Abstract
Infection of mice with sporozoites of Plasmodium berghei or Plasmodium yoelii has been used extensively to evaluate liver-stage protection by candidate preerythrocytic malaria vaccines. Unfortunately, repeated success of such vaccines in mice has not translated readily to effective malaria vaccines in humans. Thus, mice may be used better as models to dissect basic parameters required for immunity to Plasmodium-infection than as preclinical vaccine models. In turn, this basic information may aid in the rational design of malaria vaccines. Here, we describe a model of circumsporozoite-specific memory CD8 T cell generation that protects mice against multiple P. berghei sporozoite challenges for at least 19 months. Using this model we defined a threshold frequency of memory CD8 T cells in the blood that predicts long-term sterilizing immunity against liver-stage infection. Importantly, the number of Plasmodium-specific memory CD8 T cells required for immunity greatly exceeds the number required for resistance to other pathogens. In addition, this model allowed us to identify readily individual immunized mice that exceed or fall below the protective threshold before infection, information that should greatly facilitate studies to dissect basic mechanisms of protective CD8 T cell memory against liver-stage Plasmodium infection. Furthermore, the extremely large threshold in memory CD8 T cell frequencies required for long-term protection in mice may have important implications for development of effective malaria vaccines.
Infection of humans with Plasmodium species, the causative agents of malaria, results in severe morbidity and mortality in the developing world (1), effects that have stimulated intense efforts to develop efficacious vaccines. Protective CD8 T cell immunity against liver-stage Plasmodium infection has been demonstrated after vaccination of rodents with irradiated or genetically attenuated parasites and after subunit vaccination against liver-stage antigens (2–12). Immunity in rodents can last for 6–12 months (3, 4, 7, 13), but in several studies also seems to wane with time (7, 14–16). Although irradiated sporozoite vaccines also protect humans (17–19), current subunit vaccinations limit liver-stage infection but rarely prevent blood-stage parasitemia (20). Importantly, it remains unknown whether sterilizing long-term immunity to Plasmodium infection can be achieved through subunit vaccines that predominantly evoke memory CD8 T cell responses and, if so, precisely what memory T cell parameters will be required.
A single mosquito bite delivers a few hundred infectious Plasmodium sporozoites into dermal tissues (21), a fraction of which traffic to the liver and establish hepatocyte infection leading to release of blood stage parasites 2 days (P. berghei infection of mice) (22) or 6–8 days (P. falciparum infection of humans) (23) later. As such, infected cells may represent as few as 1 in 109 hepatocytes in humans and 1 in 106 hepatocytes in mice. Thus, both temporal and spatial challenges (analogous to rapidly finding a few needles in a haystack) must be overcome for Plasmodium-specific memory CD8 T cells to deal with all infected hepatocytes and prevent the symptomatic blood stage of infection. The use of mouse models of Plasmodium infection to determine how the immune system can be manipulated by immunization to overcome these challenges may have important implications for rational design of malaria vaccines. Filling this knowledge gap will require immunization models to reliably generate memory CD8 T cells that confer long-term immunity, so that the characteristics of these populations leading to protection can be defined. Here, we describe an immunization strategy that generates P. berghei circumsporozoite (CS)-specific memory CD8 T cells capable of protecting mice from multiple sporozoite challenges for at least 19 months. Studies with this model revealed that the threshold in memory CD8 T cell numbers required for long-term protection from sporozoite infection reflects a substantial fraction of the CD8 T cell compartment, a finding with potentially important implications for development of effective vaccines to protect against human malaria.
Results
Generation of CS-Specific Memory CD8 T Cells.
