Abstract
The B6.YTIR sex-reversed female mouse is anatomically normal at young ages but fails to produce offspring. We have previously shown that its oocytes go through the meiotic cell cycle up to the second metaphase; however, the meiotic spindle is not properly organized, the second meiotic division goes awry after activation or fertilization, and none of the oocytes initiate embryonic development. In the present study, we transferred the nuclei of GV-stage oocytes from XY females into the enucleated GV-stage oocytes from (B6.DBA)F1.XX females. The resultant reconstructed oocytes properly assembled second meiotic spindles after in vitro maturation and produced healthy offspring after in vitro fertilization. Some male pups inherited maternal Y chromosomes. We conclude that the cytoplasm of the XY oocyte is insufficient to support spindle formation at the second metaphase whereas its replacement with the cytoplasmic material from an XX oocyte allows normal development.
Keywords: meiotic spindle, nuclear transfer, sex chromosome aneuploidy, XY sex reversal
Age-dependent decline in female fertility is associated with an increasing incidence of aneuploidy. Developmental potential of oocytes may decrease in terms of nuclear as well as cytoplasmic factors (1). For example, reduced chiasmata or cohesion between chromatids during the prolonged prophase I arrest may lead to premature chromatid separation (2–4). On the other hand, dysfunctional cytoplasm could lead to the formation of abnormal meiotic spindles and consequent chromosomal malsegregation (5, 6). Little is known about the specific nature of such dysfunctions (7). Ooplasmic transfer from donor to recipient oocytes before in vitro fertilization (IVF) has been performed in various mammalian species. In humans, some women who were unsuccessful in previous attempts at IVF conceived and delivered babies after ooplasmic transfer (8, 9). However, this procedure does not overcome the problems that occur during oocyte maturation. Transfer of a germinal vesicle (GV) might overcome cytoplasmic insufficiencies, e.g., by allowing normal spindle formation, although this would not necessarily correct problems related to loss of chromosome cohesion between chromatids during the prolonged prophase I (10, 11). So far, however, no compelling evidence from appropriate animal models supports this approach to correcting meiotic abnormalities.
The B6.YTIR sex-reversed female mouse provides an excellent model for studying the competence of oocytes for embryonic development. This strain was established by repeating backcrosses to place the Y chromosome originating from a variant of Mus musculus domesticus caught in Tirano, Italy, (TIR) on the C57BL/6J (B6) genetic background (12). Similar sex reversal has been reported using the Y chromosomes from other variants of Mus musculus domesticus (13, 14). The YTIR chromosome appears to remain intact during backcrosses because it can initiate normal testicular differentiation on a genetic background other than B6 (12, 15, 16). Therefore, sex reversal in the B6.YTIR mouse can be attributed to a lack of coordination between the YTIR chromosome and the B6 genetic background (13, 17). The resultant XY sex-reversed females are anatomically normal at young ages but fail to produce offspring (13, 18). Our previous studies have demonstrated that the primary cause of infertility lies in the incompetence of the oocytes from these females to initiate embryonic development (19–21). The meiotic cell cycle proceeds normally up to the second metaphase (MII) in these oocytes in culture despite sex chromosome aneuploidy; however, the second meiotic division goes awry after activation or fertilization and very few oocytes reach the 2-cell stage (22). In the present study, we demonstrate that the oocytes of XY females are defective in their cytoplasm; by transferring the karyoplast of an XY oocyte into an enucleated oocyte from a normal XX female, either before or after maturation, we could make the reconstructed oocytes go through the second meiotic division and transmit the maternal Y chromosomes to healthy offspring.
Results
Correction of the Second Meiotic Spindle Assembly by Ooplasmic Replacement.
