Abstract
Mating triggers physiological and behavioral changes in females. To understand how females effect these changes, we used microarray, proteomic, and comparative analyses to characterize gene expression in oviducts of mated and unmated Drosophila females. The transition from non-egg laying to egg laying elicits a distinct molecular profile in the oviduct. Immune-related transcripts and proteins involved in muscle and polarized epithelial function increase, whereas cell growth and differentiation-related genes are down-regulated. Our combined results indicate that mating triggers molecular and biochemical changes that mediate progression from a “poised” state to a mature, functional stage.
Keywords: antimicrobial peptides, network, reproduction
Successful fertilization is the culmination of concerted interactions between oocyte and sperm. For many animals, the microenvironment of the female reproductive tract (RT) plays an important role in mediating the interaction between gametes (1). The oviducts secrete a variety of molecules that generate the correct osmolarity for supporting the production, maintenance, and modification of gametes and that protect the oviduct and the gametes/fetus from microbial infection and other stressors (2). In mammals, the oviducts secrete glycoproteins, thought to enhance sperm binding to the zona pellucida of oocytes and decrease polyspermy; protease inhibitors, which regulate proteolytic activity to protect the integrity of the zona pellucida, blastomeres, and oviductal tissues; and growth factors, which may enhance embryonic development (2).
The microenvironment of the female RT may also influence fertility in insects. Female insects store sperm in specialized organs called the spermatheca and seminal receptacle, which allows the female to fertilize eggs for days after mating. In Drosophila melanogaster, the spermathecal ducts secrete glucose dehydrogenase, which influences sperm motility as well as sperm storage and release (3). Secretory glands in the spermatheca produce lipoproteins, phospholipids, carbohydrates, and proteins that may help maintain sperm viability and maximal fertilization potential.
In Drosophila, mating may induce molecular and biochemical changes in the female RT that allow it to support a high rate of ovulation, fertilization, and oviposition (4). Shortly after mating, females begin ovulating (5). Mature oocytes become activated in the oviduct (5) in transit from the ovary to the uterus (Fig. 1A), where sperm released from the sperm storage organs enter the egg through an aperture in the eggshell called the micropyle (6). Mating induces specific physiological changes in the oviduct. In nonlaying, unmated females, a hydrated matrix is detected between the intima and the microvillar surface of the oviduct; after egg laying begins, the intima lies close to the oviduct epithelium, suggesting changes in epithelial cell activity (7). This might affect the osmolarity of the extracellular fluid in the oviduct, which is necessary to support high ovulation and egg laying rates. Major changes also occur in the peptidergic nerve termini innervating different parts of the RT, including distinct domains within the lateral and common oviducts (4). These observations suggest that the oviducts are not “passive” conduits, and that each domain in the female RT (ovary, sperm storage organs, female accessory glands, and uterus) may be regulated locally and possibly in synchrony with other domains.
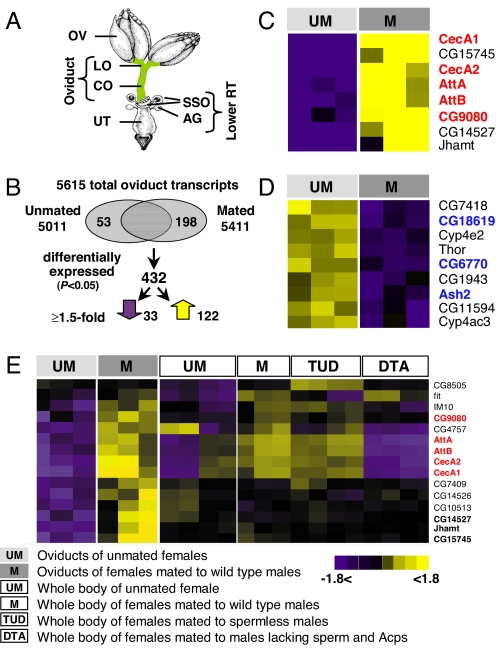
Fig. 1.
Mating induces changes in oviduct transcript expression level. (A) Schematic representation of Drosophila female RT. The oviduct is composed of the lateral oviducts (LO) and common oviduct (CO). Other organs: ovary (OV), accessory gland (AG), sperm storage organs (SSO), and uterus (UT). (B) Identification of mating-responsive transcripts. Venn diagram of transcripts from unmated or mated oviducts at 3 h after mating: Of the 5,615 total transcripts present in either sample, 53 were present only in unmated oviduct, and 198 were present only in mated oviduct. A total of 432 transcripts were significantly differentially expressed (P < 0.05); of these, 33 were down-regulated and 122 were up-regulated by ≥1.5-fold. (C–E) Clusters of genes differentially expressed (fold change ≥1.5; P < 0.05) in oviducts of unmated and mated females at 3 h after mating. Expression ratios range from −1.8 (purple) to +1.8 (yellow). (C) Genes up-regulated in mated female; most immune-related genes (red) were higher in mated oviduct. (D) Genes down-regulated in mated female; transcription factors are highlighted (blue). (E) Genes differentially expressed in oviduct and whole-body assay (8); genes from cluster shown in C are highlighted in bold labels, with immune-related genes in red labels. Schematic in A adapted from FlyBase image FBim6932540. See also Fig. S1.