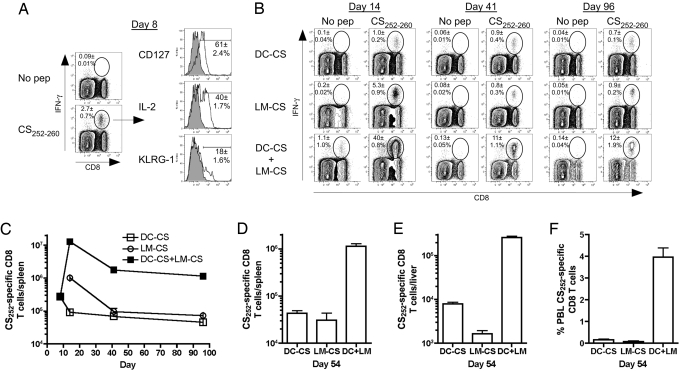
Protective immunity to infection may be influenced by both the functional attributes and numbers of memory CD8 T cells (24–26). We reasoned that the extremely low frequencies of infected hepatocytes might dictate that a large number of memory CD8 T cells would be required to ensure all infected liver cells are located and dealt with to prevent blood stage infection. To test this hypothesis, we made use of an accelerated “prime-boost” immunization strategy, developed in our laboratory, that rapidly generates large numbers of memory CD8 T cells (27). BALB/c mice initially were immunized with mature dendritic cells (DC) coated with a P. berghei epitope (CS252–260, also known as “Pb9,” DC-CS immunization) that is a target of protective CD8 T cells (8). As we reported for other epitopes (27), DC-CS immunization resulted in accelerated acquisition of memory characteristics (CD127hi, KLRG-1lo, IL-2+) by the responding CD8 T cells (Fig. 1A), including the ability to respond to booster immunization with recombinant attenuated (actA-, inlB-deficient) (28) L. monocytogenes expressing the CS252–260 epitope (here on referred to as “LM-CS252”) that is embedded within a secreted ovalbumin fusion protein and does not contain known antibody or CD4 T cell epitopes. This DC-CS + LM-CS immunization generated large frequencies (Fig. 1B) and total numbers (Fig. 1C) of effector and memory CS252-specific CD8 T cells that were >10-fold greater than generated by DC-CS or LM-CS252 immunization alone. Splenic memory CD8 T cell frequencies and total numbers (Fig. 1B and C) in all groups were maintained stably between day 41 and day 96 and, of critical importance for resistance to liver-stage Plasmodium infection, numbers of CS252-specific CD8 T cells in the liver and spleen were proportional at day 54 (Fig. 1D and E) and at day 72 (data not shown). Additionally, the frequency of CS252-specific CD8 T cells of all peripheral blood lymphocytes (PBLs) was proportional to the numbers of antigen-specific cells in the spleen and liver (Fig. 1F). Thus, DC-CS + LM-CS immunization rapidly generated large and stable populations of P. berghei-specific memory CD8 T cells in the spleen, PBL, and liver.
Fig. 1.
Generation and maintenance of P. berghei CS252-specific CD8 T cells. BALB/c mice were primed with 3 × 105 bone marrow-derived dendritic cells coated with CS252–260 (DC-CS). Seven days later DC-CS mice were boosted with 2 × 107 LM-CS252 (DC-CS + LM-CS) or naive mice were primed with 7 × 106 LM-CS252 (LM-CS). (A) Frequency and phenotype of splenic CD8 T cells that are CS252 specific as determined by ICS 8 days after DC-CS prime. (B) Frequency of splenic CD8 T cells that are CS252 specific on days 14, 41, and 96 as determined by ICS. Profiles from representative mice are shown; numbers represent mean ± SD; n = 3 per group. (C) Total number (mean ± SD, n = 3 per group) of CS252-specific CD8 T cells in the spleen on various days after initiation of immunization. Total number (mean ± SD, n = 3 per group) of CS252-specific CD8 T cells (D) in the spleen and (E) liver or (F) percent CS252-specific CD8 T cells of all PBL 54 days after initiation of immunization for the indicated groups. Data are representative of three experiments.
It should be noted that this immunizations strategy, based on a short peptide epitope prime and epitope-fusion protein boost, does not induce a detectable CD8 negative (i.e., CD4 T cell) IFN-γ response (data not shown). Also, antibodies induced by this vaccination would be directed either to the CS252-MHC class I complex (after DC-CS252 immunization) or to the short CS epitope embedded in the ovalbumin carrier protein (LM-CS252 boost). If such antibodies are induced, they are unlikely to react in a meaningful way with the conformationally intact CS protein expressed by P. berghei sporozoites. Thus, our immunization strategy permits a focus on the ability of CS252-specific CD8 T cells to provide immunity against sporozoite challenge.
DC-CS + LM-CS CD8 T Cells Prevent Blood-Stage Parasitemia.
