC tags. (B and C) Yotiao fragments were expressed in HEK293 cells and subjected to GST pull-down assay. Deletion of 808–966 of Yotiao (ΔFL) results in loss of Yotiao binding to GST–AC2-NT. (D) GST–AC2-NT binds specifically and directly to E. coli purified 808–957 (C*) but not to 953–1171 (D*). (E) IP-AC assay of AC2 association with Yotiao and fragments of Yotiao. Yotiao and corresponding fragments, shown in A, were expressed in HEK293 cells with AC2 and immunoprecipitated with mouse α-cMYC before stimulation with 50 nM Gαs and 30 nM Gβγ (P < 0.001, for Yotiao A, B, and C fragments). (F) Membranes from transfected HEK293 cells were stimulated with Gαs and Gβγ. YotiaoΔ808–966 (ΔFL) had no effect on AC2 activity, and full length Yotiao inhibited AC2 by ∼50% (P < 0.001). (G) Membranes from HEK293 cells transfected with AC2 +/− Yotiao were incubated with 5 μM purified Yotiao 808–957 or 953–1171 before stimulation with Gαs and Gβγ. Yotiao 808–957 reversed the inhibition of AC2 by Yotiao, whereas 953–1171 had no effect (P < 0.001). (H) Rat brain membranes were incubated with buffer and purified Yotiao 808–957 or 953–1171 (5 μM) before stimulation with 50 nM Gαs and 30 nM Gβγ. Membranes incubated with purified Yotiao 808–957 had 38 ± 2.9% (P < 0.001) more AC activity than membranes incubated with buffer or 953–1171.
Purified protein containing amino acid 808–957 of Yotiao also can reverse the inhibitory effect of Yotiao. Membranes prepared from cells expressing AC2 and Yotiao were incubated with 808–957 or a control fragment (Yotiao 953–1171) before stimulation with Gαs and Gβγ. The AC-binding fragment of Yotiao (808–957) reversed Yotiao inhibition of AC2 (Fig. 4G).
Yotiao 808–957 Stimulates AC Activity in Neonatal Rat Brain Membranes.
The final phase of this study was to determine whether Yotiao-AC interactions are physiologically relevant. Rat brain membranes were incubated with the purified AC disruptor fragment 808–957 or a control (953–1171) to test their effects on the endogenous proteins. The addition of 808–957 increased AC activity (over brain membranes alone or those with 953–1171) by an average of 38% ± 2.9 (Fig. 4H), indicating that endogenous Yotiao suppresses AC2 activity in rat brain.
Discussion
This study identified a subset of AC isoforms that can interact directly with the AKAP Yotiao. This association has important implications for cAMP signaling. It is increasingly appreciated that cAMP is restricted in its diffusion throughout the cell, leading to the notion of temporal and spatial regulation of cAMP signaling (13–15). Contributing to spatial regulation are AKAP scaffolding proteins, which anchor PKA in addition to a diverse set of regulatory proteins and PKA substrates. When an AKAP tethers both PKA and its substrate, the rate of substrate phosphorylation by PKA is enhanced (16). Thus, the spatial regulation of both cAMP synthesis and cAMP effector proteins such as PKA, EPACs, and cyclic nucleotide-gated ion channels provides a means to generate specificity for this commonly used soluble second messenger. Yet, it was still not clear how a restricted pool of cAMP generated by AC was specifically targeted to anchored PKA and its select subset of effectors. This problem is solved by tethering AC to the same complex.
Yotiao Selectivity for AC Isoforms.
Yotiao resides at the plasma membrane and displays major expression in brain and heart (5, 6). We show that in both brain and heart, significant AC activity is associated with Yotiao. Yet, the AC isoform found in these complexes was unknown. A screen of seven of the nine membrane-bound ACs identified a unique subset of AC isoforms capable of binding to Yotiao. AC isoforms 1, 2, 3, and 9 associate with Yotiao, whereas AC 4, 5, and 6 do not. There are few common threads among these ACs, with each displaying very different regulatory patterns. AC1 is stimulated synergistically by Gαs and Ca2+/calmodulin, and inhibited by calmodulin kinase IV, Gi, Go, and Gz (17). AC2 is activated by PKC and Gαs, with conditional stimulation by Gβγ (17). AC3 is typically considered the olfactory cyclase with stimulation by Gαs and inhibition by calmodulin kinase II and RGS2 (18). AC9 is the most unique, lacking any stimulation by forskolin, but displaying activation by Gαs and inhibition by protein phosphatase 2B (19). Even the localization within the plasma membrane differs, with AC 1 and 3 residing in lipid rafts, whereas AC 2 and 9 are largely excluded from these regions (20).