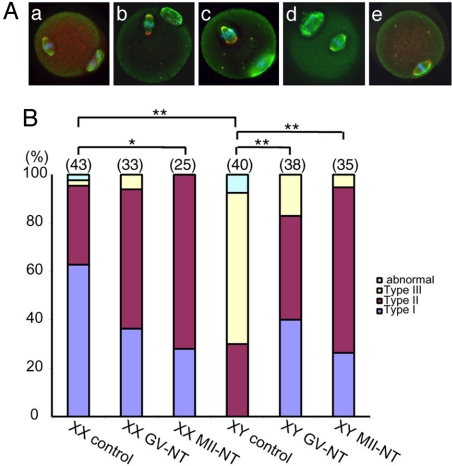
We have previously reported that abnormal second meiotic spindle is the most consistent defect observed in the MII oocytes from B6.YTIR females after in vitro maturation (IVM) (22). In the present study, we asked if the replacement of the ooplasm during IVM would correct this defect in the presence of B6.YTIR-derived chromosomes. We transferred the GV of oocytes from either XY females or their XX littermates into enucleated GV-stage oocytes from (B6.DBA)F1.XX females and allowed the reconstructed oocytes to mature in culture. We assessed the second meiotic spindles in the oocytes which reached MII. By immunolabeling of α- and γ-tubulin, major components of microtubule spindle and microtubule organizing center, respectively, we categorized the morphology of meiotic spindles into 3 types (Fig. 1A). We also validated chromosome condensation and alignment by labeling with DAPI. The typical meiotic spindle found in the XX control group was defined as Type I spindle. α-tubulin labeling showed a barrel-shaped microtubule spindle in a parallel position to the oolemma while γ-tubulin labeling was seen in punctuate foci at both poles. Two foci were often seen at each pole in the oocytes after IVM although one was more common in ovulated oocytes (data not shown). Since both types of oocytes are competent for embryonic development, we considered the second meiotic spindle with 2 γ-tubulin foci per pole as normal. The condensed chromosomes were aligned along the midzone. After IVM without nuclear transfer (control), 60% and 30% of the MII oocytes from XX females contained Type I and Type II spindles, respectively (Fig. 1B). For comparison, 30% and 60% of the MII oocytes from XY females contained Type II and Type III spindles, respectively, and none contained Type I spindles. The proportions of MII oocytes containing Type I, II, and III spindles in the XY control group were significantly different by χ2 test (P < 0.001) from those in the XX control group. In addition, the majority of MII oocytes in the XY control group were seen with meiotic spindles perpendicular to the oolemma, in comparison to parallel positions in the majority of MII oocytes in the XX control group. After the transfer of GV from XX or XY oocytes into enucleated GV-stage oocytes from F1.XX females, followed by IVM (defined as XX or XY GV-NT group, respectively), the majority of the reconstructed oocytes contained Type I and Type II spindles. The proportion of MII oocytes containing Type III spindles was higher in the XY GV-NT group than that in the XX GV-NT group, but no statistical difference was found. On the other hand, the proportions of MII oocytes containing Type I, II, and III spindles in the XY GV-NT group were significantly different (P < 0.001) from those in the XY control group. In addition, most of reconstructed oocytes in the XY GV-NT group were observed with meiotic spindles in parallel positions. Similar results were obtained by using the oocytes from (B6.C3H)F1 females as recipients (data not shown). Thus, the replacement of ooplasm during IVM improved the morphology and orientation/positioning of the second meiotic spindles in the reconstructed oocytes carrying the karyoplast from XY oocytes.
Fig. 1.
Spindle microtubules in the MII oocytes after in vitro maturation (IVM), with or without nuclear transfer. (A) Immunolabeling of α-tubulin (green) and γ-tubulin (red) counterstained with DAPI (blue). (a) Type I spindle found in the XX control group. α-tubulin labeling shows a barrel-shaped microtubule spindle in a parallel position to the oolemma. γ-tubulin labeling is seen in punctuate foci at both poles. The condensed chromosomes are aligned along the midzone. The first polar body is seen on the bottom right. (b) Type II spindle found in the XY control group. The microtubule spindle shows globally normal shape, but oriented in a perpendicular position to the oolemma. Multiple γ-tubulin foci are spread over both poles, which are considerably wider that the poles of Type I spindle. The condensed chromosomes are loosely aligned at the midzone. The first polar body is seen on the top. (c) Type III spindle found in the XY control group. The microtubule spindle is bulky, i.e., one pole is wider than the midzone, and oriented in a perpendicular position to the oolemma. Multiple γ-tubulin foci are spread over the spindle. The condensed chromosomes are scattered around the midzone. The first polar body is seen on the bottom right. (d) Type I spindle observed in the XY GV-NT group. The microtubule spindle has normal shape with 1 or 2 γ-tubulin foci at each pole. The condensed chromosomes are aligned at the midzone. The first polar body is seen on the top left. (e) Type I spindle observed in the XY MII-NT group. A barrel-shaped microtubule spindle is oriented in a parallel position to the oolemma. Two γ-tubulin foci are seen at each pole. The condensed chromosomes are aligned at the midzone. The first polar body has been removed during nuclear transfer. (B) Percentages of MII oocytes containing each type of meiotic spindles. The total number of oocytes examined is indicated on the top of each column. *, P < 0.01; **, P < 0.001 by χ2 test. Type I, II, and III spindles are defined above. The “abnormal” spindle is defused or contains tripolar microtubule spindles.
To examine whether the meiotic stage of nuclear transfer is critical for the correction of meiotic spindle assembly, we transferred the MII chromosomes within a small amount of ooplasm (here termed MII chromosomes) of IVM-oocytes from XY or XX females into enucleated MII-stage oocytes ovulated from F1.XX females and further incubated the reconstructed oocytes for 1 h (defined as MII-NT group). We used ovulated oocytes since they can be easily prepared. Of the reconstructed oocytes, 30% contained Type I and 70% contained Type II spindles, regardless of the origin of MII chromosomes (Fig. 1B). There was a significant decrease (P < 0.01) in the proportion of MII oocytes containing Type I spindles in the XX MII-NT group compared to that in the XX control group. This tendency was also found in the XX GV-NT group, suggesting that the proportion of Type I and Type II spindles was affected by artificial manipulation such as nuclear transfer. Nonetheless, the proportions of MII oocytes containing Type I, II, and III spindles in the XY MII-NT group were significantly different (P < 0.001) from those in the XY control group and comparable to those in the XX MII-NT group. We conclude that the ooplasm controls the second meiotic spindle assembly in this model regardless of the meiotic stage of nuclear transfer. Therefore, the abnormal second meiotic spindles observed in the MII oocytes from XY females can be attributed to defects in their cytoplasm. The rapid correction of meiotic spindle assembly after nuclear transfer at the MII stage can be attributed to the dynamic turnover of microtubule polymerization and depolymerization (23). We consistently observed, in addition to abnormal meiotic spindles, loosely organized metaphase chromosomes in the MII oocytes from XY females after IVM. Our current results suggest that the alignment of metaphase chromosomes can be secondary to the abnormal assembly of microtubule spindles and can be corrected by the replacement of the ooplasm. This conclusion is consistent with the model of spindle assembly in the mouse oocyte (24).