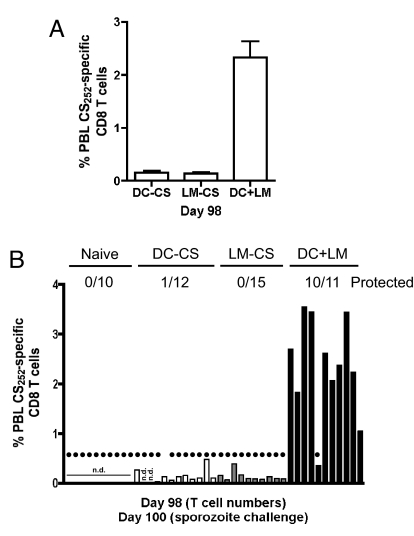
To evaluate individual-to-individual variability and to mimic sampling of humans, frequencies of memory CS252-specific CD8 T cells of all PBL were determined at day 98 in individual mice. DC-CS- and LM-CS252-immunized mice had <0.2% CS252-specific PBL, whereas DC-CS + LM-CS mice exhibited >2% CS252-specific PBL (Fig. 2A). This frequency represented a substantial fraction (>21%) of circulating CD8 T cells in the DC-CS + LM-CS mice (data not shown). All naïve and LM-CS252-immune and 11 of 12 DC-CS-immune mice developed blood stage parasitemia after sporozoite challenge (Fig. 2B). In contrast, 10/11 DC-CS + LM-CS mice were protected, with blood-stage parasitemia observed only in the mouse that had the lowest frequency of CS252-specific PBL (<0.5%). These data suggest that immunity to liver-stage parasites may depend on the numbers of antigen-specific memory CD8 T cells.
Fig. 2.
CS252-specific memory CD8 T cells afford protection for >3 months against a P. berghei sporozoite challenge. (A) Frequency of CS252-specific CD8 T cells in the PBL 98 days after priming. Data (mean ± SD) are from 10 to 12 mice per group. (B) Percentage total PBL that are CS252-specific CD8 T cells as determined by ICS in individual mice from the indicated immunization group before challenge. Filled circles indicate naïve or immune mice that developed blood-stage malaria after a challenge with 1000 P. berghei sporozoites. Numbers represent protected mice/total challenged for each group. n.d., not determined.
CS-Specific Memory CD8 T Cells Afford Long-Term Sterile Immunity.
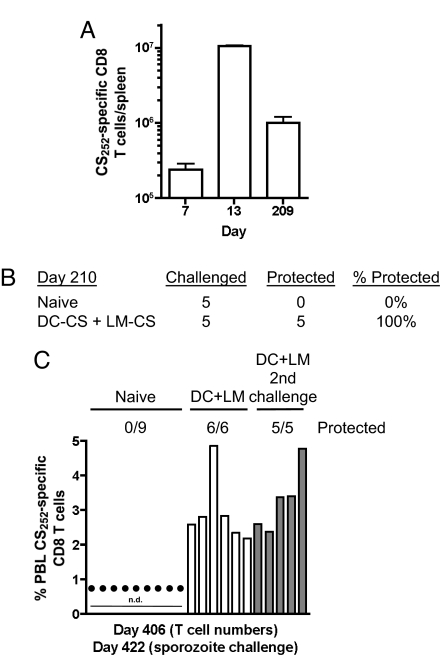
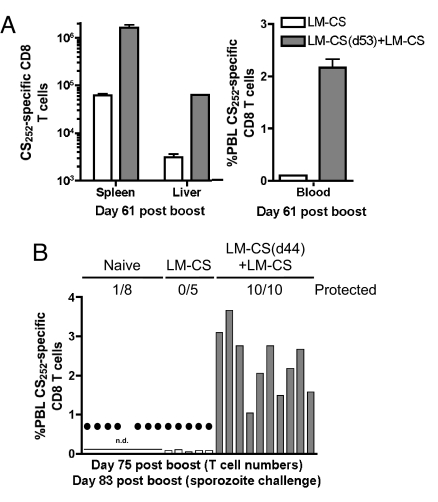
Although many vaccination strategies are successful in protecting rodents from sporozoite challenge for several months (29), the feasibility of long-term immunity based solely on memory CD8 T cells remains unknown. A representative analysis of DC-CS + LM-CS mice revealed ≈106 CS252-specific CD8 T cells per spleen on day 209 (Fig. 3A), a number similar to that observed at day 41 (Fig. 1). One hundred percent of additional mice from this group were protected from sporozoite challenge on day 210, indicating that CD8 T cells in DC-CS + LM-CS mice can protect for at least 7 months (Fig. 3B). Immunity in malaria-endemic areas must protect the host from repeated sporozoite exposures. To address this issue and to evaluate further the duration of memory and protection, CS252-specific PBL were determined at day 406 in the 5 mice that had resisted challenge at day 210 and in an additional group