Heifetz and Wolfner (4) hypothesized that, before mating, the female RT of Drosophila does not possess maximal biosynthetic capacity and secretory activity but is “poised” and waiting for a signal provided by mating to continue development. Molecular profiling of female whole-body and lower RT (soma that store sperm) suggests that mating does induce physiological changes (8, 9). To further understand how the female reproductive system achieves maximal functionality to support a high fertility rate, we examined the effect of mating on the oviduct (soma in which eggs are activated). We hypothesize that mating directly or indirectly induces transcriptional and translational changes, transforming the oviduct from a resting state to a physiological state that can sustain a high rate of ovulation of properly activated fertilizable eggs. Here, we provide molecular evidence for such a developmental switch within the oviduct.
Results
Mating Induces Up-Regulation of Immune-Related Transcripts in the Female Oviduct.
Mating in Drosophila triggers profound changes in female behavior and physiology (5). Females begin to oviposit 3 h after mating, suggesting that the female oviduct undergoes significant physiological changes during this period to prepare for supporting massive egg activation and movement. To identify genes whose expression changes after mating, we analyzed RNA extracted from the oviduct (Fig. 1A) of unmated and mated females at 3 h after mating. The expression profile of unmated oviducts revealed 5,011 transcripts as “present” [Fig. 1B; see supporting information (SI) Methods]. This set is enriched for genes involved in structural constituents of the ribosome, nucleotide binding, protein binding, transporter activity, translation regulator activity, and actin binding (for P values see Table S1).
We next examined differences between unmated and mated female oviducts. From the mated group, 5,411 transcripts were present. Of all transcripts in either group (5,615 in total), 0.95% (53/5,615) were detected only in the oviduct of unmated females (Figs. 1B and S1A). These transcripts showed overrepresentation of genes involved in serine-type, endopeptidase activity (Table S1), whose down-regulation may eliminate enzymatic activity that could interfere with oviduct maturation or seminal fluid activity after mating. We also detected 3.5% of the transcripts (198/5,615) only in the mated oviduct (Fig. 1B). These transcripts were enriched for genes involved in helicase activity, nucleic acid binding, and ATP-dependent helicase activity (Table S1). A total of 432 transcripts showed significantly different expression levels in unmated vs. mated oviduct (P < 0.05), of which 122 (28%) increased and 33 (7.6%) decreased by 1.5-fold or more after mating (Figs. 1B and S1A and Table S2). Thus, mating promotes the appearance of many new transcripts as the oviduct shifts to sustained egg laying, and differentially expressed transcripts tend to be up-regulated. Because 3 h after mating is the beginning of the highly active oviduct phase (5), these changes are likely to be essential to achieve a highly functional oviduct.
To compare expression profiles of these 432 transcripts, we clustered genes based on similarities in expression (Fig. S1 B and C). The cluster exhibiting the largest increase after mating (Fig. 1C) was enriched for immune response genes (red labels in Fig. 1C; Table S1). The cationic peptides cecropin A1 and A2 (cecA1; 12-fold) and glycine-rich peptides attacin A and B (att A; 4-fold) are active in the humoral immune response and belong to a family of peptides primarily targeting Gram-negative bacteria (10). CG9080 encodes a small peptide that is strongly induced in response to immune challenge mediated by relish and spaetzle (11). Also in this cluster is juvenile hormone acid methyltransferase (Jhamt), a juvenile hormone (JH) biosynthesis enzyme (12), whereas several genes involved in JH catabolism were down-regulated, suggesting that mating induces changes in hormonal activity in the oviduct.
A second distinct cluster consisted of the most strongly down-regulated transcripts in mated females (Fig. 1D), including three encoding putative transcription factors (Fig. 1D, blue). CG18619 has a basic-leucine, zipper domain and high conservation of human cAMP-responsive, element-binding, protein-like 2 (13). CG6770 is homologous to mammalian P8 transcriptional regulator, which is implicated in pancreatic growth and regeneration (14). Absent, small, or homeotic discs 2 (Ash2), a member of the trithorax group, is a positive regulator of homeotic genes whose targets include cell cycle, cell proliferation, and cell adhesion genes (15). This cluster also includes thor, a homolog of mammalian PhasI/4E-BP. Thor plays a role in immune response and regulation of cell size and can act as a translational inhibitor by binding to eIF-4E and other factors (16). Down-regulation of genes in this cluster might be required to control cell growth and to complete differentiation of the oviduct tissues after mating, thus allowing efficient egg activation and fertilization. Two cytochrome P450 genes (cyp4e2 and cyp4ac3) are also down-regulated. CYPs catalyze enzymatic reactions in detoxification and/or biosynthetic pathways, and some CYPs are involved in JH biosynthesis in other insects (17); it is possible, therefore, that these changes reflect hormonal changes in the oviduct related to egg laying.
