Abstract
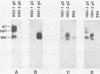
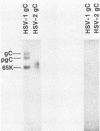
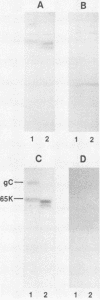
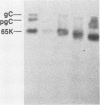
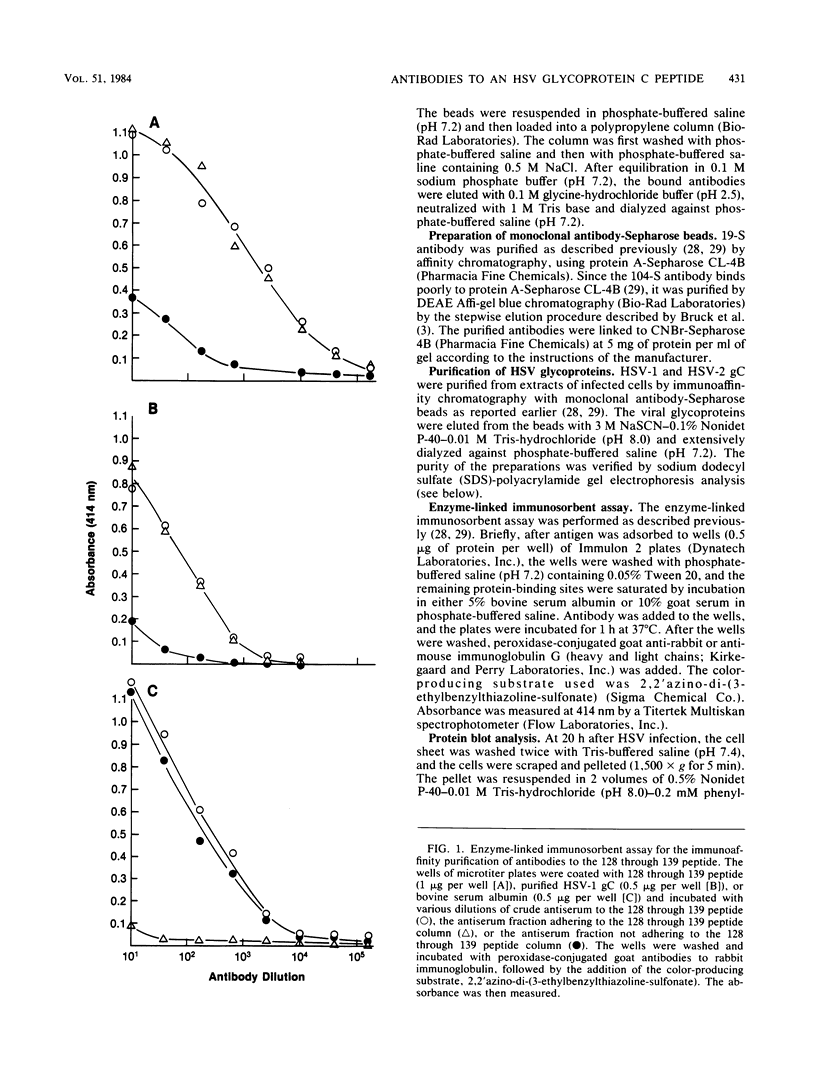
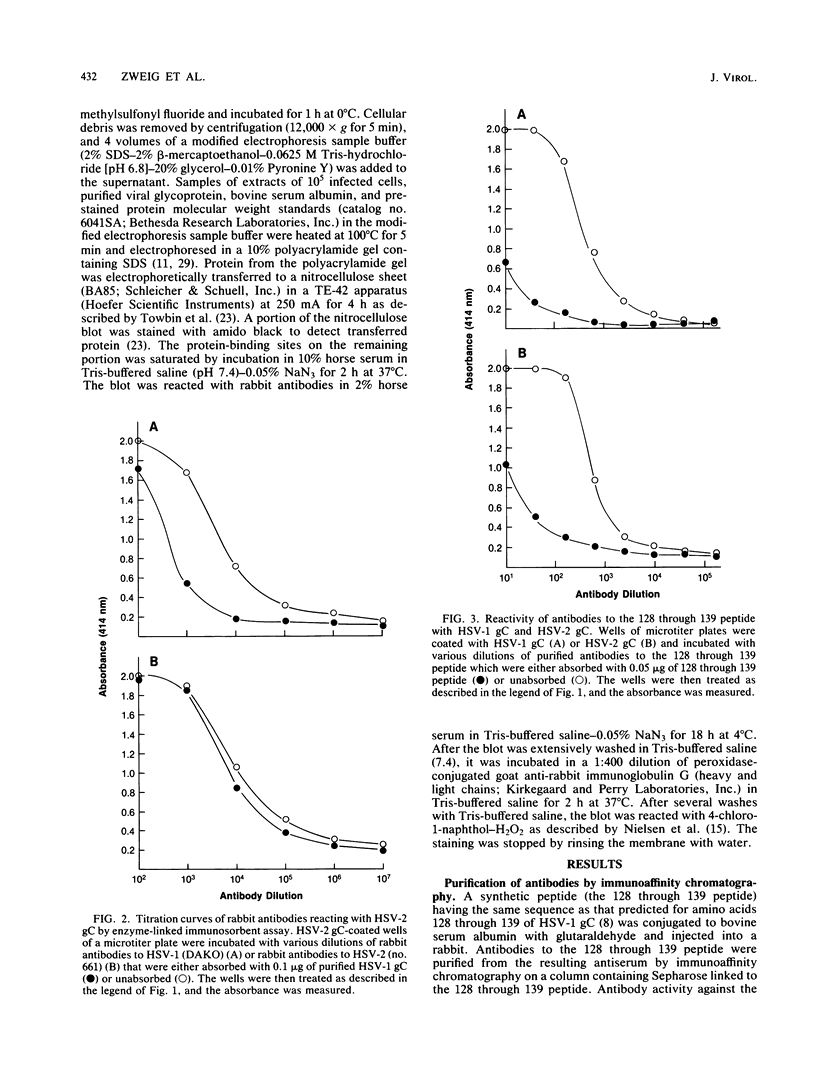
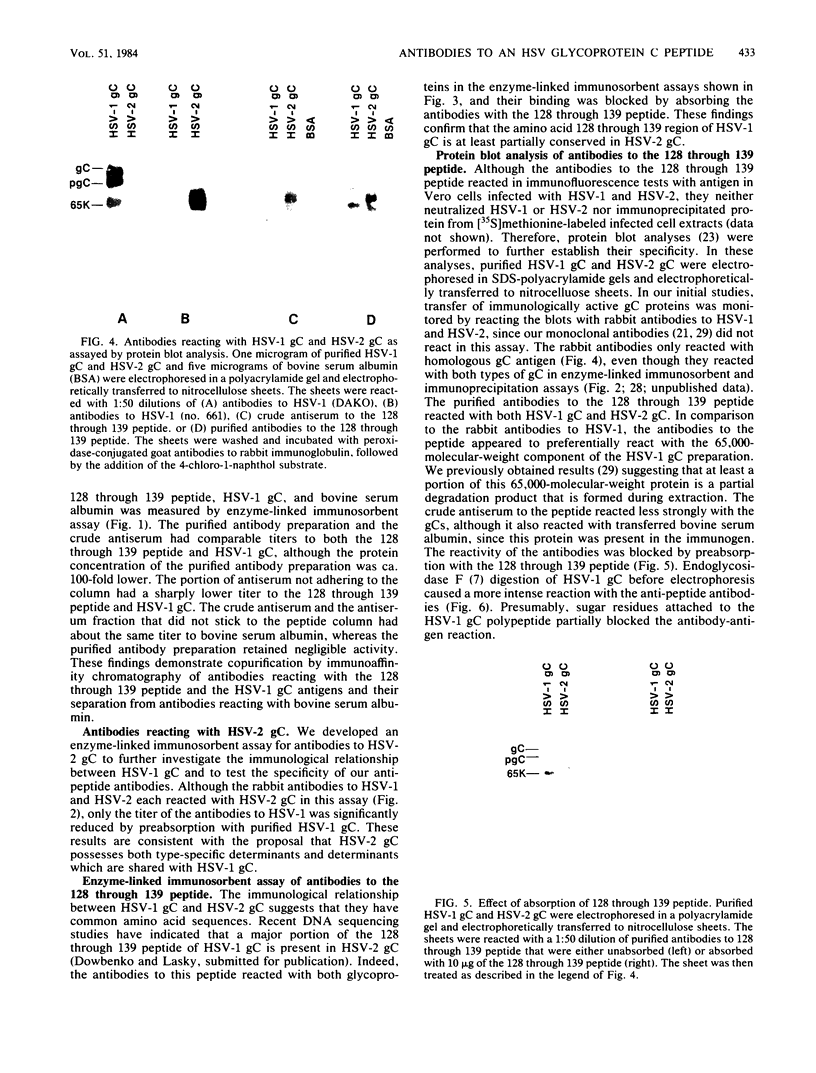
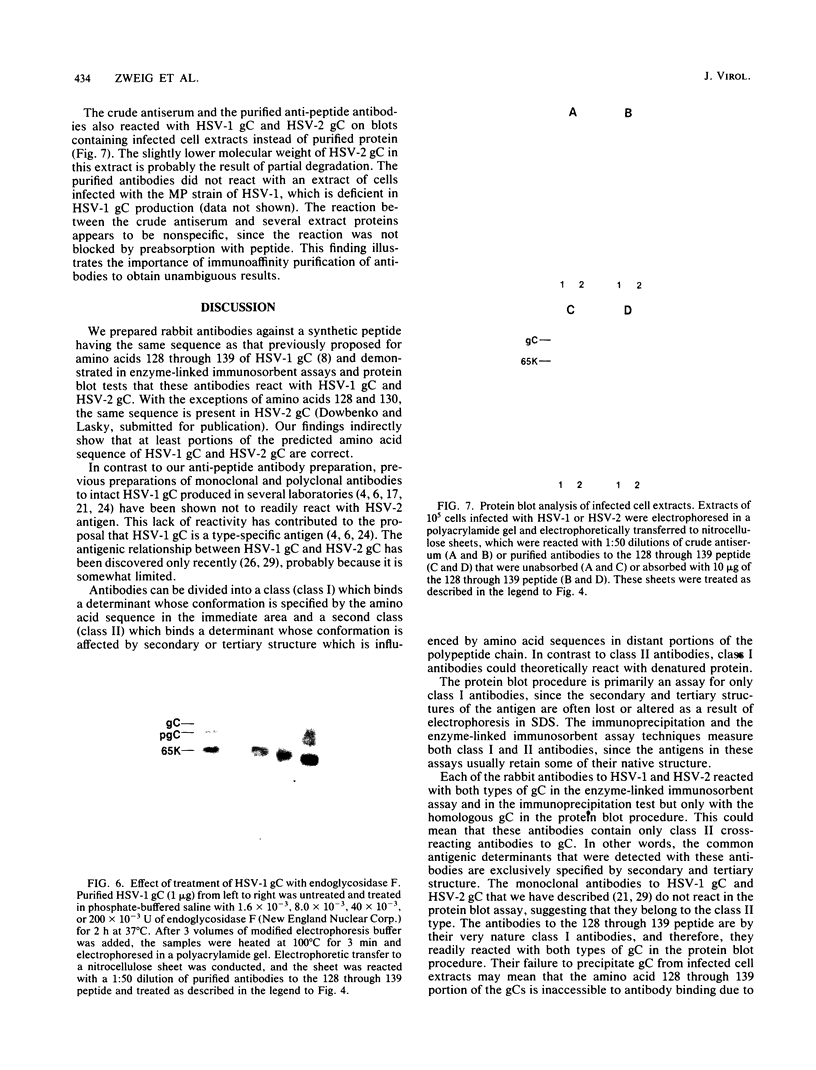
Nucleotide sequence and mRNA localization studies have allowed the prediction of the amino acid sequence of herpes simplex virus type 1 (HSV-1) glycoprotein C (gC). We immunized a rabbit with a conjugate of bovine serum albumin and a synthetic peptide having the same sequence as that deduced for amino acids 128 through 139 of HSV-1 gC. A very similar amino acid sequence has been predicted to exist in the related product, herpes simplex virus type 2 (HSV-2) gC, which was formerly designated gF. Preparations of crude antiserum and immunoaffinity-purified antibodies were obtained and shown to react in enzyme-linked immunosorbent assays with purified HSV-1 gC and HSV-2 gC. Although these antibodies did not detectably immunoprecipitate proteins from radiolabeled infected cell extracts, they reacted with HSV-1 gC and HSV-2 gC that were electrophoretically transferred to