Abstract
Sperm aging is known to be detrimental to reproductive performance. However, this apparently general phenomenon has seldom been studied in an evolutionary context. The negative impact of sperm aging on parental fitness should constitute a strong selective pressure for adaptations to avoid its effects. We studied the impact of sperm aging on black-legged kittiwakes (Rissa tridactyla), a monogamous seabird. Kittiwakes comprise a model system because (i) of evidence that females eject their mates' sperm to prevent fertilization by sperm that would be old and degraded by the time of fertilization and result in reduced reproductive performance and (ii) the lack of extra-pair fertilization in this species makes cryptic female choice an unlikely explanation of postcopulatory sperm ejection by females. We experimentally manipulated the age of the sperm fertilizing kittiwake eggs by fitting males with anti-insemination rings for variable periods of time preceding egg-laying. We found evidence that sperm aging negatively affected four sequential stages of reproduction: fertilization potential, rate of embryonic development, embryonic mortality, and chick condition at hatching. These results may be produced by a continuum of a single process of sperm aging that differentially affects various aspects of development, depending on the degree of damage incurred to the spermatozoa. The marked impact of sperm age on female fitness may thus drive postcopulatory sperm ejection by females. These results provide experimental evidence of deleterious effects of sperm aging on a nondomestic vertebrate, underlining its taxonomic generality and its potential to select for a wide array of adaptations.
Keywords: egg development, fertility, reproduction, sperm competition, sperm senescence
All cells undergo senescence. Spermatozoa, however, are especially susceptible to aging. Their highly concentrated nucleic DNA and reduced cytoplasm may result in DNA damage that is more likely to accumulate without repair (1). Furthermore, their high metabolic activity results in substantial exposure to oxidative stress, leading to extensive cellular damage over time (2).
Numerous studies have shown negative consequences of the functional decline of aging sperm on different aspects of reproduction, such as sperm motility, fertilization potential, or embryonic survival (3). For utilitarian reasons, the link between sperm aging and reproductive failure has mostly been studied in domestic livestock (4) and medicine (5). Early experimental studies in poultry show that embryo mortality increases with sperm age (6), with older sperm causing earlier developmental arrest (7). Additionally, Dharmarajan (8) showed that sperm aging is associated with slower embryonic development. Sperm aging has also been linked to reduced reproductive output in such taxa as marine invertebrates, Drosophila spp., and amphibians (3, 9). However, with the exception of several insect studies (10–12), this link has only rarely been addressed from an evolutionary perspective.
Our aim was to test the effects of sperm aging on reproduction by using an experimental approach in a wild bird species, the black-legged kittiwake (Rissa tridactyla). Whereas most previous studies on sperm aging focused on one or two aspects of reproduction, our experiment was designed to examine its effects at four levels of reproduction: fertilization potential, rate of embryonic development, embryonic survival rate, and chick condition at hatching.
Kittiwakes comprise a model system for two reasons. First, there is evidence that females eject their mates' sperm to prevent fertilization by sperm that would be old and degraded by the time of fertilization and result in reduced reproductive performance (13). Second, unlike in other species where postcopulatory sperm ejection by females is a form of cryptic mate choice (14, 15), in kittiwakes this explanation is unlikely because (i) females have never been observed soliciting extra-pair copulations, (ii) <1% of all copulations were extra-pair and all were initiated by males (16), and (iii) a DNA study revealed that extra-pair fertilizations are nonexistent or very rare in this socially monogamous species (16).
We manipulated sperm age by fitting males with anti-insemination rings (ARs) that prevented sperm transfer by preventing cloacal contact between mates during copulation attempts, a method successfully applied to songbirds (17). By varying the number of days these rings were worn before fertilization, we were able to obtain eggs fertilized by sperm of varying minimum ages and monitor the development of the embryo. To control for any potential effects caused by the rings themselves, the experimental males were compared with a second group of males fitted with control rings (CRs) that did not prevent them from inseminating their mates. This method allowed us to examine the impact of sperm aging on reproductive performance and whether this impact may comprise a selective pressure shaping adaptations that counter negative effects. More specifically, our experiment tested the hypothesis that female kittiwakes eject sperm to avoid the detrimental effects of fertilization by old sperm (13).
The ability to study kittiwakes in the field has provided the opportunity to evaluate how representative the study of sperm aging in domestic fowl may be of wild and phylogenetically distant species. Domestic chickens (Gallus gallus) and black-legged kittiwakes constitute contrasting model systems. Whereas chickens have been artificially selected for thousands of generations to produce eggs daily, kittiwakes produce only two eggs per year. Because of this and other differences, it is important to assess the degree to which results obtained from artificially selected domestic fowl are representative of nondomestic species.
Results
Efficacy of ARs.