Improvement in the Cell Cycle Progression by Ooplasmic Replacement.
We performed reciprocal karyoplast exchange between the GV-stage oocytes from XY females and those from their XX littermates to obtain further evidence that the cytoplasm of XY oocytes is responsible for the incompetence of the oocyte nucleus for embryonic development. We activated the reconstructed oocytes by the treatment with 5 mM SrCl2 for 4 h. The results were consistent with our conclusions from the above experiments; very few or none of the reconstructed oocytes reached the 2-cell stage (one out of 53 oocytes in the XX GV-NT group and none in 28 oocytes in the XY GV-NT group) when the cytoplasm was provided from XY oocytes whereas ≈20% of the reconstructed oocytes did so (4 out of 26 oocytes in the XX GV-NT group and 10 out of 62 oocytes in the XY GV-NT group) when the cytoplasm was provided from XX oocytes, regardless of the origin of nuclei. However, this rate of embryonic development was much lower than that in non-manipulated oocytes from XX females (≈90%) (22) and prevented us from further investigations.
By contrast, when we transferred the GV of oocytes from either XY females or their XX littermates into the enucleated GV-stage oocytes from F1.XX females, followed by IVM, 51% of the oocytes in the XY GV-NT group reached the 2-cell stage in 24 h postactivation (Table 1). Although this rate of embryonic development was significantly lower than the 74% of the oocytes in the XX GV-NT group, both rates were much higher than those in reciprocal karyoplast exchange. Furthermore, when the enucleated MII oocytes ovulated from F1.XX females were used as the recipients, 94% of oocytes in the either XY or XX MII-NT group reached the 2-cell stage in 24 h postactivation (Table 1). Therefore, the cytoplasm of the oocytes ovulated from F1.XX females has a far higher potential to support cell cycle progression in the reconstructed oocyte. On the other hand, the cytoplasm of the oocytes from B6 females appears to be vulnerable to physically denudation or nuclear manipulation during IVM. Since we did not have an option of reciprocal karyoplast exchange, we conducted serial nuclear transfer to maximize the developmental competence of the karyoplast in reconstructed oocytes (25).
Table 1.
Development of reconstructed oocytes after parthenogenetic activation
| Composition |
No. of oocytes (%) |
|||
|---|---|---|---|---|
| Karyoplast | Cytoplasm | Reconstructed | Matured | 2-cell |
| Nuclear transfer at the GV-stage (GV-NT) | ||||
| XX | F1.XX | 86 | 81 (94) | 60 (74) |
| XY | F1.XX | 68 | 67 (96) | 34* (51) |
| Nuclear transfer at the MII-stage (MII-NT) | ||||
| XX | F1.XX | 16 | 15 (94) | |
| XY | F1.XX | 17 | 16 (94) | |
*, P < 0.05
Development of Reconstructed Oocytes after Fertilization.
To validate the competence of the reconstructed oocytes for the second meiotic division as well as for the embryonic development, we removed the MII chromosomes from the reconstructed oocytes in GV-NT groups and transferred them into enucleated MII-stage oocytes ovulated from F1.XX females. These re-reconstructed oocytes were fertilized in vitro and their development in culture was monitored for 4 days. Similar proportions of the re-reconstructed oocytes were fertilized and reached the 8-cell stage whether they carried the karyoplasts from XY or XX oocytes (Table 2). This rate of embryonic development was much higher than that we have previously observed in unmanipulated oocytes from XY females (22). Furthermore, most of the 2-cell-stage embryos developed from the re-reconstructed oocytes appeared morphologically normal whereas those from unmanipulated oocytes were asymmetrical and often contained multinuclei (22). Therefore, replacement of the ooplasm during IVM enabled the nuclei from XY oocytes to go through the second meiotic division into embryonic development. The proportions of the re-reconstructed oocytes in the XY GV-NT group that reached the morula and blastocyst stages were lower but not statistic different from those of the re-reconstructed oocytes in the XX GV-NT group (Table 2). All blastocyst embryos were transferred into the uteri of pseudopregnant females. A total of 13 (3 female and 10 male) healthy pups were delivered from the re-reconstructed oocytes in the XY GV-NT group and all but one male pup developed into adulthood.
Table 2.