of unchallenged DC-CS + LM-CS mice (Fig. 3C). Both groups exhibited >2% CS252-specific PBL and were protected from sporozoite challenge at day 422 (Fig. 3C). Both groups of mice maintained high levels of CS252-specific CD8 T cells (>2% of all PBL on average), and all resisted additional sporozoite challenges at day 455, day 485, and day 565 (19 months after immunization, data not shown). Interestingly, we did not observe “boosting” in memory cell frequencies in these groups despite the repeated challenges (data not shown). This may result from the very large memory populations already present in the DC-CS + LM-CS immune mice or suggest that the amount of antigen present in the challenges was insufficient to cause noticeable boosting in memory numbers. However, it is possible that the repeated challenge infections induced antibody and CD4 T cell responses that contributed to resistance of multiply challenged mice. These issues are under investigation. Over multiple experiments, 136/141(>96%) of DC-CS + LM-CS mice were protected from initial sporozoite challenge from day 28 to day 422 after immunization. Thus, CD8 T cell protection can last for >14 months, and immunity is maintained up to 19 months in the face of multiple sporozoite challenges. Importantly, the ability to generate large numbers of CS252-specific memory CD8 T cells and sterilizing immunity to sporozoite challenge was not limited to DC-CS + LM-CS immunization but also could be achieved by boosting LM-CS252-immune mice at >40 days with a higher dose of LM-CS252 (LM-CS + LM-CS data in Fig. 4). These data demonstrate that single-epitope specific-memory CD8 T cells can provide long-term sterilizing immunity against Plasmodium sporozoite challenge.
Fig. 3.
Long-term protection against multiple P. berghei sporozoite challenges. BALB/c mice were primed with 5 × 105 splenic DC (sDC)-CS and boosted with 2 × 107 LM-CS252. (A) Total number (mean ± SD, n = 3 mice per day) of CS252-specific CD8 T cells in the spleen on various days after the start of immunization. (B) Naïve or DC-CS + LM-CS mice were challenged with 1000 P. berghei sporozoites on day 210. (C) The percent of total PBL that are CS252-specific CD8 T cells at day 406 after immunization was determined by ICS from individual unchallenged or previously challenged DC-CS + LM-CS immunized mice. Naïve and immune mice were challenged with 1000 P. berghei sporozoites at day 422. Filled circles indicate mice that developed blood-stage malaria. Numbers represent protected mice per total challenged for each group. n.d., not determined.
Fig. 4.