nitrocellulose membranes from polyacrylamide gels. These results confirm that HSV-1 gC and HSV-2 gC are immunologically related and also define a specific portion of HSV-1 gC that is conserved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Ching C. Y., López C. A type-specific antiserum induced bya major herpesvirus type 1 glycoprotein. J Immunol Methods. 1980;32(4):383–391. doi: 10.1016/0022-1759(80)90030-7. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., Manchester K. L., Towbin H., Gordon J., Thomas G. The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem. 1982 Oct 25;257(20):12316–12321. [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Savage T., Roizman B., Heine J. W. Immunological specificity of the glycoproteins of herpes simplex virus subtypes 1 and 2. J Gen Virol. 1972 Oct;17(1):31–48. doi: 10.1099/0022-1317-17-1-31. [DOI] [PubMed] [Google Scholar]
- Schneweis K. E., Nahmias A. J. Antigens of Herpes simplex virus type 1 and 2-immunodiffusion and inhibition passive hemagglutination studies. Z Immunitatsforsch Exp Klin Immunol. 1971 Jun;141(5):471–487. [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Bauer H., Birr C., Pipkorn R. Antibodies against synthetic peptides as a tool for functional analysis of the transforming protein pp60src. Cell. 1983 Sep;34(2):587–596. doi: 10.1016/0092-8674(83)90391-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F., Norrild B. Crossed immunoelectrophoresis of a herpes simplex virus type 1-specific antigen: immunological and biochemical characterization. J Infect Dis. 1978 Nov;138(5):639–643. doi: 10.1093/infdis/138.5.639. [DOI] [PubMed] [Google Scholar]
- Yeo J., Killington R. A., Watson D. H., Powell K. L. Studies on cross-reactive antigens in the herpesviruses. Virology. 1981 Jan 30;108(2):256–266. doi: 10.1016/0042-6822(81)90434-7. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Bladen S. V., Showalter S. D., Hampar B. Detection in antisera of antibodies that cross-react with herpes simplex virus type 1 glycoprotein gC. Infect Immun. 1983 Aug;41(2):482–487. doi: 10.1128/iai.41.2.482-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Showalter S. D., Bladen S. V., Heilman C. J., Jr, Hampar B. Herpes simplex virus type 2 glycoprotein gF and type 1 glycoprotein gC have related antigenic determinants. J Virol. 1983 Jul;47(1):185–192. doi: 10.1128/jvi.47.1.185-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]