Development of reconstructed oocytes after IVF and transfer into foster mothers
| Composition |
No. of oocytes (%) |
No. of embryos (% of fertilized oocytes) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karyoplast | Cytoplasm | Reconstructed | Matured | Rereconstructed | Fertilized† | 2-cell | 4-cell | 8-cell | Morula | Blastocyst | Pups |
| Nuclear transfer at the GV-stage (GV-NT) | |||||||||||
| XX | F1.XX | 40 | 39 (98) | 32 | 29 (91) | 29 (100) | 29 (100) | 28 (97) | 28 (97) | 24 (83) | 6 (21) |
| XY | F1.XX | 68 | 65 (96) | 55 | 52 (95) | 52 (100) | 51 (98) | 47 (90) | 42 (81) | 37 (71) | 13 (25) |
| Nuclear transfer at the MII-stage (MII-NT) | |||||||||||
| XX | F1.XX | 73 | 62 (85) | 61 (98) | 55 (89) | 55 (89) | 47 (76) | 35 (56) | 19 (31) | ||
| XY | F1.XX | 104 | 87 (84) | 83 (95) | 79 (91) | 66 (76) | 42* (48) | 25* (29) | 6** (7) | ||
*, P < 0.001; **, P < 0.05.
†Excluding polyspermy.
To address whether the ooplasmic replacement during IVM is essential for improving the competence of the oocyte nucleus for embryonic development, we subjected the reconstructed oocytes in MII-NT groups to IVF and embryonic development. Similar proportions of the reconstructed oocytes in either XY or XX MII-NT groups were fertilized and reached the 4-cell stage (Table 2). However, a smaller proportion of the reconstructed oocytes in the XY MII-NT group reached the 8-cell stage, and significantly smaller proportions (P < 0.001) reached the morula and blastocyst stages. After transfer into the uteri of pseudopregnant females, 6 male pups were born (one accidentally died). The remaining 5 pups developed into adulthood. This proportion of live-born was also significantly smaller (P < 0.05) than in the XX MII-NT group. When Cesarean section was performed near the delivery day, many fetuses in the XY MII-NT group were found absorbed while all fetuses in the XX MII-NT group were alive and healthy. These results suggest that the proper assembly of the second meiotic spindle is associated with successful progression in the second meiotic division, but not sufficient to lead to full embryonic development. We speculate that the competence of the oocyte nucleus for embryonic development was compromised in the defective cytoplasm of XY oocytes during IVM.
Transmission of Maternal Y Chromosomes to Male Pups.
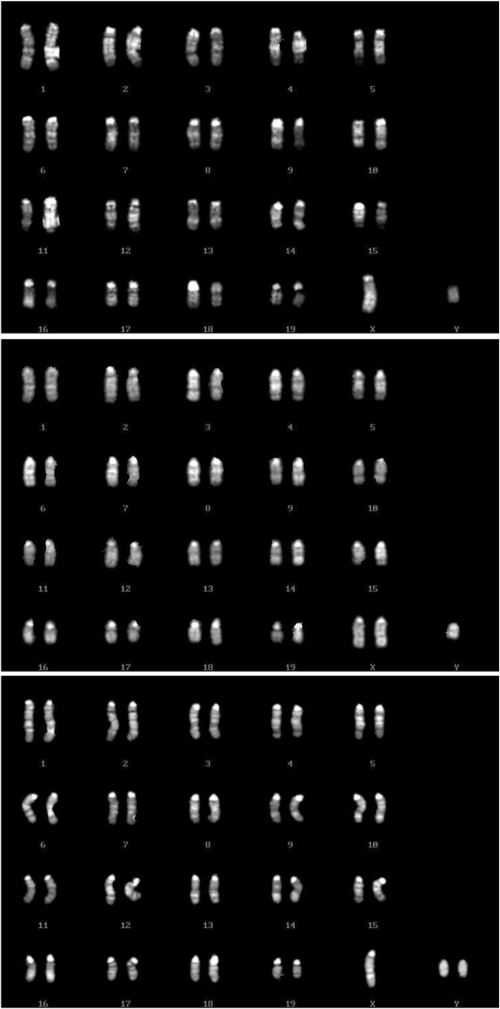
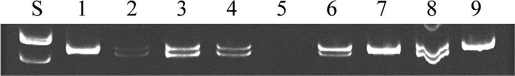
Our previous studies have shown that unpaired X and Y chromosomes segregate independently but meiotic drive occurs at the first meiotic division in the oocytes from B6.YTIR females (22). Consequently, MII oocytes retain X, Y, XY, and no sex chromosomes at the ratio of 0.35, 0.35, 0.24, and 0.06, respectively. Of the expected zygotes, YY and OY embryos are anticipated to die during preimplantation development. If the remaining embryos have an equal chance to survive, the anticipated offspring would be dominated by males (74%), carrying Y chromosomes of either maternal or paternal origin. Our current results were consistent with this expectation. When the nuclei were transferred at the GV-stage (GV-NT groups), 77% and 23% of live-born pups were phenotypical male and female, respectively (Table 3). Of 3 female pups, 2 were XX and one was XO. Of 10 male pups, 3 were XY whereas 6 had sex chromosome aneuploidy, either XXY or XYY (Fig. 2). One died too soon to be karyotyped. Since the XY oocyte carried the YTIR chromosome whereas the sperm carried the YDBA chromosome, we distinguished them by the polymorphism of the Zfy2 sequence (21, 26). The mouse Zfy gene has 2 copies, Zfy-1 and Zfy-2; the YTIR chromosome carries both copies of an identical molecular size at 618 base pairs (bp) whereas the YDBA chromosome has the Zfy-2 copy with an 18 bp deletion (Fig. 3). All 3 XY male pups carried sperm-derived YDBA chromosomes whereas 2 XXY male pups carried oocyte-derived YTIR chromosomes (Table 3). Three XYY male pups carried both sperm- and oocyte-derived Y chromosomes. One XYY male pup carried both Y chromosomes of oocyte origin, most likely as a consequence of an error at the second meiotic division in the oocyte carrying a single Y chromosome.