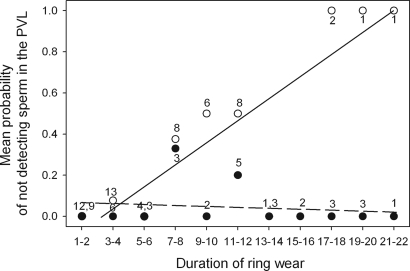
Anti-insemination ring (AR) pairs laid a significantly higher proportion of eggs in which no sperm or holes were found in the perivitelline layer (PVL) than control ring (CR) pairs [(mean ± SE) AR: 24.19% ± 5.46, n = 62; CR: 5.0% ± 3.48, n = 40; generalized linear mixed model (GLMM), F1,102 = 4.0, P = 0.05]. Furthermore, the AR wear duration had a highly significant negative effect on the probability of our finding sperm in the PVL (logistic regression: X1,372 = 19.70, P < 0.0001), whereas no such effect was found for the CR eggs (logistic regression: X1,252 = 0.16, P = 0.69) (Fig. 1). CR eggs showed the same level of hatching success as unmanipulated eggs [(mean ± SE) CR: 87%± 5.56, n = 38; unmanipulated: 83%± 5.0, n = 58; GLMM, F1,96 = 0.28, P = 0.60). Thus, the ARs were apparently effective in preventing insemination, whereas CRs allowed sperm transfer.
Fig. 1.
Mean probability of an egg not receiving sperm (indicated by the absence of spermatozoa or holes in the PVL) according to the duration of wear of the ring (number of days from the fitting of the device until two days before laying, when we assume fertilization occurs) for AR eggs (open circles) and CR eggs (filled circles). Numbers represent the number of eggs for each category of duration of wear. When there are two numbers, they represent the sample size for ARs and CRs, respectively. The interaction between treatment and duration of wear was highly significant (GLMM, F1,102 = 60.36, P < 0.0001, n = 102 eggs, with nest included as a random variable).
Effect of Treatment on Egg Mass.
There was no significant difference in egg mass [(means ± SE) AR: 49.86 ± 0.51 g; CR 49.06 ± 0.82 g; GLMM, corrected for egg rank, F1,85 = 1.40, P = 0.24) according to the experimental treatment, indicating that the treatment did not cause females to alter their reproductive investment in terms of egg size. Additionally, because egg mass reflects female quality in kittiwakes (18) and other seabirds (19, 20), this finding suggests that the treated pairs were randomly selected with regard to individual quality.
Effect of Treatment on Hatching Rate.
Because females may not have been inseminated before the fitting of the ARs or may have actively ejected or passively lost stored sperm (21) during the experiment, hatching failure in some eggs may have been caused by a lack of sperm. To ensure that sperm age, and not sperm presence, was the only factor manipulated, we excluded all eggs in which no sperm were detected (15 AR and 2 CR eggs), yielding a sample size of 41 AR eggs and 36 CR eggs.
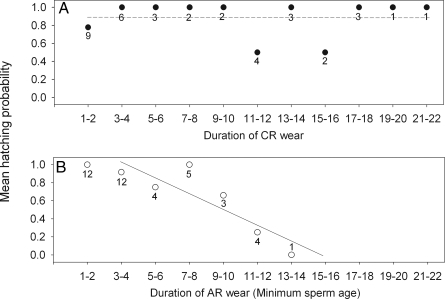
We found that hatching probability decreased significantly with duration of AR wear (logistic regression, X1,262 = 15.22, P < 0.0001) (Fig. 2B), whereas no such effect was found for CR eggs (logistic regression, X1,242 = 0.07, P = 0.79) (Fig. 2A), suggesting that sperm age has a strong deleterious effect on hatching.
Fig. 2.
Mean hatching probability according to duration of wear (number of days from the fitting of the ring until fertilization) of CR (A) and AR (B). In B, duration of wear is equal to minimum sperm age. Numbers represent the number of eggs for each category of duration of wear. The interaction between treatment and duration of wear was significant (GLMM, F1,25 = 12.02, P = 0.002, n = 77 eggs, with nest included as a random variable). When we removed the data point of 13–14 days in B, the interaction remained significant (F1,24 = 11.63, P = 0.0024).
The eggs that failed to hatch belonged to two categories: eggs where an embryo developed but died before hatching (Category I) and eggs where sperm were found in the PVL but where the blastodisc showed no sign of fertilization (Category II). When we excluded the eggs of Category II from the analysis, thus considering only embryonic survival, we found that hatching probability decreased significantly with the duration of AR wear (logistic regression, X1,192 = 5.16, P = 0.02), whereas no effect was found for CR eggs (logistic regression, X1,192 = 0.33, P = 0.56), suggesting that sperm aging has a deleterious effect on embryonic survival. An important consideration is whether the eggs of Category II received sperm in sufficient numbers to be fertilized, a possibility we next examined by studying sperm numbers and their dynamics.
Effect of Treatment on Numbers of Sperm and Holes.