LM-CS252-primed and LM-CS252-boosted BALB/c mice are protected against a P. berghei sporozoite challenge. (A) In an initial experiment to evaluate conventional prime-boost responses, BALB/c mice were primed with 7 × 106 LM-CS252 and boosted with 2 × 107 LM-CS252 53 days later (LM-CS(d53)+LM-CS). On the day of the LM-CS boost, naive BALB/c mice were primed with 7 × 106 LM-CS252 (LM-CS). (A) Total number (mean ± SD, n = 3 per group) of CS252-specific CD8 T cells in the spleen and liver and percentage of PBL at day 61 after boost or first immunization was determined by ICS. (B) In a second experiment BALB/c mice were primed with 7 × 106 LM-CS252 and boosted with 2 × 107 LM-CS252 44 days later (LM-CS(d44)+ LM-CS). On the day of the LM-CS252 boost, naive BALB/c mice were primed with 7 × 106 LM-CS252 (LM-CS). Percentage of total PBL that were CS252-specific CD8 T cells at day 75 after the last immunization was determined by ICS in individual mice. Filled circles indicate naïve or immune mice that developed blood-stage malaria after a challenge with 800 P. berghei sporozoites on day 83 after boost. Numbers represent protected mice per total challenged for each group. n.d., not determined
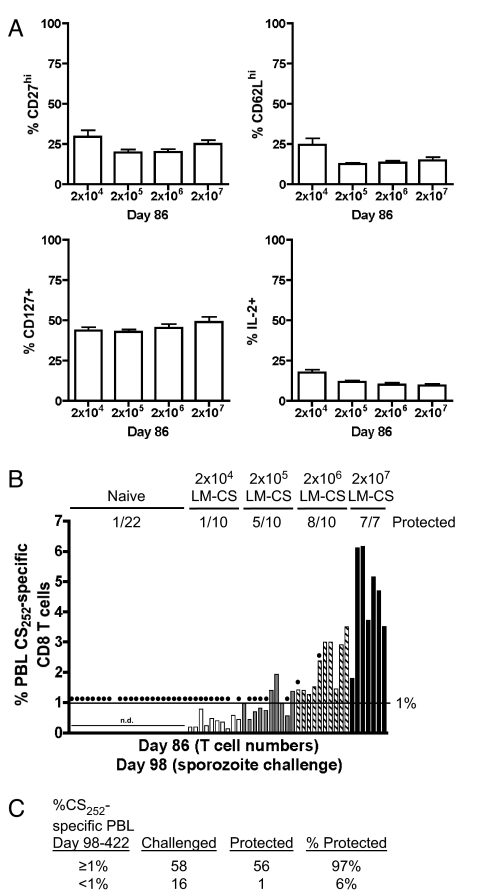
Defining the Threshold Frequency of Memory CD8 T Cells Required for Sterilizing Immunity.
The reliable long-term protection afforded by DC-CS + LM-CS immunization suggests that this model may be well suited to dissect the basic parameters of CD8 T cell immunity to liver-stage Plasmodium infection. Initially, we sought to determine whether a threshold frequency of protective memory CD8 T cells could be defined with this immunization model. BALB/c mice were primed with DC-CS and then boosted with a range of LM-CS252 (from 2 × 104-2 × 107) to stimulate different magnitudes of CD8 T cell memory. Because DC-CS, LM-CS252, and DC-CS + LM-CS immunization stimulated memory CD8 T cells that exhibited some differences in phenotype (for example, CD27 and CD62L expression and IL-2 production) [supporting information (SI) Fig. S1], we investigated whether boosting the mice with different doses of LM-CS252 altered the phenotype of the CS252-specific CD8 T cells. Importantly, the surface expression of certain memory markers (CD27, CD62L, and CD127) was similar in all groups, as was the fraction of CS252-specific memory cells that could produce IL-2 after in vitro stimulation (Fig. 5A and Fig. S2). Thus, differences in the resistance of individual immunized mice probably would be based on the number of memory CD8 T cells. The average frequency of memory CD8 T cells in the PBL of each group increased with increasing doses of LM-CS252 boosting, as did the frequency of protected mice in each group (Fig. 5B). Because there was individual-to-individual variation within each group, and the phenotypes of the memory CD8 T cells were similar, we evaluated all immunized mice based on the frequency of CS252-specific CD8 T cells and protection. Strikingly, 20 of 22 DC-CS + LM-CS mice that had ≥ 1% CS252-specific PBL were protected from sporozoite challenge at day 98 after immunization, whereas only 1 of 16 similarly immunized mice with <1% CS252-specific PBL were protected (Fig. 5B). When we include all DC-CS + LM-CS mice in which memory CD8 T cells have been evaluated in the blood at day 98 or later, our data demonstrate a 97% chance of protection from sporozoite challenge in mice containing ≥ 1% CS252-specific PBL and a 6% chance of protection in mice with <1% CS252-specific PBL (Fig. 5C). Thus, long-term sterilizing immunity to P. berghei infection in this model requires maintenance of CS252-specific memory CD8 T cells that exceed a large (≥ 1%) but definable frequency of PBL (Fig. 5C).
Fig. 5.