Table 3.
Inheritance of sex chromosomes in the pups developed from the reconstructed oocytes carrying the nuclei from XY oocytes
| Phenotype | Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nuclear transfer at the GV-stage (GV-NT) | |||||||||
| Karyotype | XO | XX | XY | XXY | XYY | ND* | |||
| Maternal | O | X | X | XY | XY | YY | Y + ? | ||
| Paternal | X | X | Y | X | Y | X | X | ||
| No. of pups | 1 | 2 | 3 | 2 | 3 | 1 | 1 | ||
| Sex ratio | 23% | 77% | |||||||
| Nuclear transfer at the MII-stage (MII-NT) | |||||||||
| Karyotype | XY | XXY | XYY | ||||||
| Maternal | X | XY | XY | ||||||
| Paternal | Y | X | Y | ||||||
| No. of pups | 0 | 1 | 3 | 1 | |||||
| Sex ratio | 0% | 100% | |||||||
*One male pup died soon after birth, and therefore, its karyotype was not determined. The Zfy polymorphism indicated the presence of only the YTIR-chromosome in this pup.
Fig. 2.
Examples of karyotype analyses in male pups. (Top) 40XY karyotype. (Middle) 41XXY karyotype. (Bottom) 41XYY karyotype.
Fig. 3.
PCR analyses of the Zfy polymorphism. Lane S, 100 bp ladder. Lane 1, B6.YTIR mother. Lane 2, (B6.DBA)F1.XY father. Lane 3 and 4, male pups from the reconstructed oocytes which carried the karyoplast from XX oocytes. Lane 5, a female pup from the reconstructed oocyte which carried the karyoplast from an XY oocyte. Lane 6–9, male pups from the reconstructed oocytes which carried the karyoplast from XY oocytes. The ratio of 618 bp band intensity against 600 bp band intensity is near 1.0 in lanes 2, 3, 4, and 8 whereas it is 2.6 in lane 6, suggesting the presence of both YTIR and YDBA chromosomes in this sample.
Similar results were obtained when the nuclei were transferred at the MII stage, although the number of pups was limited (Table 3). One XY male pup carried sperm-derived YDBA chromosome whereas 3 XXY male pups carried oocyte-derived YTIR chromosomes. One XYY male pup inherited both sperm- and oocyte-derived Y chromosomes.
The fertility of pups developed from the reconstructed oocytes was largely as expected. Both XX and XO females were fertile, so were XY males carrying sperm-derived Y chromosomes. XXY males were infertile as the presence of 2 X chromosomes is incompatible with the survival of spermatogonia (27). The fertility of XYY males carrying both sperm- and oocyte-derived Y chromosomes was variable (28, 29). The XYY male carrying 2 oocyte-derived Y chromosomes was infertile. Unfortunately, we have not yet obtained XY males carrying oocyte-derived Y chromosomes.
Discussion
Our current results demonstrate a dominant effect of the cytoplasm during oocyte maturation over the competence of the nucleus for embryonic development in the XY oocyte. The replacement of the ooplasm at the GV-stage corrected the second meiotic spindle assembly, leading to successful second meiotic division and embryonic development. The aneuploidy of sex chromosomes at the first meiotic division blocked neither meiotic progression nor embryonic development, and the distribution of sex chromosomes was faithfully reflected into the offspring. On the other hand, the replacement of the ooplasm at the MII stage also corrected the second meiotic spindle assembly and second meiotic division; however, the competence of the oocyte nucleus for embryonic development was not fully installed. We found that significantly larger proportions of embryos in the XY MII-NT group were lost during development compared to the XX MII-NT group, whereas no difference was found in GV-NT groups. Therefore, the presence of proper cytoplasm during maturation is critical for the competence of the oocyte nucleus for embryonic development. The cytoplasm of ovulated oocytes, compared to IVM oocytes, is far superior to support embryonic development. Therefore, in our studies, the successful embryonic development in the reconstructed oocytes can be partly attributed to the use of ovulated oocytes as the recipient. Nonetheless, the developmental incompetence of the XY oocyte nucleus could not be overcome by the replacement of the cytoplasm at the MII stage. Further studies are needed to determine the cause of embryonic loss in the XY MII-NT group.