In four of the AR eggs that failed to hatch, we found no visible signs of fertilization. However, we counted 118, 476, 878, and 1,752 spermatozoa in the PVL of these eggs. One explanation for hatching failure may be that these eggs had not received sufficient sperm at the time of fertilization. It is not known how many sperm are required to fertilize eggs of nearly all wild species, including kittiwakes. Furthermore, these eggs were opened after 11 days of incubation (see Materials and Methods), producing an inevitable decrease in the detectability of sperm. To study the effects of our treatment and incubation time on the number of sperm and holes counted, we built a reference by examining eggs from nonmanipulated and manipulated pairs after different incubation durations. Thus, we were able to estimate the number of sperm that were initially present in the PVL of the experimental eggs before the onset of incubation. We found that 12 of the 13 AR eggs contained numbers of sperm within the range of unmanipulated fertile eggs, 93% of which hatched [supporting information (SI) Fig. S1], providing evidence that even though the treatment reduced the number of sperm reaching AR eggs, these eggs typically received sufficient sperm to be fertilized.
Effect of Treatment on Rate of Embryonic Development.
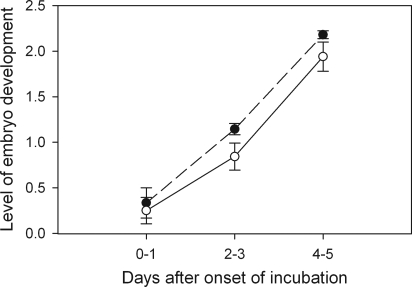
We used egg candling to examine the rate of early development of the embryo. There was no difference in the rate of embryonic development between nonmanipulated eggs and CR eggs (GLMM, F1,126 = 0.08, P = 0.77, using nest as a random variable). This finding indicates that the device itself (ring and harness) had no negative effect on development. Thus, we combined both control groups (CO) and compared them with the AR group. The rate of embryo development was significantly higher in the CO group than in the AR group during the first 5 days of development (GLMM, F1,149 = 9.82, P = 0.002, using nest as a random variable) (Fig. 3). From the age of 6 days onward, most eggs were in a sufficiently advanced state of development that any possible differences in development became indistinguishable (Fig. S2), and, as expected there were no significant differences among the treatments (GLMM, F1,113 = 2.32, P = 0.13).
Fig. 3.
Level of development of embryos from AR (open dots,, continuous line) and CR (filled dots, dashed line) eggs according to the number of days after onset of incubation. The level of development was obtained by using a candling technique and was measured by using a predefined scoring system ranging from 0–3 (see Fig. S2). Error bars represent the standard error.
Effect of Treatment on Chick Condition at Hatching.
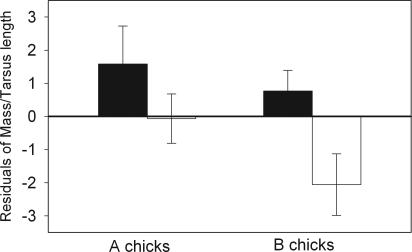
We found no effect of the experimental treatment on hatchling condition in both years combined (GLMM, F1,59 = 0.54, P = 0.46, using mass as the dependent variable, tarsus length as the independent covariable, and nest as a random variable). However, because there appeared to be a year effect on chick condition (F1,59 = 4.35, P = 0.052), we examined each year separately. Additionally, because “B chicks,” hatching from the second egg, tended to be in poorer condition than “A chicks” (F1,11 = 3.67, P = 0.08), we also separately compared the effect of the experimental treatment on A and B chicks. Examining a small sample in 2005, we found no significant difference in body condition between AR and CR chicks regardless of egg rank [generalized linear model (GLM), F1,21 = 0.5, P = 0.49, n = 15]. In 2004, we found a difference in hatchling condition between AR and CR treatments which was significant in B (GLM, F1,16 = 5.36, P = 0.03) but not in A chicks (GLM, F1,16 = 1.44, P = 0.25) (Fig. 4). Although we found no significant effect of the duration of AR wear on chick condition (GLM, F1,39 = 0.87, P = 0.37), the mean sperm age for AR B chicks was 4.5 ± 0.7 days (n = 12, range: 1–10 days), whereas in the CR group, it was likely that eggs were fertilized by young sperm (<1 day old).
Fig. 4.
Mean chick-body condition on hatching day according to treatment in 2004 in CR (filled bars) and AR (open bars) chicks. A chicks hatched from the first egg of a clutch and B chicks from the second. Error bars represent the standard error.
Sperm Age According to the Fate of the Eggs.
Within the AR group we investigated the mean minimum sperm age in three different categories: unfertilized eggs in which sperm were found (Category I), eggs in which the embryos developed but did not survive to hatching (Category II), and eggs that hatched (Category III). We found a significant difference in mean sperm age between these categories (GLMM, F2,13 = 9.18, P = 0.003, using nest as a random variable), with the highest sperm age in Category I (10.3 ± 0.66 days), the lowest in Category III (4.3 ± 0.51 days), and an intermediate value in Category II (7.0 ± 2.65 days).