Previous female whole-body assays have shown that 160 mating-responsive genes were regulated by Acps, 548 by sperm, and 1,072 by other mating components (8). To determine which mating components regulate gene expression changes in the oviduct, we compared the whole-body data (8) with our oviduct data (Table S2). We found 66 mating-responsive genes that were differentially expressed in both whole-body (4%; 66/1,780) and oviduct (15%; 66/432) assays. Of these 66 genes, 11 were mediated by Acps, 28 by sperm, and 27 by other mating components. This suggests that only a small portion of the whole-body mating response reflects the response in the oviduct, and that changes in transcript level in the oviduct are mainly mediated by sperm and by non-Acp/nonsperm factors as seen in whole-body (8) and lower RT assays (9). Several mating-responsive genes showed more extreme changes in the oviduct than in whole-body assays (e.g., cecA1; Fig. 1E, red labels; Fig. S1D) or exhibited a change only in the oviduct (e.g., CG14527; Fig. 1E, bold labels). This suggests either that the signal in whole-body assays represents a dilution of the oviduct signal with RNA from other body parts, as shown in ref. 18, or, more intriguingly, a mating-induced shift in the focus of the immune response from whole-body to the RT, as has also been suggested in ref. 19.
Mating Induces Increase of Cytoskeletal-Associated Proteins.
We used multidimensional protein identification technology (MudPIT) and peptide counting as a semiquantitative measure (see SI Methods) to characterize the oviduct proteome and to determine how the proteome changes in oviducts at 3 h after mating relative to unmated oviducts. We identified a total of 187 proteins, 89 of which were reproducibly detected in the oviduct of both unmated and mated females (SI Methods). Eighteen percent (16/89) of these proteins increased (≥ 2-fold) and 1% (1/89) decreased (≤ 0.5-fold) in abundance in the oviduct after mating (SI Methods; Table S3 A and B). Gene Ontology (GO) terms related to cytoskeleton, developmental process, and muscle contraction were significantly overrepresented in the set of 89 expressed proteins. Structural/cytoskeletal functions were specifically enriched in the set of 16 increased proteins (Table S1); the majority of these could be classified as either muscle-specific or involved in epithelial cell morphogenesis, which may indicate regulatory changes affecting epithelial and muscle activity and/or structural properties that would lead to functional changes in the oviduct environment after mating. Western blot analysis of a subset of these oviduct mating-responsive proteins [e.g., muscle LIM protein at 84B (Mlp84B), adducin-like protein (HTS-R1), neuroglian (Nrg), or coracle (Cora)] confirmed that their abundance increased after mating (Table S3C).
The oviduct muscles are visceral muscles that are supercontractile, meaning they have Z-bands with perforations that enlarge during contraction (20). With mating, muscle proteins that increased in abundance in the oviduct include α-actinin (Actn), Bent (Bt), Paramyosin (Prm), and Mlp84B. Actn is specific to the perforated Z-band of supercontractile muscle, where it cross-links actin filaments from adjacent sarcomeres (21). Bt localizes to A-bands in all muscles, interacts with myosin heavy chain, and contributes to muscle stiffness, a property needed for generating optimum power (22). Prm, which functions in the formation and assembly of fibers into myofibrils, is required for producing optimal force and power transduction. Mlp84B localizes to the z-line/I-band interface where it interacts with Actn and in the nucleus where it promotes muscle-specific gene expression. Mlp84B might also have a role in monitoring the integrity of contractile activity (23).
Mating increased abundance of proteins associated with epithelial morphogenesis including α- and β-spectrin (Spec), Cora, HTS-R1, and Nrg. Different Spec isoforms localize to different domains of the epithelial cell plasma membrane: αβ-Spec resides at the basolateral surface, whereas αβH-Spec is restricted to the apical domain (24). αβ-Spec contributes to polarized membrane organization by binding membrane proteins at the cell surface. Cora and Nrg localize to septate junctions, which regulate solute flow between adjacent cells (25). This polarized organization of epithelial cells is essential for their function, including transport of ions and other molecules through the epithelial layer.