Because of the diversity of ACs identified, it is not surprising that we report different mechanisms in place to regulate the activity of AC-AKAP complexes. AC 2 and 3 are clearly inhibited by Yotiao, identifying this anchoring protein as a protein inhibitor of AC2. Inhibition of AC3 only occurs upon stimulation with Gαs and forskolin, but not Gαs alone. In contrast, AC 1 and 9 activity is unaffected by interaction with the anchoring protein, at least under the stimulatory conditions tested. Yotiao, therefore, appears to merely serve as a scaffold for AC 1, 3, and 9 under select stimulatory states to bring PKA in proximity to the cAMP synthesis machinery. However, the possibility exists that regulation by Yotiao may occur in activation states not yet screened. In contrast, Yotiao seems to play a clear regulatory role for AC2.
The N terminus of AC appeared to be the most logical binding site for Yotiao. This region is highly variable and could serve to differentiate ACs. Not only did the N terminus of AC2 (AC2-NT) bind to Yotiao, but it also served as a competitive inhibitor for Yotiao binding and inhibition of AC2. However, AC2-NT could not reverse the inhibition of AC3 by Yotiao, suggesting that different AC isoforms may have unique binding sites on Yotiao.
Mapping studies identified a region from 808–957 of Yotiao that bound directly to AC2-NT. No other proteins map to this region of Yotiao, representing a unique binding site for AC2. This fragment of Yotiao serves as a competitive inhibitor of Yotiao-AC2 interactions, reversing AC2 binding and inhibition, but not binding of AC1 or AC9 (data not shown). To explore the physiological meaning of the interaction between Yotiao and AC2, we examined the regulation of AC2 activity by Yotiao in brain. Using the Yotiao 808–957 fragment to disrupt Yotiao–AC2 interactions, we show a 38% increase in adenylyl cyclase activity in brain membranes when stimulated with Gαs plus Gβγ. Thus, there appears to be a tonic inhibition of AC2 by Yotiao at endogenous protein levels in brain. Whether this interaction is a dynamic and regulated process is still unclear.
Role of AC Isoforms in Yotiao Complexes.
Yotiao anchors a number of regulatory proteins and effectors including PP1, NMDA receptors, IP3 receptors, and heart potassium channel subunit KCNQ1 (5, 7, 9, 12). In brain, PKA phosphorylation of NMDA receptors leads to increased channel activity. All of the AC isoforms associated with Yotiao are expressed in various brain regions and, thus, are possible candidates for roles in Yotiao-localized NMDA signaling in brain. The regulation of AC 1 and 9 by calcium-sensitive pathways could provide nicely regulated feedback loops in the Yotiao–NMDA receptor complex. In contrast, AC2 is relatively calcium-insensitive. Addition of AC2-NT fully blocked the association of AC activity with Yotiao in brain. This suggests that much of Yotiao in brain is associated with AC2. However, we cannot rule out the possibility that AC2-NT can block interactions with AC 1 or 9 as well, although under the stimulatory conditions used, we would not expect to measure much AC9 activity. Additional studies will be required to determine the relative contributions of AC isoforms in complex with Yotiao.
In heart, the role for Yotiao regulation is quite clear. Mutations that decrease the affinity of KCNQ1 for Yotiao result in decreased PKA phosphorylation and activity of the I(Ks) channel, giving rise to long QT syndrome (8). Normal I(Ks) channel activity is stimulated by sympathetic activity in cardiac myocytes (12). In the heart, AC 2 and 3 are expressed in cardiac fibroblasts, whereas AC1 has been reported only in the sino-atrial node (21, 22). AC3 is present mainly in the developing heart with decreased expression in adulthood (23). Of the Yotiao-interacting AC isoforms identified, only AC9 and AC2 have expression in cardiac myocytes (24, 34). The presence of ACs in this complex could play an important role in the regulation of the cardiac action potential. It is possible that AC2 and Yotiao interact in heart fibroblasts, but the physiological or pathophysiological function of that interaction would be hard to define at this time, particularly in terms of I(Ks).
In addition to a role in signal transduction, several AKAPs facilitate the trafficking of their anchored cargo, including that of membrane bound proteins such as aquaporin-2, the NMDA receptor, and β-adrenergic receptors (25–29). AC2 is targeted to the plasma membrane as part of a preassembled signaling complex that includes the β2-adrenergic receptor (30). AKAPs such as Yotiao may not only provide the spatial regulation for PKA, but may also facilitate AC trafficking.
Summary.