Discussion
Our objective was to examine the reproductive consequences of sperm aging in a wild vertebrate species. We manipulated sperm age by using ARs (17), which were effective in preventing insemination during the period they were worn by males. The longer the duration of AR wear, the less likely we were to detect sperm in the PVL of the eggs, suggesting that females were not able to replenish their sperm stores after the AR was fitted on their mates. No such effect was found in the CR group, indicating that CRs enabled normal insemination during the time they were worn by males. This condition implies that for the AR group, eggs were fertilized by sperm inseminated before the fitting of the ring. Consequently, duration of AR wear may be considered as equivalent to minimum sperm age. Our experimental design allowed us to identify deleterious effects of sperm aging on four stages of reproduction: fertilization potential, rate of embryo development, embryo mortality, and chick condition at hatching.
Fertilization Potential.
Some eggs of AR pairs with long durations of ring wear showed no sign of fertilization. Nevertheless, spermatozoa and points of hydrolysis caused by spermatozoa were found in the PVL of some of these eggs, indicating that they were exposed to sperm as they passed through the female tract. It is possible that the lack of fertilization was because of insufficient sperm numbers. However, two lines of evidence suggest that this possibility is unlikely. First, sperm counts according to duration of incubation indicate that the 11 days during which these eggs were incubated caused as much as a fourfold underestimation of the number of sperm initially present in the PVL and by extension, at the time of fertilization (see SI Text). Second, when correcting for the decrease in detection of spermatozoa with incubation, we found that 12 of 13 AR eggs that showed no visible sign of fertilization contained sperm in numbers within the range of unmanipulated fertilized eggs (Fig. S1). As a result, it seems that past a certain age, the damage incurred by the sperm is deleterious to the extent that they lose their ability to fertilize. This effect has been suggested by a number of studies (see ref. 3 for review), including in poultry (6, 7). Those studies did not, however, account for sperm numbers and thus were unable to separate effects of reduced sperm numbers and sperm aging. The decrease in fertilization potential shown here may be explained by decreased sperm motility (9), inability of the spermatozoa to mount an acrosome reaction (22), or dysfunction of the very process of fertilization at the prometaphase stage (23). It is also possible that fertilization successfully occurred but that the damage was such that embryonic development ceased at a very early stage. In mice, it was shown that DNA-damaged sperm can fertilize oocytes at the same rate as intact-DNA sperm but that the damage may reduce blastocyst formation to < 5% (24).
Rate of Embryo Development.
The second developmental step associated with a harmful effect of sperm aging was slowed embryonic development in its earliest stages. Detrimental effects of sperm aging on embryonic development and survival have been reported in several different vertebrate and invertebrate taxa (3), including domestic chickens, in which it was noted that eggs fertilized by older sperm produced embryos with retarded development and numerous lesions in the central nervous system, as well as in the vascular system (8).
Embryo Mortality.
Our findings in kittiwakes are consistent with early experimental studies of domestic chickens, where it was shown that embryo mortality increases with sperm age (6), with older sperm causing earlier developmental arrest (7). The processes explaining deleterious effects on both embryo development and survival are not well known, although mechanisms such as chromosomal structure rearrangements (25) or lack of sperm chromatin decondensation (26) have been suggested.
Chick Condition at Hatching.
Lastly, this experimental study provides evidence that in kittiwakes, old sperm may also lead to reduced offspring condition. It is possible that the sperm damage that causes slower embryonic development and embryo mortality may also cause chicks to be in poorer condition if it occurs to a lesser degree and the embryo survives. Two elements from the results give us some insight on the way in which sperm aging may act on chick condition. First, we found a difference in body condition between chicks that were sired by AR- and CR-wearing males in 2004 but not with a small sample size in 2005. It appears that 2004 was a year of low food availability in our population, in that fledging success was less than half that of 2005 (unpublished data). Second, within the 2004 sample, the reduction in chick condition in the experimental treatment was significant only for B chicks, which hatch from smaller eggs and therefore receive fewer nutrients. These two findings suggest that sperm aging may interact with the nutrient content in the egg. It may be that the higher nutrient level in all A and B eggs of 2005 compensated for the deleterious effects of sperm age. Conversely, these effects were exacerbated when nutrient levels were particularly low, as was the case for the 2004 B eggs. Our result in 2004 supports correlative evidence of an impact of sperm age on offspring condition obtained in the same species (13) and may, to our knowledge, comprise the first experimental evidence of an effect of sperm aging on offspring condition.
Degree of Impact According to Sperm Age.