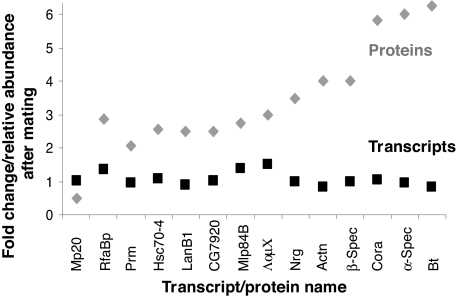
To examine whether changes in protein abundance correlated to changes in transcript level, we compared the list of 89 oviduct proteins detected in the unmated and/or mated group with the list of 5,615 transcripts that were present in either group. We found that the abundance of 19% (17/89) of the oviduct-expressed proteins cannot be explained by transcript abundance (Fig. 2 and Table S3 A and B), similar to observations on yeast transcriptional and translational regulation by Lu et al. (26). Interestingly, these 17 proteins are the mating-responsive, oviduct proteins (Table S3A). Transcripts for most of them (13/17) were present, but none changed significantly in expression level (Fig. 2). This suggests that the oviduct response after mating includes a posttranscriptional component, as observed in the lower female RT (9). Thus, the proteome analysis identified 17 additional mating-responsive genes and insights about the oviduct response to mating.
Fig. 2.
Expression of RNA transcripts does not mirror level of mating-responsive proteins. Relative abundance of protein expression (gray diamonds) and fold-changes in transcript level (black squares) at 3 h after mating. Shown are proteins that increased or decreased by at least 2-fold and whose corresponding transcripts were detected as present on at least two arrays of either unmated or mated groups. See also Table S3.
Although we have not identified the complete proteome present in the oviduct, our analysis detected proteins that were reproducibly expressed in the oviduct of unmated and mated females and proteins with the most abundant changes in the oviduct after mating. Together, transcriptional and proteomic profiling revealed that transcription factors involved in cell growth and differentiation are down-regulated, whereas proteins involved in muscle contractile activity and polarized epithelial function are increased in the oviduct after mating.
Molecular Interactions Reveal Functional Cohesion Among Mating-Responsive Proteins and Link Polarized Epithelial Cell Functions with Actin-Based Cytoskeletal Processes.
To further characterize potential functional themes among oviduct gene products, we analyzed them in the context of a composite Drosophila protein interaction network from high-throughput interactome mapping projects (see SI Methods and Table S4 for details). We examined two topological properties in the interaction network that correlate with functional relatedness: proximity (how many intervening interactions separate two gene products) and neighborhood cohesiveness (how often gene products interact with shared partners). Briefly, we calculated the characteristic path length (CPL, or average shortest distance) and mutual clustering coefficient (Cvw, a measure of neighborhood cohesiveness) for all possible pairs in each oviduct gene set (proteins or transcripts, either expressed or up-regulated in the oviduct) compared with sets of the same size selected at random from the interactome map (SI Methods). We found that proteins, but not transcripts, showed significant connectivity in the Drosophila network by both measures (low CPL and high Cvw) (Table S5 and Fig. S2 A and B). This suggests that the posttranscriptional response to mating tends to involve functionally related molecules, whereas the transcriptional response spans a range of different functional themes, consistent with the GO term analysis. Networks including interologs (inferred protein–protein interactions) from human, worm, and yeast (SI Methods) showed similar results (Table S5 and Fig. S2 A and B).
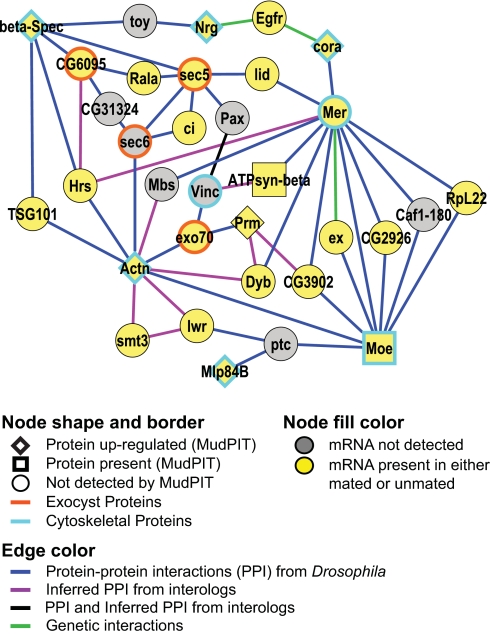
To gain a better understanding of the molecular context in which these proteins function, we inspected the “nearest neighbor networks” (NNNs) of oviduct gene products in the interactome map (SI Methods, Fig. S2 C and D, and SI File 1). These NNNs contain all direct protein–protein interactions between oviduct gene products and all of their interaction partners and reveal subnetworks with putative functional interdependencies within them. For example, epithelial polarity relies on polarized delivery of proteins and lipids to distinct membrane domains (27). The exocyst is a tethering complex that mediates vesicle transport to various compartments and is involved in transcytosis and polarized exocytosis in epithelial cells (28). In Drosophila, the exocyst helps maintain epithelial cell polarity by delivering the cell adhesion molecule E-cadherin to the lateral membrane (29). The subnetwork in Fig. 3 reveals interactions that link exocyst components (Sec5, Sec6, Exo70, and CG6095) with cytoskeletal proteins involved in establishing epithelial cell polarity (e.g., β-Spec and Actn) and molecules that regulate proliferation [Tumor suppressor protein 101 (TSG101) and Cubitus interruptus (ci)]. Additional cytoskeletal proteins in this subnetwork not identified here by MudPIT may also function in the oviduct muscle; because the composite interaction data are not specific to this tissue type, further work will be needed to delineate those subnetworks most relevant to the oviduct. The combined data suggest testable hypotheses to determine whether and how regulation of proliferation, transcytosis, and polarization of the epithelial cytoskeleton may be causally linked. Our results support the idea that active tissue remodeling takes place in the oviduct epithelium and musculature in response to mating, consistent with observed physiological changes in the epithelium (7) and known roles of muscle-specific proteins in muscle architecture and function (discussed above).