Numerical requirements for sterile immunity mediated by CS252-specific CD8 T cells. BALB/c mice were primed with 2.5 × 105 splenic DC-CS and boosted with titrating doses of LM-CS252 (2 × 104, 2 × 105, 2 × 106, or 2 × 107). (A) Phenotype of PBL CS252-specific CD8 T cells as determined by ICS at day 98. Data (mean ± SD) are from 10 mice per group except for 2 × 107, in which n = 7. (B) Before sporozoite challenge the percentage of total PBL CS252-specific CD8 T cells was determined in individual mice by ICS. Naïve and immune mice were challenged with 1000 P. berghei sporozoites. Filled circles indicate mice that developed blood-stage malaria. n.d. = not determined. (C) Cumulative results from DC-CS + LM-CS immunized mice in which the frequency of PBL CS252-specific CD8 T cells was determined before challenge with sporozoites.
Immunity to P. berghei Requires More CD8 T Cells than Other Pathogens.
Although it is clear that the magnitude of CD8 T cell memory can influence resistance to infection (25, 26), there are few data comparing the actual numbers of antigen-specific T cells required for protection against diverse pathogens. To address this issue, we determined that adoptive transfer of 10,000 memory CD8 T cells reduced infection by the liver pathogen Listeria monocytogenes by ≈100-fold (Fig. S3). Similarly, adoptive transfer of ≈85,000 memory CD8 T cells dramatically decreased virus titers after lymphocytic choriomeningitis virus infection (30). Finally, the presence of ≈50,000 memory CD8 T cells in the spleen converted a lethal L. monocytogenes infection into a sublethal infection that was cleared from all mice (26). This last situation is analogous to the biological “bar” that must be overcome for CD8 T cell protection against Plasmodium infection, in which elimination of all infected hepatocytes is required for survival of the mouse. Thus, the 1% of CS252-specific PBL threshold (equivalent to >106 in spleen and >2 × 105 in liver) required for long-term sterilizing immunity to liver-stage Plasmodium infection is 100-1000-fold higher than the numbers of memory CD8 T cells required for meaningful immunity against a bacterial or viral pathogen.
Discussion
In this study, we developed a model of epitope-specific immunization to generate large memory CD8 T cell responses capable of protecting mice from sporozoite challenges. Although several studies have shown that protection from challenge at short intervals after boosting correlates with large CD8 T cell responses (6, 8, 9, 31, 32), our results extend the field in at least 3 ways. First, we demonstrate that memory CD8 T cells specific for Plasmodium liver-stage antigens are capable of providing long-term sterilizing immunity, approaching the entire lifespan of the laboratory mouse. Protection lasting >6 months has been described only for immunization with irradiated or genetically attenuated sporozoite immunizations, suggesting that long-term immunity after subunit vaccination may not be possible. Our results clearly argue against this notion. Second, our results also reveal that a large but definable threshold of memory CD8 T cells is required for protection against sporozoite challenge. Importantly, this threshold greatly exceeds the number of memory CD8 T cells required for protection against specific bacterial and viral pathogens. These results suggest that the biology of the pathogen will affect the number of memory CD8 T cells required for resistance to infection. In the case of Plasmodium infection, the low frequency of infected hepatocytes in combination with the requirement of preventing even 1 infected cell from releasing blood-stage parasites provides a challenge to the immune system that requires commitment of a substantial fraction of the CD8 T cell repertoire to achieve sterilizing immunity. Third, these data describe a novel model system that should facilitate studies to address how CD8 T cells provide immunity against liver-stage Plasmodium infection.
Our results were generated with an epitope-specific immunization protocol in inbred mice, and this scenario is unlikely to have immediate relevance as a vaccine strategy in humans. Although the mouse in general and our approach specifically may have limitations as a preclinical model, the results still may have relevance for understanding why subunit vaccines that evoke sterilizing immunity against human malaria have been difficult to obtain. For example, accumulating data from human clinical trials show that current prime-boost immunizations generate Plasmodium-specific T cell responses in the range of 0.1% of PBL at the peak after boosting and <0.01% at memory stages (20, 33–36). These frequencies are 10-fold and 100-fold lower than required to protect mice from Plasmodium infection and consist mainly of CD4 T cells, which may explain why these vaccines delay the onset but rarely prevent blood-stage parasitemia (20, 36). Clearly, delayed onset of blood-stage parasitemia indicates partial protection by these vaccines, and such partial protection could have real benefits in malaria-endemic areas. However, results from our model suggest that additional or stronger booster immunizations may be required to generate memory CD8 T cell frequencies that exceed the threshold for optimal resistance to Plasmodium infection in humans. Furthermore, it remains to be determined whether immunization against multiple liver-stage antigens will decrease the large frequency of CS-specific memory CD8 T cells required for sterilizing immunity. The model system we describe here is ideally suited to address this question.