In future studies, we plan to identify the insufficient or detrimental factor(s) in the XY oocyte by comparing the cytoplasmic components between XY and XX (or F1.XX) oocytes and to delineate how such factor influences the assembly of the second metaphase spindles. How the rotation/positioning of spindles is regulated by the cytoplasmic components is intriguing but largely unknown (30). We previously reported that phosphorylated MAPK but not cyclin B1 was slightly lower in the MII oocytes from XY females (22). DOC1R and MISS are known to be MAPK substrates and to be pivotal for organization of spindle poles (31, 32). To explore maternal factors deficient in the XY oocyte, analyses of DOC1R, MISS, and other factors associated with organization of spindle poles in oocytes (e.g., NuMA, Spindlin, MEK1/2) (33–35) would be informative. In particular, the dosage of X-encoded gene products may be inadequate in the XY oocyte (36). It is generally accepted that the single X chromosome is sufficient for female fertility because the XO female mouse is invariably fertile. However, a part of the single X chromosome may attain a transcriptionally inactive form due to a lack of pairing during the pachytene stage of meiotic prophase (37, 38). Furthermore, the presence of Y-encoded gene products, which are highly homologous but not identical to their X-encoded homologues, may alter the levels of functional proteins. Some X-encoded genes are known to play pivotal roles in female reproduction (e.g., spindlin family genes, Bmp15, Ube2a) (39–42) and need to be examined. It is conceivable that the altered dosage of sex-chromosome-encoded genes may influence directly or indirectly other genes which are essential for spindle formation during oocyte maturation.
Materials and Methods
Mouse.
All animal experiments were conducted in accordance with the Guide to the Care and Use of Experimental Animals issued by the Canadian Council on Animal Care and the Guidelines for Proper Conduct of Animal Experiments as promulgated by the Science Council of Japan and with approvals from the Animal Research Committee of McGill University and from the Tokyo University of Agriculture Institutional Animal Care and Use Committee. B6.YTIR progeny were produced and genotyped as described previously (22). (B6.DBA)F1 mice were purchased from Clea.
Nuclear Transfer Between GV-Stage Oocytes and Subsequent Maturation in Culture.
Female mice at 25–29 days postpartum were injected i.p. with 5 IU equine CG (eCG) (Sigma–Aldrich) each and killed 45–47 h later. Oocytes in cumulus cell complexes were isolated from the antral follicles of their ovaries and denuded mechanically by repeated pipetting through fine glass needles in the MEM-α medium (GIBCO/Life Science) supplemented with 240 μM dibutyryl cyclic-AMP (dbc-AMP) (Sigma-Aldrich). The denuded oocytes were further incubated in the MEM-α medium supplemented with dbc-AMP and 5% FBS (GIBCO) for 2 h until perivitelline space was formed between zona and oocytes. Nuclear transfer was performed as described previously (43, 44). Before nuclear transfer, the zona pellucida of the GV-stage oocytes was slit with a glass knife along 10–20% of the circumference in the M2 medium (45) containing 240 μM dbc-AMP. The GV was removed with a minimal amount of cytoplasm from the oocyte in the M2 medium containing 10 μg/ml cytochalasin B and 0.1 μg/ml colcemid (both from Sigma-Aldrich), and then a GV was introduced with Sendai virus (HVJ) into the perivitelline space of an enucleated GV-stage oocyte. The reconstructed oocytes were cultured in the MEM-α medium supplemented with 300 ng/ml follicle stimulating hormone (Sigma-Aldrich), 25 μg/ml sodium pyruvate, 5% FBS, and antibiotics (all from GIBCO) for 21 h as described previously (22).
Nuclear Transfer Between MII-Stage Oocytes and Subsequent Incubation in Culture.
(B6.DBA)F1 mice at 8–12 weeks postpartum were injected with 5 IU eCG and 48 h later with 5 IU human CG (hCG) (Sankyo). MII oocytes were collected from the oviducts 14–16 h after the hCG injection. As the recipient oocytes, MII chromosomes were removed from the ovulated oocytes in the M2 medium containing 5 μg/ml cytochalasin B. Then, the MII chromosomes (karyoplast) of either the reconstructed oocytes or non-manipulated oocytes after IVM were transferred into the enucleated recipient oocytes. The fusion of karyoplast and ooplasm was induced by inactivated HVJ as described previously (43, 44).
Parthenogenetic Activation of MII-Oocytes with SrCl2.
After IVM, the reconstituted oocytes which had extruded the first polar body (= MII stage) were transferred into a Ca2+-free M16 medium (45) containing 5 mM SrCl2 and incubated for 4 h. The oocytes were then washed 3 times and cultured in the basic M16 medium for 20 h.
In Vitro Fertilization and Embryonic Development.
Spermatozoa were collected from the caudal epididymis of (B6.DBA)F1 virgin males at 10–14 weeks postpartum and capacitated in a TYH medium under oil at 37 °C for 1.5 h as described previously (43). The re-reconstructed and reconstructed oocytes were transferred into the TYH medium containing diluted spermatozoa under oil and further incubated for 4 h. After washings, the oocytes were incubated in the M16 medium for up to 4 days. The embryos were then transferred into the uterine horns of (B6.DBA)F1 females at 2.5 days of pseudopregnancy.
Immunocytochemical Labeling of Microtubule Spindles.