Fig. 3.
Drosophila oviduct-related subnetwork of molecular interactions between exocyst and actin cytoskeletal components. A subnetwork from Fig. S2C (NNN of up-regulated oviduct proteins) showing all direct links between members of the exocyst complex (orange borders) and actin-related cytoskeletal proteins (aqua borders). Indirect links through other interaction partners of these proteins are also shown. Some known interactions between exocyst subunits are absent because the interactome map is incomplete. See also Fig. S2D and SI File 1. The full interactome network is provided in Table S4 and can be browsed interactively online by using N-Browse (37) (www.gnetbrowse.org).
Mating Elicits a Unique Reproductive Signature in the Female Oviduct.
Because the oviduct is composed of secretory epithelia and visceral circular muscle fibers, which are tissue types also present in other somatic organs, we asked whether the expression profile we observed after mating is unique to the oviduct. We compared mating-responsive genes from the oviduct with expression profiles of other reproductive soma, including ovary and testis (30), lower RT (9), and mouse oviduct (31); the expression profile of a nonreproductive somatic tissue, the Malpighian tubule (32); and with UniGene ESTs of head, fat body, hemocytes, and salivary gland. Of the 449 mating-responsive transcripts and proteins, 80 (18%) were detected only in the oviduct. Notably, over half of these (46/80) represent genes of unknown function. Thus, 369 transcripts (82%) were present or enriched in other datasets and could be used for further comparisons. Strikingly, 83% (307/369) of these were expressed in the head, which may reflect common themes and regulatory mechanisms.
Only seven genes show enriched expression in all reproductive soma (oviduct, ovary, lower RT, and testis), most of which are cytoskeleton-related (Fig. S3A, bold and italicized labels). Comparing oviduct with other female reproductive tissues, only 12% (44/369) of mating-responsive, oviduct genes found in other somatic tissues are overrepresented in lower RT (5.7%; 21/369) or ovary soma (9.2%; 34/369) (Fig. S3A, red). The latter are involved in ATP binding, mRNA binding, and regulation of transcription, potentially indicating common regulatory themes between soma that support egg development (ovary) and egg activation (oviduct). More oviduct mating-responsive genes are expressed in testis soma (12.7%; 47/369) than in ovary soma or lower RT (with ATP binding, metabolism, and immune-related function highly represented), possibly reflecting the tubular structure of these organs. Alternatively, cross-talk between the RT soma and sperm might lead to sperm-induced, somatic gene expression that is essential for processes contributing to the special environment in the testis and oviduct.
Only two mating-responsive genes in the oviduct were expressed in Malpighian tubules, ducts with nonreproductive functions (Fig. S3A, italics). The cytochrome cyp4e2 is found only in these two tissues, whereas α-esterase-3 (α-est3; a carboxylesterase) is also expressed in head and salivary gland (Fig. S3 A and B). Thus, although both tissues energize fluid secretion, the oviduct epithelia express a unique combination of transporters and ion channels, likely reflecting different mechanisms of transport or secretory regulation (e.g., neural, humoral, and local control) essential for maintaining osmolarity.
To determine whether mating and immune challenge elicit an immune response via common or distinct mechanisms, we also looked at genes induced on immune challenge (11). We found that 10% (35/369) of mating-responsive, oviduct genes enriched or present in other somatic tissues are also enriched in response to immune challenge and vice versa (35/400; Fig. S3 A and B). This suggests that regulatory pathways mediating mating-dependent and immune responses after infection largely differ but may share some common components.
Although limited by the few available datasets, our comparative analysis revealed a set of mating-responsive genes unique to the oviduct, as well as transcripts enriched in other reproductive but not nonreproductive soma. Mating-responsive genes in the oviduct thus define a reproductive signature that differentiates the oviduct “domain” from other RT “domains” and likely specifies a developmental program that sets the final establishment of its positional and functional identity.
Cec Has a Unique Expression Pattern Within the RT and Is Up-Regulated After Mating in the Lower Common Oviduct.