It is clear that at least two different AKAPs can interact with a unique set of AC isoforms. With nine mammalian AC isoforms and >30 subfamilies of AKAPs, the possible combinations of AC–AKAP interactions are staggering. In addition, the regulatory patterns for individual AC isoforms are quite complex, and it is unclear whether scaffolding to AKAPs may change these patterns in vivo, facilitating some modes of regulation while inhibiting others. The real challenge will be to determine what combination of ACs, PKA substrates, and AKAPs are important in varying pathways and tissues, and perhaps more importantly, the physiological consequence of these unique signaling modules.
Materials and Methods
Plasmids.
Yotiao pEGFP, Yotiao 808–957 pet30a, and Yotiao 953–1171 pet30a were previously described (7). cMYC-Yotiao and Yotiao-V5His were constructed by transferring the Yotiao coding region from Yotiao pEGFP into pCMV5myc-1 or pCDNA3.1/V5-His-TOPO, respectively. C-MYC–tagged fragments of Yotiao were constructed by PCR and inserted into the pCMV5myc-1 vector. All constructs were confirmed by DNA sequencing. GST–AC2-NT was a generous gift from Dr. Nathan Dascal.
Transfections.
HEK293 cells were seeded at 5 × 106 cells per 10-cm dish 1 day before transfection with Lipofectomine 2,000 and analyzed 48 h later. Plasmid DNA (10 μg of total) used to transfect 10-cm dishes included: Yotiao (5 μg), AC5 (2 μg), all other ACs (5 μg), and pCDNA3.
Protein Purification.
Gαs-H6 was expressed in E. coli and activated with [35S]GTPγS as previously described (31). β1γ2-H6 was purified from Sf9 cells (32). Hexa-histidine- and GST-tagged proteins were purified on Ni-NTA agarose (Qiagen) and glutathione resin (Amersham) in buffers lacking detergents as previously described (31). Proteins were dialyzed into buffer containing 50 mM Hepes pH 8.0, 100 mM NaCl, 5% glycerol, 2 mM DTT, and 1 mM EDTA, and stored at −80°C.
Antibodies.
Rabbit α-Yotiao antibody was generated against a purified H6-tagged portion of Yotiao (amino acid 808–957) by Sigma Genosys. Additional antibodies included V5-agarose (Sigma), mouse α-cMYC (Santa Cruz), and mouse α-GST (Invitrogen).
Preparation of Membranes.
After transfections, HEK293 cells were rinsed with PBS and resuspended in 20 mM Hepes, 1 mM EDTA, 2 mM MgCl2, 1 mM DTT, 250 mM sucrose, and protease inhibitors (HMED plus sucrose). The cells were allowed to swell for 10 min on ice, Dounce homogenized, and centrifuged at 1,800 × g to pellet the nuclei. Membranes were subjected to centrifugation for 20 min at 60,000 × g, resuspended in HMED and sucrose, and analyzed for protein concentration. Membranes (30 μg per reaction) were immediately assayed for adenylyl cyclase activity (33).
Preparation of Tissue Extracts.
Rat brains from 1- to 2-day old Sprague–Dawley rats were rinsed, quartered, and Dounce homogenized in buffer containing 250 mM sucrose, 20 mM Hepes pH 8.0, 1 mM EDTA, 2 mM MgCl2, 1 mM DTT, and protease inhibitors. The homogenate was centrifuged at 2,000 × g to remove nuclei, followed by 60,000 × g to collect membranes. Membranes were stored at −80°C. For extracts, membranes were diluted to 10 mg/ml with lysis buffer (50 mM Hepes pH 7.4, 1 mM EDTA, 1 mM MgCl2, 150 mM NaCl, 0.5% C12E9, and protease inhibitors), homogenized, and centrifuged to remove the insoluble fraction. The remaining supernatant was analyzed for protein content. Rat heart extracts were processed similarly, except that tissue was homogenized first with a polytron in buffer lacking detergent, followed by Dounce homogenization with 1% Triton X-100. The heart extracts were used immediately for experiments.
Immunoprecipitation-Adenylyl Cyclase Assay.
Assays were performed essentially as described (4). Detailed methods can be found in SI Materials and Methods.
GST-Pull Down Assays.
Lysates from transfected HEK293 cells (500 μg) or purified proteins (1 μM) were incubated with GST (50 μg) or GST–AC2-NT (50 μg) for 30 min at 4°C. Glutathione agarose (30 μl of packed resin) was added to each sample for 2 h. Samples were washed three times with wash buffer and analyzed by Western blotting.
Statistical Analysis.
Data were analyzed by using a single-factor ANOVA from an average of at least three experiments, each performed in duplicate or triplicate. Experiments were considered significant for P values <0.05.
Supplementary Material
Acknowledgments.