Alternatively, vaccination of humans to achieve the large frequencies of memory CD8 T cells that are required for sterilizing immunity to Plasmodium infection in mice may not be feasible. In this regard, CD8 T cells are not the only effectors of immunity to Plasmodium infection, and efforts are underway to develop vaccines that also engage Plasmodium-specific CD4 T cells and antibodies and that are capable of targeting multiple stages of the parasite infection (23). Importantly, the relationships between these various arms of the immune response resulting in the most effective resistance to Plasmodium infection are unknown. The model system described here is well suited for determining these relationships, because it permits quantitative assessment of whether and how Plasmodium-specific antibodies and CD4 T cells decrease the threshold frequencies of CS-specific CD8 T cells required for protective immunity.
In addition, the precise mechanisms and pathways required for CD8 T cell immunity to liver-stage Plasmodium infection remain to be defined. The DC-CS + LM-CS immunization approach used here provides an informative and reliable model in which immune and susceptible mice can be identified before infection. The ability to differentiate prospectively between resistant and susceptible subjects provides a level of resolution that is particularly important for liver-stage studies because the host must be killed for tissue sampling before the outcome of challenge is known. This feature of the model will facilitate studies to address in detail the molecular and cellular features of long-term CD8 T cell protection against liver-stage Plasmodium infection. In turn, this basic information should be useful in devising the most efficacious malaria vaccines.
Methods
Mice and Immunizations.
BALB/c mice were housed at the University of Iowa and Iowa State University animal care units. Mice were primed with DC (2.5 × 105-5 × 105) coated with CS252–260 or with LM-CS252 (7 × 106) through i.v. injections. DC-primed mice were boosted 7 days later with 2 × 107 LM-CS252 or with titrating doses of LM-CS252 (2 × 104, 2 × 105, 2 × 106, or 2 × 107). (Detailed methods are available in SI Text.)
Quantification of Antigen-Specific T Cells.
The total number of spleen CS252-specific CD8 T cells was determined by ICS for IFN-γ after 5 h of incubation in brefeldin A in the presence or absence of CS252–260. Total liver CS252-specific CD8 T cells and the percent of PBL that were CS252-specific were determined by ICS for IFN-γ after 5 h of incubation in brefeldin A in the presence or absence of CS252–260-coated P815 cells.
Mosquito Infections.
Anopheles stephensi (Liston) strain STE2 (MR4–128, MR4, ATCC) were reared in controlled environments (27°C ± 1°C and 80% ± 5% relative humidity) and a 16:8-hour photoperiod. Mosquitoes were fed on anesthetized mice ≈3 days after subpassage or when parasitemia reached 5–20%. To confirm infection before sporozoite collection, oocyst prevalence and intensity were monitored 7–14 days after exposure.
Sporozoite Challenge.
P. berghei (ANKA strain clone 234) sporozoites were isolated from the salivary glands of infected A. stephensi. Naïve and immunized mice were challenged with 1000 sporozoites i.v. unless otherwise noted.
Identification of Protected Mice.
Thin blood smears were performed 7 to 12 days after sporozoite challenge. Parasitized red blood cells were identified by Giemsa stain. Protected mice were defined as those not having blood-stage parasites.
Supplementary Material
Acknowledgments.
We thank members of the J.T.H. laboratory for helpful discussion and S. Perlman for critical comments on the manuscript. We thank the Malaria Research and Reference Reagent Resource Center for providing us with Anopheles stephensi eggs (donated by William E. Collins) and Susan Paskewitz for advice in culturing parasites in mosquitoes. Work in the J.T.H. laboratory is supported by grants from the National Institutes of Health and by support from the Department of Microbiology and Carver College of Medicine, University of Iowa.