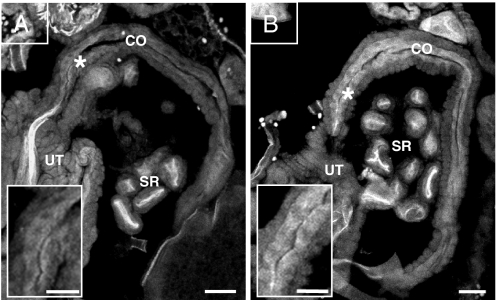
Polarization of oviduct epithelial cells is important for maintaining optimal osmolarity for supporting activation of released eggs and for secretion of membrane active peptides, such as antimicrobial peptides (AMPs) that are produced mainly on the epithelial surface. CecA1 mRNA was strongly induced (12-fold) at 3 h after mating (Fig. 1C). To better understand the function of oviduct epithelial cells, we examined the spatial distribution of cec mRNA and Cec protein and asked whether a change in transcript level is paralleled by an increase in protein level. In situ hybridization to frozen sections of unmated females revealed cec mRNA expression throughout the oviduct epithelia (Fig. 4A). After mating, the spatial range of cec mRNA was unaltered, but the level of cec mRNA increased significantly in the lower common oviduct (P < 0.0002; Fig. 4B).
Fig. 4.
The expression level of cec is significantly increased after mating in the lower part of the common oviduct. (A and B) Digoxigenin-labeled antisense RNA probe for cecA1 hybridized to longitudinal cryosection through the female body (see Fig. S4 for control and schematic): (A) Unmated female. (B) Female mated to WT male at 3 h after mating. Note that cec is transcribed throughout the unmated female oviduct (A), but is strongly up-regulated in the lower part of the common oviduct (CO) at 3 h after mating (B, asterisk). Inset in A and B show high magnification of cec expression in a region of the lower CO marked by asterisk. (Scale bar, 20 μm); n = 15 females for each treatment; (Inset scale bar, 10 μm); UT, uterus; SR, seminal receptacle.
Using transgenic flies expressing GFP under the control of cecA1 promoter (33), we compared Cec-GFP fluorescence intensity in unmated and mated females. Tzou et al. (33) reported that the expression of cec-GFP mRNA and Cec-GFP protein, verified by Northern and Western blot analysis, was similar in kinetics of induction to that of the endogenous gene products. We detected Cec-GFP in the oviduct epithelia (Fig. S5), but it was excluded from the middle part of the oviduct (Fig. S5 C–E). It seems likely that the difference we see between Cec-GFP and cec mRNA expression patterns reflects the expression of cecA2 mRNA, which is also present in the female oviduct and can be detected with the cec in situ probe we used. Like cec transcript, the spatial range of Cec-GFP fluorescence within the RT was unaltered after mating (Fig. S5). However, in the lower common oviduct, quantification showed that mating induced a significant increase in fluorescence intensity (P < 0.001; Fig. S5D), consistent with the in situ data for cec mRNA. An observed increase in lateral oviducts (Fig. S5B) was not statistically significant.
The expression pattern of cec, along with the increase in cec transcript and Cec protein levels after mating, demonstrate that polarized epithelial cells along the oviduct are fully functional. To determine whether the spatial localization of cec is unique, we examined other AMPs detected in our microarray in the oviduct (attacin, defensin, diptericin, drosocin, and drosomycin) and lower RT (metchnikowin) (9). We found that each AMP is expressed in a spatially defined subpopulation of epithelial cells in multiple regions of the RT (Fig. S6). Although each AMP has its own unique spatial pattern, they partially overlap, and within each region of the RT, functionally distinct domains express a different combination of AMPs (Fig. S6C). Human oviduct and uterus also secrete AMPs, accompanied by mucus and a variety of peptide mediators, such as chemokines and cytokines, which regulate the traffic and activity of immune cells (34). The localization of AMPs in distinct domains in the RT and the large quantities of cec observed after mating provide evidence that in Drosophila, as in human (34), epithelial cells play an important role in maintaining a microbe-free environment in the oviduct and contribute to physiological homeostasis of the oviduct microenvironment.
Discussion
Through combined transcriptional and proteomic profiling, we have shown that mating induces transcriptional and translational changes in the epithelium and musculature of the oviduct that advance their maturation state. Mating promotes changes in actin-based cytoskeletal organization and induces immune-related transcripts such as AMPs that likely contribute to creating the optimal environment for successful fertilization. Examination of one immune-related transcript, the AMP cec, revealed a highly ordered spatial expression pattern within the RT that is distinct from other AMPs examined. This pattern is suggestive of a “plug” that protects the entrances to the oviduct and ovaries from foreign matter, although cec and other peptides may have additional, unknown roles. We hypothesize that the epithelial cells and musculature of the unmated female oviduct are poised for rapid response to an extrinsic cue (mating), and that this cue triggers tissue remodeling and functional changes (e.g., contractile muscle activity and polarized secretion from the epithelium of components such as lubricants, solutes and AMPs) that are essential for proper oviduct function, including egg activation and transport. Anatomical analysis reveals that morphological changes in innervation, muscle, and epithelium are distinguishable as early as 6 h after mating (Kalpenikov, Rivlin, Hoy, and Heifetz, unpublished data), thus the oviduct appears to be poised at both a molecular and structural level. We propose that these changes represent the last stage of muscle and epithelial development that prepare the oviduct for reproductive function. One advantage of poised oviduct development may be to conserve metabolic energy until sperm are available.