MII oocytes were fixed for 30 min at room temperature in the microtubule-stabilizing buffer (46) containing 2% formaldehyde (EM Science) and processed for immunolabeling with a mouse monoclonal anti-α-tubulin antibody (Cedarlane Lab) and a rabbit anti-γ-tubulin antibody (Sigma-Aldrich), followed by a goat anti-mouse IgG antibody conjugated with biotin and a goat anti-rabbit IgG antibody conjugated with rhodamine, and finally with avidin conjugated with FITC (all from Pierce Endogen) as described previously (22). The oocytes were mounted in Vectashield (Vector) supplemented with 0.3 μg/ml 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Behringer-Mannheim). The slides were examined under a fluorescence microscope (Olympus). Color overlays of corresponding reflected light and fluorescent optical section images were made for each confocal z-series, using the “Overlay Images” feature in Metamorph (Molecular Devices). Maximum intensity projections of the overlay z-series were then generated over a range of projection angles using the “3-D Reconstruction” function in Metamorph. The resulting projections were then saved as a stack file.
Fertility.
The fertility of offspring was tested by caging them with (B6.DBA)F1 male or female mice individually. The presence of copulation plug was checked regularly and the delivery of pups confirmed their fertility.
Karyotype Analysis.
The karyotype of each mouse was identified by using lymphocytes as described previously (47). The peripheral blood was collected from each adult mouse and cultured in the RPMI1640 medium supplemented with 20% FBS, 3 μg/ml Con A, 10 μg/ml lipopolysaccharide, 55 μM 2-mercaptoethanol, and antibiotics for 72–96 h. The blood cells were then treated with 0.075 M KCl solution at room temperature for 20 min. A fresh mixture of methanol and acetic acid (3:1) was applied to the slides to spread the metaphase chromosomes. For each mouse, the number of chromosomes was counted in 10–50 metaphase preparations after staining with 5% Giemsa for 5 min. The karyotype was determined in 3 to 7 metaphase preparations after staining with 0.01 μg/ml Hoechst 33258 for 5 min, followed by 5.0 μg/ml quinacrine mustard for 20 min.
PCR Analysis of the Zfy Polymorphism.
The origin of Y chromosomes in male pups was identified by PCR analysis of the polymorphism in a Y-encoded gene Zfy (26). Total DNA was isolated, amplified with Zfy primers, and size-fractionated by 5% polyacrylamide gel electrophoresis in Tris-borate buffer and visualized by ethidium bromide staining as described previously (21).
Statistical Analyses.
All experiments were repeated at least twice. Significant differences among pooled results were analyzed by χ2 test.
Acknowledgments.
We are grateful to Dr. Chian (McGill University) for allowing us to use his micromanipulation facility. This study was supported by grants from CIHR (Canada) to T.T., BRAIN (Japan) to T.K., and The Ministry of Education, Science, Culture and Sports (Japan) to Y.O. and T.K.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.M.M. is a guest editor invited by the Editorial Board.
References
- 1.Navot D, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- 2.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 3.Wolstenholme J, Angell RR. Maternal age and trisomy—a unifying mechanism of formation. Chromosoma. 2000;109:435–438. doi: 10.1007/s004120000088. [DOI] [PubMed] [Google Scholar]
- 4.Hodges CA, et al. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- 5.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 6.Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: Possible damage to the spindle apparatus. Reprod Biomed Online. 2002;5:117–124. doi: 10.1016/s1472-6483(10)61613-6. [DOI] [PubMed] [Google Scholar]
- 7.Malter HE, Cohen J. Ooplasmic transfer: Animal models assist human studies. Reprod Biomed Online. 2002;5:26–35. doi: 10.1016/s1472-6483(10)61593-3. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, et al. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 9.Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7:428–435. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, et al. In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil Steril. 1999;71:726–731. doi: 10.1016/s0015-0282(98)00549-4. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi T, et al. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum Reprod. 1999;14:1312–1317. doi: 10.1093/humrep/14.5.1312. [DOI] [PubMed] [Google Scholar]
- 12.Nagamine CM, Taketo T, Koo GC. Studies on the genetics of tda-1 XY sex reversal in the mouse. Differentiation. 1987;33:223–231. doi: 10.1111/j.1432-0436.1987.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 13.Eicher EM, Washburn LL, Whitney JB, III, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science. 1982;217:535–537. doi: 10.1126/science.7089579. [DOI] [PubMed] [Google Scholar]
- 14.Biddle FG, Nishioka Y. Assays of testis development in the mouse distinguish three classes of domesticus-type Y chromosome. Genome. 1988;30:870–878. doi: 10.1139/g88-140. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Taketo T. Normal onset, but prolonged expression, of Sry gene in the B6YDOM sex-reversed mouse gonad. Dev Biol. 1994;165:442–452. doi: 10.1006/dbio.1994.1266. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht KH, Young M, Washburn LL, Eicher EM. Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics. 2003;164:277–288. doi: 10.1093/genetics/164.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taketo T, et al. Expression of SRY proteins in both normal and sex-reversed XY fetal mouse gonads. Dev Dyn. 2005;233:612–622. doi: 10.1002/dvdy.20352. [DOI] [PubMed] [Google Scholar]