Our experimental manipulation of sperm age has provided evidence of a wide range of deleterious effects varying in stage and severity. When we compared the mean sperm age of eggs according to their fate, we found a gradation whereby eggs that showed no sign of development were fertilized by older sperm than those that hatched, whereas sperm of eggs producing failed embryos were at an intermediate age. This finding seems to indicate that the older the sperm, the earlier the stage at which reproduction is affected and thus the more severe the impact on normal development. Thus, it is plausible that the different effects observed constitute the continuum of a single phenomenon, whereby the same process of aging creates deleterious effects on different aspects of reproduction, depending on the degree of damage incurred to the spermatozoa.
Generality of the Deleterious Effects of Sperm Aging.
Despite marked life history differences between kittiwakes and poultry, the deleterious effects shown in this study are remarkably concordant with those found in poultry (6–8) and in other taxa, ranging from invertebrates to mammals. Old sperm have been found to cause slower embryonic development in the sea urchin Arbacia punculata and abnormal development in a marine worm Nereis limbata. Aging sperm is also associated with early developmental arrest and embryonic mortality in both aforementioned species, as well as in Drosophila melanogaster and in the frog Xenopus laevis (3, 9). These deleterious effects found at various stages of ontogeny closely match those we have found in this study in a single species, although taxonomically distant to those cited above. The detrimental impact of sperm aging on reproduction thus appears to be a general phenomenon among sexually reproducing animals.
Evolution of Counteradaptations.
Given the apparent ubiquity of sperm aging, a number of strategies countering these deleterious effects are likely to have evolved (27). Accordingly, physiological adaptations have been identified in males (28) and females (29) that help reduce the functional deterioration rate of spermatozoa. Moreover, behavioral mating strategies limiting the use of old sperm would confer a substantial reproductive advantage and are therefore likely to be favored by selection. In this respect, our experimental results support the interpretation from a correlative study (13) that sperm ejection by female kittiwakes was aimed at avoiding fertilization by old sperm (30). They also allow us to exclude alternative explanations of female sperm ejection, such as that, by discarding early inseminations, females minimize the number of parasites or pathogens transmitted via copulation (31). Using similar logic, the semen itself may be harmful to females (32, 33). Either pathogens or certain compounds in semen might therefore reduce reproductive success, producing a selective advantage to females that eject extraneous inseminations. Both of these hypotheses predict that a reduced number of inseminations should increase reproductive success; however our experiment produced the opposite results. Therefore, the avoidance of old sperm appears to be a likely cause of sperm ejection by female kittiwakes.
Given the occurrence of sperm ejection, the question is raised why old sperm reached the eggs when females might have ejected them. This may have occurred in the eggs that received no sperm. However, many eggs did receive sperm, because the maximum sperm ejection rate was 40% (13). Another question raised is why pairs copulate long before laying if associated costs are so high. This issue has been addressed by Helfenstein et al. (34), who noted that even the rare occurrence of extra-pair copulations may induce sperm competition and produce trade-offs between paternity assurance and the effects of old sperm.
Consequences of Sperm Aging on Reproductive Strategies.
More generally, our results have ramifications for the study of sperm competition and sexual selection. For example, choice of young sperm may be a more parsimonious explanation of female choice of successful males in lekking species, that is, by choosing males that copulate frequently, females may increase the probability of being inseminated by freshly produced sperm (27). Fertilization by young sperm may thus suggest an explanation of female mate copying. Further, it may provide an additional explanation of why the copulation rate in most bird species peaks soon before fertilization (35), as occurs in kittiwakes (16). By increasing their copulation rate soon before fertilization, pairs may increase the proportion of young to old sperm. Finally, sperm aging may comprise the elusive mechanism that causes the last of several males that copulate with one female to obtain most fertilizations (36). In other words, last-male sperm precedence might be explained by “young sperm precedence.”
Conclusions
Our study experimentally examined the effects of sperm aging at multiple developmental stages in a nondomestic vertebrate. The specific mechanisms causing the detrimental effects at each step remain largely unknown and require further study. However, it appears that the multiple effects observed in this study may form a continuum in which sperm senescence affects different stages of reproduction, depending on the degree of cellular damage incurred by the sperm. The marked similarity in effects of sperm aging on reproduction observed in a large number of taxa of contrasting life-history traits and reproductive biology highlights the generality of this process, which has not yet received the attention it merits in an evolutionary context. Sperm aging has important consequences on fitness and may constitute a selective pressure driving the evolution of a wide array of physiological and behavioral strategies to reduce its impact.
Materials and Methods
Study Population.
The study was conducted in the breeding seasons (May–July) of 2004–2006 on a colony of approximately 1,000 black-legged kittiwakes nesting on an abandoned U.S. Air Force radar tower on Middleton Island (59° 26′N, 146° 20′ W), Gulf of Alaska. The approximately 400 nest sites created on the upper walls were viewable from inside the tower through sliding one-way mirrors. This setup enabled us to easily capture and monitor the breeders.
Experimental Procedures.