The authors thank Dr. Nathan Dascal (Tel Aviv University, Israel) for providing the GST–AC2-NT clone, Dr. Rachna Sadana (University of Texas Health Science Center, Houston) for helpful discussions and for providing purified G proteins, and Kathryn Hassell for excellent technical assistance. This work was supported by the National Institutes of Health Grants GM60419 to C.W.D. and GM48231 to J.D.S., and the Leducq Foundation Grant 06CVD02 (J.D.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712100105/DCSupplemental.
References
- 1.Hirsch AH, Glantz SB, Li Y, You Y, Rubin CS. Cloning and expression of an intron-less gene for AKAP 75, an anchor protein for the regulatory subunit of cAMP-dependent protein kinase II beta. J Biol Chem. 1992;267:2131–2134. [PubMed] [Google Scholar]
- 2.Wong W, Scott JD. AKAP signaling complexes: Focal points in space and time. Nature Reviews Mol Cell Biology. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 3.Dodge-Kafka KL, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman AL, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt PH, et al. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J Biol Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- 7.Westphal RS, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 8.Marx SO, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 9.Tu H, Tang TS, Wang Z, Bezprozvanny I. Association of type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J Biol Chem. 2004;279:19375–19382. doi: 10.1074/jbc.M313476200. [DOI] [PubMed] [Google Scholar]
- 10.Dudman JT, et al. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner LE, Li WH, Yule DI. Phosphorylation of type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases: a mutational analysis of the functionally important sites in the S2+ and S2− splice variants. J Biol Chem. 2003;278:45811–45817. doi: 10.1074/jbc.M306270200. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Kass RS. Dual roles of the A kinase-anchoring protein Yotiao in the modulation of a cardiac potassium channel: A passive adaptor versus an active regulator. Eur J Cell Biol. 2006;85:623–626. doi: 10.1016/j.ejcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DMF, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci USA. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linder JU. Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci. 2006;63:1736–1751. doi: 10.1007/s00018-006-6072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinnarajah S, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409:1051–1055. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- 19.Antoni FA, Smith SM, Simpson J, Rosie R, Fink G, Paterson JM. Calcium control of adenylyl cyclase: the calcineurin connection. Adv Second Messenger Phosphoprotein Res. 1998;32:153–172. doi: 10.1016/s1040-7952(98)80010-4. [DOI] [PubMed] [Google Scholar]
- 20.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrom RS, et al. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem. 2003;278:24461–24468. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- 22.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582:1195–1203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrand N, Pessah M, Frayon S, Marais J, Garel JM. Olfactory receptors, Golf alpha and adenylyl cyclase mRNA expressions in the rat heart during ontogenic development. J Mol Cell Cardiol. 1999;31:1137–1142. doi: 10.1006/jmcc.1999.0945. [DOI] [PubMed] [Google Scholar]
- 24.Cui H, Green RD. Cell-specific properties of type V and type IX adenylyl cyclase isozymes in 293T cells and embryonic chick ventricular myocytes. Biochem Biophys Res Commun. 2001;283:107–112. doi: 10.1006/bbrc.2001.4725. [DOI] [PubMed] [Google Scholar]
- 25.Jo IH, et al. AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex. Am J Physiol-Renal Physiol. 2001;281:F958–F965. doi: 10.1152/ajprenal.2001.281.5.F958. [DOI] [PubMed] [Google Scholar]
- 26.Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A kinase-anchoring proteins (AKAPs) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao JC, Wang HY, Malbon CC. Src docks to A-kinase anchoring protein gravin, regulating beta 2-adrenergic receptor resensitization and recycling. J Biol Chem. 2007;282:6597–6608. doi: 10.1074/jbc.M608927200. [DOI] [PubMed] [Google Scholar]
- 28.Fraser ID, et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 29.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta(1)-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281:33537–33553. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- 30.Dupre DJ, Baragli A, Rebois RV, Ethier N, Hebert TE. Signaling complexes associated with adenylyl cyclase II are assembled during their biosynthesis. Cell Signal. 2007;19:481–489. doi: 10.1016/j.cellsig.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Salim S, Sinnarajah S, Kehrl JH, Dessauer CW. Identification of RGS2 and type V adenylyl cyclase interaction sites. J Biol Chem. 2003;278:15842–15849. doi: 10.1074/jbc.M210663200. [DOI] [PubMed] [Google Scholar]
- 32.Kozasa T, Gilman AG. Purification of recombinant G proteins from Sf9 cells by hexa-histidine tagging of associated subunits. Characterization of α12 and inhibition of adenylyl cyclase by αz. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 33.Dessauer CW. Kinetic analysis of the action of P-site analogs. Methods Enzymol. 2002;345:112–126. doi: 10.1016/s0076-6879(02)45011-2. [DOI] [PubMed] [Google Scholar]
- 34.Ping P, Anzai T, Gao M, Hammond HK. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.