We do not yet understand how these changes are triggered, but they could be mediated by a few key regulators. In some insects, maturation of the female RT is mediated by JH after eclosion. In locusts, JH mediates morphological and functional changes in the ovipositor muscle fibers, inducing changes in the structural organization and contractile activity of myofibrils, as well as a massive change in their mitochondria and sarcoplasmic reticulum (35). JH also mediates changes in the secretory epithelium of the oviduct that stimulate secretion (36). Whether JH or other hormones are directly or indirectly involved in mediating the changes we observed in the oviduct tissues in Drosophila remains to be determined.
Comparison with other reproductive and nonreproductive tissues sheds light on the oviduct's specialized function. After mating, the oviduct bears transcripts not found in other tissues examined and differs from other nonreproductive tubular secretory epithelia, such as Malpighian tubules, as well as other reproductive tissues, such as the ovary and lower RT. Thus, each region of the reproductive soma appears to bear a unique molecular signature that specifies its specialized function. Combining expression and network analysis provides an opportunity to begin probing, at a systems level, the functional modules underlying the maturation of oviduct tissues and their interdependencies. We conclude that the oviduct signature after mating evidences unique properties that enable the complex coordination of nerve, muscle, and epithelial cells to support the release of fertilizable eggs.
Materials and Methods
See SI Methods for detailed descriptions of all procedures.
Flies.
WT flies were Canton S. Transgenic flies expressing GFP under the control of each AMP promoter (Pepx-GFP) (33) were provided by Jean-Luc Imler (Centre National de la Recherche Scientifique). All flies were kept in a 12-h light/dark cycle at 23 ± 2°C. On eclosion, females and males were collected on ice and held separately for 3 d.
Sample Preparation.
Unmated females and males were placed together and timed from the initiation of mating. At the end of copulation, females were removed, held singly for 3 h, and then placed on ice. The upper female RT tissues (Fig. 1A) were dissected on ice, collected into TRIzol (Invitrogen), and placed at −80°C until processing of RNA and protein. To minimize variation, the same flies were used to prepare RNA and protein. Three independent biological samples, each consisting of pooled tissues from 400 to 500 females, were created for each treatment.
Microarray Assays.
Total RNA was extracted and processed for hybridization (9). Each sample was hybridized to oligonucleotide Drosophila Genome (GeneChip) DrosGenome1 arrays (Affymetrix). Labeling and hybridization were performed by the Department of Biological Services, Weizmann Institute.
Network Analysis.
A composite Drosophila protein–protein interaction map with 7,736 links among 5,400 proteins was created from the union of three published interaction maps. An augmented map including human, worm, and yeast interologs (inferred interactions from orthologous interacting protein pairs) contained a total of 10,230 links among 6,022 proteins. Topological parameters were calculated for each oviduct subnetwork by using only the subset of proteins present in the Drosophila interactome network (±interologs).
Proteomic Assays.
Proteins were isolated from the phenol-ethanol supernatant obtained after precipitation of DNA from TRIzol according to the manufacturer's protocol. Protein analysis and identification were carried out at the Smoler Proteomics Center (Technion, Haifa, Israel).
Proteomic Analysis.
To determine the direction (increase or decrease) of changes in protein abundance, we performed semiquantitative proteomics by using the “peptide count” technique (the total number of peptides identified from a protein in a given LC/LC-MS/MS analysis). We compared the relative abundance of each protein in our dataset (89 proteins) in mated vs. unmated oviducts.
Supplementary Material
Acknowledgments.
We thank T. Ziv, E. Barnea, and A. Admon for assistance with protein analysis and helpful discussions; N. Friedman and N. Shefi for assistance with genomic analysis and helpful discussions; M. Bender, P. Mack, and P. Rivlin for discussions; J. Kriger, H. Breer, and M. Tater for insights into cryosection and in situ hybridization; D. Hultmark for cecropin plasmids and helpful suggestions; J.-L. Imler for the cec-GFP transgenic flies; A. Grundwag for helpful insights; and special thanks to U. Tram, M. Bender, I. Carmel, V. Nagalakshmi, and anonymous reviewers for careful reading of this paper. This research was supported by The United States-Israel Binational Agricultural Research and Development Fund research Grant 3492, Vigevani Fund, and Sacker Award (to Y.H). E.Z. and Y.G. were supported by a fellowship from The Center of Absorption in Science, Ministry of Absorption, The Government of Israel. K.C.G. was supported by NYSTAR Grant C040066 and US Department of the Army Grant 23RYX-3275-N605.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE12332).