We captured males after they and their mates had initiated nest building and fitted them with ARs designed to prevent cloacal contact, and thus insemination, during copulation. These devices consisted of a rubber ring 2.5 cm in diameter and 1.5 cm in height, glued to the feathers and skin around the cloaca and reinforced with a harness (see Fig. S3). We adapted this method from Michl et al. (17, see also 37). The harness, which comprised elastic straps (Elastoplast), was fitted around the tail and the back of the bird, causing the ring to remain fixed in place for the duration of the experiment. From inside the tower, we observed the male at least twice a day (from a maximum distance of 20 cm) (Fig. S3b) to verify that the AR remained fixed. To control for any potential effects caused by the ARs, a second group of males was fitted with CRs which were identical to the ARs, except that the ring was much thinner (4 mm) and did not prevent males from inseminating their mates. Once their clutch was complete, most males were recaptured and the devices were removed. All males that we were not able to recapture eventually lost their rings and harnesses by the end of the reproductive season through desquamation of skin and feather molting.
The rings were inconspicuous, as they were covered by the surrounding feathers and they allowed males to defecate normally (Fig. S3). We detected no sign of stress or unusual behavior. CR males displayed normal mating behavior with copulations comprising several cloacal contacts. AR males were regularly seen mounting their mates and attempting cloacal contacts, but unlike CR males, they were never observed succeeding.
The banding of all males allowed us to ensure that no individual males were used in both years of the manipulation. Because kittiwakes are breeding-site faithful (38) and we used different nest sites in the two years, we assume that the 31% (39 of 126) of females that were unbanded in the first year were also used only once, as was the case for all banded females.
Rings were fitted randomly over a period of 16 days preceding egg-laying. As a result, in the AR group, eggs that were fertilized were done so by sperm that were inseminated before the fitting of the AR. The duration of AR wear (time between the fitting of the AR and fertilization) was therefore equal to the minimum age of the sperm available to fertilize the egg. We were thus able to study the rates of embryonic development and embryonic mortality and the hatching success of eggs fertilized by spermatozoa that ranged in age from 0–14 days. Because the exact duration between the fertilization and the laying of the egg is unknown in most wild species, including kittiwakes, we assumed that it occurred between 1 and 2 days before the egg was laid (29). Thus, males that wore a ring for <2 days before egg laying were excluded from the analyses. The experimental procedure was approved by the U.S. Fish and Wildlife Service.
Counting Spermatozoa and Holes in the PVL.
We estimated sperm numbers to evaluate the effect of reduced sperm numbers versus sperm aging on fertilization. To determine whether eggs with no visible sign of development (20 AR eggs and 4 CR eggs) were exposed to sperm at the site of fertilization, we counted the number of spermatozoa trapped in the PVL of the egg yolk by using a standard technique (39) (see SI Text for further details). Because incubation time is known to affect sperm counts (40), we carried out a side study (see SI Text for details) to estimate sperm numbers correcting for the duration of incubation.
Embryonic Development.
On the day of laying, all eggs were numbered with a nontoxic indelible marker and were measured and weighed to the nearest gram. To control for any potential effects in incubation behavior caused by the rings, eggs were removed from the manipulated pairs and either incubated by unmanipulated pairs (in 2004) or placed in artificial incubators (in 2005). The hatching rate of artificially incubated CO eggs did not differ from that of naturally incubated CO eggs [(mean ± SE) artificial: 92.5% ± 3.2, n = 66; natural: 93.7 ± 2.5, n = 95; corrected for egg rank, GLM, F1,161 = 0.20, P = 0.66].
Embryonic development was monitored by candling eggs one to three times at various stages between their laying date and the age of 11 days. Eggs from the same clutch were candled at the same time to minimize disturbance of incubation behavior. By monitoring over 100 eggs, we found that normal development is characterized by an initial darkening and spreading of the chorioallantoic membrane (2–3 days after the beginning of incubation) until the egg becomes nearly opaque (≈8–11 days). Embryonic development was measured by using scores ranging from 0 (freshly laid egg, no development) to 3 (egg totally opaque) (Fig. S2). In a pilot season in 2003, we encountered a small number of eggs that showed no sign of development until 10 days after laying. This exceptionally long period was likely because of a combination of factors such as a delay in the onset of incubation as well as an unusually thick or dark shell that made it difficult to detect early development. To include every potentially fertilized egg, all eggs were conservatively incubated for at least 11 days. Those that did not exhibit “yolk spreading” 11 days after being laid were opened and placed in a Petri dish and were frozen at −20°C for subsequent sperm count analyses. Eggs that showed yolk spreading within 11 days were left to develop normally. After that stage, if the egg emitted an odor, if no embryo movement was detected, or if the egg did not hatch within 32 days (all successfully hatched chicks did so within 30 days), the egg was opened and the embryo was photographed and weighed to determine the stage of developmental arrest. Artificially incubated eggs were placed with foster parents as soon as external pipping occurred. All chicks were weighed, measured (tarsus length), and blood sampled within 12 h of hatching.