- 18.Taketo-Hosotani T, et al. Development and fertility of ovaries in the B6. YDOM sex-reversed female mouse. Development (Cambridge, UK) 1989;107:95–105. doi: 10.1242/dev.107.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Merchant-Larios H, Clarke HJ, Taketo T. Developmental arrest of fertilized eggs from the B6. YDOM sex-reversed female mouse. Dev Genet. 1994;15:435–442. doi: 10.1002/dvg.1020150506. [DOI] [PubMed] [Google Scholar]
- 20.Amleh A, Ledee N, Saeed J, Taketo T. Competence of oocytes from the B6. YDOM sex-reversed female mouse for maturation, fertilization, and embryonic development in vitro. Dev Biol. 1996;178:263–275. doi: 10.1006/dbio.1996.0217. [DOI] [PubMed] [Google Scholar]
- 21.Amleh A, Taketo T. Live-borns from XX but not XY oocytes in the chimeric mouse ovary composed of B6. YTIR and XX cells. Biol Reprod. 1998;58:574–582. doi: 10.1095/biolreprod58.2.574. [DOI] [PubMed] [Google Scholar]
- 22.Villemure M, et al. The presence of X- and Y-chromosomes in oocytes leads to impairment in the progression of the second meiotic division. Dev Biol. 2007;301:1–13. doi: 10.1016/j.ydbio.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Gorbsky GJ, Simerly C, Schatten G, Borisy GG. Microtubules in the metaphase-arrested mouse oocyte turn over rapidly. Proc Natl Acad Sci USA. 1990;87:6049–6053. doi: 10.1073/pnas.87.16.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Obata Y, Maeda Y, Hatada I, Kono T. Long-term effects of in vitro growth of mouse oocytes on their maturation and development. J Reprod Dev. 2007;53:1183–1190. doi: 10.1262/jrd.19079. [DOI] [PubMed] [Google Scholar]
- 26.Nagamine CM, Chan KM, Kozak CA, Lau YF. Chromosome mapping and expression of a putative testis-determining gene in mouse. Science. 1989;243:80–83. doi: 10.1126/science.2563174. [DOI] [PubMed] [Google Scholar]
- 27.Mroz K, Carrel L, Hunt PA. Germ cell development in the XXY mouse: Evidence that X chromosome reactivation is independent of sexual differentiation. Dev Biol. 1999;207:229–238. doi: 10.1006/dbio.1998.9160. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez TA, Burgoyne PS. Evidence that sex chromosome asynapsis, rather than excess Y gene dosage, is responsible for the meiotic impairment of XYY mice. Cytogenet Cell Genet. 2000;89:38–43. doi: 10.1159/000015559. [DOI] [PubMed] [Google Scholar]
- 29.Turner JM, et al. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell. 2006;10:521–529. doi: 10.1016/j.devcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Brunet S, Maro B. Germinal vesicle position and meiotic maturation in mouse oocyte. Reproduction. 2007;133:1069–1072. doi: 10.1530/REP-07-0036. [DOI] [PubMed] [Google Scholar]
- 31.Terret ME, et al. DOC1R: A MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development (Cambridge, UK) 2003;130:5169–5177. doi: 10.1242/dev.00731. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre C, et al. Meiotic spindle stability depends on MAPK-interacting and spindle-stabilizing protein (MISS), a new MAPK substrate. J Cell Biol. 2002;157:603–613. doi: 10.1083/jcb.200202052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compton DA. Focusing on spindle poles. J Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- 34.Oh B, et al. SPIN, a substrate in the MAP kinase pathway in mouse oocytes. Mol Reprod Dev. 1998;50:240–249. doi: 10.1002/(SICI)1098-2795(199806)50:2<240::AID-MRD15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Yu LZ, et al. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle. 2007;6:330–338. doi: 10.4161/cc.6.3.3805. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- 37.Turner JM, et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet. 2005;37:41–47. doi: 10.1038/ng1484. [DOI] [PubMed] [Google Scholar]
- 38.Alton M, Lau MP, Villemure M, Taketo T. The behavior of the X- and Y-chromosomes in the oocyte during meiotic prophase in the B6. YTIR sex-reversed mouse ovary. Reproduction. 2008;135:241–252. doi: 10.1530/REP-07-0383. [DOI] [PubMed] [Google Scholar]
- 39.Oh B, Hwang SY, Solter D, Knowles BB. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development (Cambridge, UK) 1997;124:493–503. doi: 10.1242/dev.124.2.493. [DOI] [PubMed] [Google Scholar]
- 40.Yan C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 41.Baarends WM, et al. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol. 2005;25:1041–1053. doi: 10.1128/MCB.25.3.1041-1053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roest HP, et al. The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice. Mol Cell Biol. 2004;24:5485–5495. doi: 10.1128/MCB.24.12.5485-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kono T, et al. Epigenetic modifications during oocyte growth correlates with extended parthenogenetic development in the mouse. Nat Genet. 1996;13:91–94. doi: 10.1038/ng0596-91. [DOI] [PubMed] [Google Scholar]
- 44.Obata Y, et al. Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryogenesis. Development (Cambridge, UK) 1998;125:1553–1560. doi: 10.1242/dev.125.8.1553. [DOI] [PubMed] [Google Scholar]
- 45.Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo. New York: Cold Spring Harbor Lab; 1986. [Google Scholar]
- 46.Messinger SM, Albertini DF. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- 47.Nesbitt MN, Francke U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma. 1973;41:145–158. doi: 10.1007/BF00319691. [DOI] [PubMed] [Google Scholar]