Footnotes
Conflict of interest statement: P.L. is an employee of ANZA Therapeutics, Inc, which owns intellectual property covering the compositions and methods described in this manuscript. In addition, ANZA employees hold stock and/or stock options in the company. The remaining authors disclose no known financial conflicts.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805452105/DCSupplemental.
References
- 1.Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 3.Jobe O, et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. J Infect Dis. 2007;196:599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarun AS, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 5.Li S, et al. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T cell-mediated protective immunity against malaria. Proc Natl Acad Sci USA. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider J, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 7.Sedegah M, et al. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: Characterization of effector and memory CD8(+)-T cell populations. Infect Immun. 2002;70:3493–3499. doi: 10.1128/IAI.70.7.3493-3499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RJ, et al. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J Immunol. 2004;172:3094–3100. doi: 10.4049/jimmunol.172.5.3094. [DOI] [PubMed] [Google Scholar]
- 9.Tao D, et al. Yellow fever 17D as a vaccine vector for microbial CTL epitopes: Protection in a rodent malaria model. J Exp Med. 2005;201:201–209. doi: 10.1084/jem.20041526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji M, Zavala F. Peptide-based subunit vaccines against pre-erythrocytic stages of malaria parasites. Mol Immunol. 2001;38:433–442. doi: 10.1016/s0161-5890(01)00079-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji M, Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003;19:88–93. doi: 10.1016/s1471-4922(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 12.Ophorst OJ, et al. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect Immun. 2006;74:313–320. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douradinha B, et al. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int J Parasitol. 2007;37:1511–1519. doi: 10.1016/j.ijpara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Nussenzweig R, Vanderberg J, Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil Med. 1969;134:1176–1182. [PubMed] [Google Scholar]
- 15.Pacheco ND, McConnell E, Beaudoin RL. Duration of immunity following a single vaccination with irradiated sporozoites of Plasmodium berghei. Bull World Health Organ. 1979;57(Suppl 1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 16.Winger LA, Sinden RE. Immunoprotection in mice susceptible to waning memory against the pre-erythrocytic stages of malaria after validated immunisation with irradiated sporozoites of Plasmodium berghei. Parasitol Res. 1992;78:427–432. doi: 10.1007/BF00931700. [DOI] [PubMed] [Google Scholar]
- 17.Clyde DF. Immunity to falciparum and vivax malaria induced by irradiated sporozoites: A review of the University of Maryland studies, 1971–75. Bull World Health Organ. 1990;68(Suppl):9–12. [PMC free article] [PubMed] [Google Scholar]
- 18.Rieckmann KH. Human immunization with attenuated sporozoites. Bull World Health Organ. 1990;68(Suppl):13–16. [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 20.Webster DP, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA. 2005;102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Kebaier C, Vanderberg J. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect Immun. 2007;75:5532–5539. doi: 10.1128/IAI.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturm A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 23.Todryk SM, Hill AV. Malaria vaccines: The stage we are at. Nat Rev Microbiol. 2007;5:487–489. doi: 10.1038/nrmicro1712. [DOI] [PubMed] [Google Scholar]
- 24.Seder RA, Darrah PA, Roederer M. T cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 25.Pope C, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 27.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 28.Brockstedt DG, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci USA. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill AV. Pre-erythrocytic malaria vaccines: Towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- 30.Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert SC, et al. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–1045. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Aseguinolaza G, et al. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expressing a CD8+-T cell epitope derived from the circumsporozoite protein of Plasmodium yoelii. J Virol. 2003;77:11859–11866. doi: 10.1128/JVI.77.21.11859-11866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bejon P, et al. Alternating vector immunizations encoding pre-erythrocytic malaria antigens enhance memory responses in a malaria endemic area. Eur J Immunol. 2006;36:2264–2272. doi: 10.1002/eji.200636187. [DOI] [PubMed] [Google Scholar]
- 34.Imoukhuede EB, et al. Safety and immunogenicity of the malaria candidate vaccines FP9 CS and MVA CS in adult Gambian men. Vaccine. 2006;24:6526–6533. doi: 10.1016/j.vaccine.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Bejon P, et al. Early gamma interferon and interleukin-2 responses to vaccination predict the late resting memory in malaria-naive and malaria-exposed individuals. Infect Immun. 2006;74(11):6331–6338. doi: 10.1128/IAI.00774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun P, et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.