Data Analyses.
We included in the analyses only the pairs where ARs and CRs remained in place from the time they were fixed until 2 days before egg-laying and where eggs were properly incubated by the foster parents and undamaged, yielding a total of 102 eggs from 62 clutches. Because most clutches comprised two eggs, the differences in fertilization, hatching and development rate could possibly be explained by parents investing more in either the first or second egg. Furthermore, including eggs from the same nest may lead to biases in the analyses because of pseudoreplication. To avoid these effects, we (i) compared experimental and CO eggs and (ii) carried out analyses by using GLMMs (41) (the GLIMMIX Macro and SAS (1999) were used in the case of discrete variables), adding the nest as a random effect. In analyses where this was not possible because of small sample sizes, we analyzed data by using only the first laid egg of each clutch. For all analyses, we verified the normality of the distribution of data and the homogeneity of variance across groups. Data were analyzed by using SAS (SAS Institute).
SI.
A graph of the log number of spermatozoa nuclei counted on the PVL of eggs according to the duration of incubation is available in Fig. S4. Table S1 lists the numbers of sperm nuclei counted in AR eggs incubated for a range of durations.
Supplementary Material
Acknowledgments.
We thank A. Ramey, E. Vigneron, A. Lambrechts, M. Kryloff, M. du Toit, and C. Gouraud for their assistance in the field; J.-P. Brillard for help with sperm-counting techniques; and T. Pizzari and T. R. Birkhead for constructive comments on previous versions of the manuscript. Experiments were carried out in accordance to United States' laws and under permits from the U.S. Fish and Wildlife Service and State of Alaska. This study was financed by a 4-year grant from the French Polar Institute Paul-Emile Victor (“Program Arctique 429” 2004–2007). E. Danchin and R. H. Wagner were funded by a Centre National de la Recherche Scientifique (Projets Internationaux de Coopération Scientifique no. 2410) grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.E.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803067105/DCSupplemental.
References
- 1.Vishwanath R, Shannon P. Do sperm cells age? A review of the physiological changes in sperm during storage at ambient temperature. Reprod Fert Dev. 1997;9:321–331. doi: 10.1071/r96088. [DOI] [PubMed] [Google Scholar]
- 2.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–1436. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 3.Tarin JJ, Perez-Albala S, Cano A. Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Update. 2000;6:532–549. doi: 10.1093/humupd/6.6.532. [DOI] [PubMed] [Google Scholar]
- 4.Salisbury GW, Hart RG. Gamete aging and its consequences. Biol Reprod Supplement. 1970;2:1–13. [PubMed] [Google Scholar]
- 5.Bomsel-Helmreich O. The aging of gametes, heteroploidy, and embryonic death. Int J Gynaecol Obstet. 1976;14:98–104. doi: 10.1002/j.1879-3479.1976.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 6.Lodge JR, Fechheimer NS, Jaap RG. The relationship of in vivo sperm storage interval to fertility and embryonic survival in the chicken. Biol Reprod. 1971;5:252–257. doi: 10.1093/biolreprod/5.3.252. [DOI] [PubMed] [Google Scholar]
- 7.Nalbandov A, Card LE. Effect of stale sperm on fertility and hatchability of chicken eggs. Poultry Sci. 1943;22:218–226. [Google Scholar]
- 8.Dharmarajan M. Effects on the embryo of staleness of the sperm at the time of fertilization in the domestic hen. Nature. 1950;165:398. [Google Scholar]
- 9.Lanman JT. Delays during reproduction and their effects on the embryo and fetus. 1. Aging of sperm. N Engl J Med. 1968;278:993–999. doi: 10.1056/NEJM196805022781806. [DOI] [PubMed] [Google Scholar]
- 10.Jones TM, Elgar MA. The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc Royal Soc London Series B-Biol Sci. 2004;271:1311–1318. doi: 10.1098/rspb.2004.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhardt K, Siva-Jothy MT. An advantage for young sperm in the house cricket Acheta domesticus. Am Naturalist. 2005;165 doi: 10.1086/430010. [DOI] [PubMed] [Google Scholar]
- 12.Snook RR, Hosken DJ. Sperm death and dumping in Drosophila. Nature. 2004;428:939–941. doi: 10.1038/nature02455. [DOI] [PubMed] [Google Scholar]