This article contains supporting information online at www.pnas.org/cgi/content/full/0710997105/DCSupplemental.
References
- 1.Scott MJ, Pan LL, Cleland SB, Knox AL, Heinrich J. MSL1 plays a central role in assembly of the MSL complex, essential for dosage compensation in Drosophila. EMBO J. 2000;19:144–155. doi: 10.1093/emboj/19.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buhi WC, Alvarez IM, Kouba AJ. Secreted proteins of the oviduct. Cells Tissues Organs. 2000;166:165–179. doi: 10.1159/000016731. [DOI] [PubMed] [Google Scholar]
- 3.Iida K, Cavener DR. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J Exp Biol. 2004;207:675–681. doi: 10.1242/jeb.00816. [DOI] [PubMed] [Google Scholar]
- 4.Heifetz Y, Wolfner MF. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc Natl Acad Sci USA. 2004;101:6261–6266. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 6.Miller A. In: Biology of Drosophila. Demerec M, editor. Plainview, NY: Cold Spring Harbor Lab Press; 1950. pp. 420–531. [Google Scholar]
- 7.Mahowald AP, Goralski TJ, Caulton JH. In vitro activation of Drosophila eggs. Dev Biol. 1983;98:437–445. doi: 10.1016/0012-1606(83)90373-1. [DOI] [PubMed] [Google Scholar]
- 8.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imler JL, Bulet P. Antimicrobial peptides in Drosophila: Structures, activities and gene regulation. Chem Immunol Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- 11.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fassler J, et al. B-ZIP Proteins Encoded by the Drosophila Genome: Evaluation of Potential Dimerization Partners. Genome Res. 2002;12:1190–1200. doi: 10.1101/gr.67902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallo GV, et al. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997;272:32360–32369. doi: 10.1074/jbc.272.51.32360. [DOI] [PubMed] [Google Scholar]
- 15.Beltran S, et al. Transcriptional network controlled by the trithorax-group gene ash2 in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:3293–3298. doi: 10.1073/pnas.0538075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernal A, Kimbrell DA. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc Natl Acad Sci USA. 2000;97:6019–6024. doi: 10.1073/pnas.100391597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helvig C, Koener JF, Unnithan GC, Feyereisen R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA. 2004;101:4024–4029. doi: 10.1073/pnas.0306980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 19.Fedorka KM, Linder JE, Winterhalter W, Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc Biol Sci. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton CA, et al. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:4–17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigoreaux JO, Saide JD, Pardue ML. Structurally different Drosophila striated muscles utilize distinct variants of Z-band-associated proteins. J Muscle Res Cell Motil. 1991;12:340–354. doi: 10.1007/BF01738589. [DOI] [PubMed] [Google Scholar]
- 22.Bullard B, Linke WA, Leonard K. Varieties of elastic protein in invertebrate muscles. J Muscle Res Cell Motil. 2002;23:435–447. doi: 10.1023/a:1023454305437. [DOI] [PubMed] [Google Scholar]
- 23.Kadrmas JL, Beckerle MC. The LIM domain: From the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- 24.Dubreuil RR, Maddux PB, Grushko TA, Macvicar GR. Segregation of Two Spectrin Isoforms: Polarized Membrane-binding Sites Direct Polarized Membrane Skeleton Assembly. Mol Biol Cell. 1997;8:1933–1942. doi: 10.1091/mbc.8.10.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu P, Vogel C, Wang R, Yao X, Marcotte EM. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat Biotechnol. 2007;25:117–124. doi: 10.1038/nbt1270. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 28.Tuma PL, Hubbard AL. Transcytosis: Crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 29.Langevin J, et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:355–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Parisi M, et al. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol. 2004;5:R40–R40.18. doi: 10.1186/gb-2004-5-6-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazeli A, Affara NA, Hubank M, Holt WV. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol Reprod. 2004;71:60–65. doi: 10.1095/biolreprod.103.026815. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69–R69.21. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzou P, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 34.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunological Reviews. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 35.Rose U. Morphological and functional maturation of a skeletal muscle regulated by juvenile hormone. J Exp Biol. 2004;207:483–495. doi: 10.1242/jeb.00754. [DOI] [PubMed] [Google Scholar]
- 36.Lauverjat S, Girardie A. Female genital pathways (oviducts and pseudocolleterial glands) of the Locust migratoria. I. Ultrastructural study of imaginal development. Role of corpora allata (In French) Gen Comp Endocr. 1974;23:325–339. doi: 10.1016/0016-6480(74)90076-8. [DOI] [PubMed] [Google Scholar]
- 37.Lall S, et al. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol. 2006;16:460–471. doi: 10.1016/j.cub.2006.01.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.