- 13.Wagner RH, Helfenstein F, Danchin E. Female choice of young sperm in a genetically monogamous bird. Proc Royal Soc London Series B-Biol Sci. 2004;271:S134–S137. doi: 10.1098/rsbl.2003.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzari T. Evolution: Sperm ejection near and far. Curr Biol. 2004;14:R511–R513. doi: 10.1016/j.cub.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Pizzari T, Birkhead TR. Female feral fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- 16.Helfenstein F, Tirard C, Danchin E, Wagner RH. Low frequency of extra-pair paternity and high frequency of adoption in black-legged kittiwakes. Condor. 2004;106:149–155. [Google Scholar]
- 17.Michl G, Török J, Griffith SC, Sheldon BC. Experimental analysis of sperm competition mechanisms in a wild bird population. Proc Nat Acad Sci USA. 2002;99:5466–5470. doi: 10.1073/pnas.082036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas CS. The relationships between breeding experience, egg volume and reproductive success of the kittiwake rissa-tridactyla. Ibis. 1983;125:567–574. [Google Scholar]
- 19.Amundsen T, Stokland JN. Egg size and parental quality influence nestling growth in the shag. Auk. 1990;107:410–413. [Google Scholar]
- 20.Reid WV, Boersma PD. Parental quality and selection on egg size in the magellanic penguin. Evolution. 1990;44:1780–1786. doi: 10.1111/j.1558-5646.1990.tb05248.x. [DOI] [PubMed] [Google Scholar]
- 21.Birkhead TR, Fletcher F. Depletion determines sperm numbers in male zebra finches. Animal Behaviour. 1995;49:451–456. [Google Scholar]
- 22.Miller BJ, Graves CN, Lodge JR. Effect of in vitro storage of bovine spermatozoa on acrosomal integrity, proteolytic activity, binding, and initiation of penetration of oocytes. Gamete Res. 1988;20:53–65. doi: 10.1002/mrd.1120200106. [DOI] [PubMed] [Google Scholar]
- 23.Smith AL, Lodge JR. Interactions of aged gametes—in vitro fertilization using in vitro-aged sperm and in vivo-aged ova in the mouse. Gamete Res. 1987;16:47–56. doi: 10.1002/mrd.1120160106. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Munne S, Estop A. The effect of in vitro aging on mouse sperm chromosomes. Hum Reprod. 1991;6:703–708. doi: 10.1093/oxfordjournals.humrep.a137412. [DOI] [PubMed] [Google Scholar]
- 26.Bjorndahl L, Kvist U. Loss of an intrinsic capacity for human-sperm chromatin decondensation. Acta Physiol Scandinavica. 1985;124:189–194. doi: 10.1111/j.1748-1716.1985.tb07651.x. [DOI] [PubMed] [Google Scholar]
- 27.Siva-Jothy MT. The young sperm gambit. Ecol Lett. 2000;3:172–174. [Google Scholar]
- 28.Breque C, Surai P, Brillard JP. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol Reprod Dev. 2003;66:314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- 29.Bakst MR, Wishart GJ, Brillard JP. Oviducal sperm selection, transport, and storage in poultry. Poultry Sci Rev. 1994;5:117–143. [Google Scholar]
- 30.Dean R, Bonsall MB, Pizzari T. Aging and sexual conflict. Science. 2007;316:383–384. doi: 10.1126/science.1142201. [DOI] [PubMed] [Google Scholar]
- 31.Westneat DF, Rambo TB. Copulation exposes female red-winged blackbirds to bacteria in male semen. J Avian Biol. 2000;31:1–7. [Google Scholar]
- 32.Brooks R, Jennions MD. The dark side of sexual selection. Trends Ecol Evol. 1999;14:336–337. doi: 10.1016/s0169-5347(99)01689-4. [DOI] [PubMed] [Google Scholar]
- 33.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila-melanogaster females is mediated by male accessory-gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 34.Helfenstein F, Wagner RH, Danchin E. Sexual conflict over sperm ejection in monogamous pairs of kittiwakes Rissa tridactyla. Behav Ecol and Sociobiol. 2003;54:370–376. [Google Scholar]
- 35.Birkhead T, Moller AP. Sperm Competition in Birds. evolutionary Causes and Consequences. San Diego: Academic; 1992. [Google Scholar]
- 36.Birkhead TR. In: Sperm Competition and Sexual Selection. Birkhead TR, Møller AP, editors. San Diego: Academic; 1998. [Google Scholar]
- 37.Fossoy F, Johnsen A, Lifield JT. Evidence of obligate female promiscuity in a socially monogamous passerine. Behav Ecol Sociobiol. 2006;60:255–259. [Google Scholar]
- 38.Fairweather JA, Coulson JC. Mate retention in the kittiwake, Rissa-tridactyla, and the significance of nest-site tenacity. Anim Behav. 1995;50:455–464. [Google Scholar]
- 39.Wishart GJ. Regulation of the length of the fertile period in the domestic fowl by numbers of oviducal spermatozoa, as reflected by those trapped in laid eggs. J Reprod Fertility. 1987;80:493–498. doi: 10.1530/jrf.0.0800493. [DOI] [PubMed] [Google Scholar]
- 40.Small AO, Schlusser K, Ryan CJ, Jamieson IG. Detecting sperm on the perivitelline membrane of incubated turkey eggs and its implications for research on fertility problems in endangered species. Wildlife Res. 2000;27:635–637. [Google Scholar]
- 41.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. NC: Cary; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.