Abstract
The three isoforms of PIP5KI (α, β, and γ) synthesize PI4,5P2 (PIP2) by phosphorylating PI4P. Therefore, it is not clear why platelets, like all eukaryotic cells, have more than one isoform. To test the hypothesis that PIP5KI isoforms have nonoverlapping functions, we generated a murine line containing a null mutation of PIP5KIβ and analyzed the effect on platelet signaling. PIP5KIβ-null mice had normal platelet counts. In contrast to platelets lacking PIP5KIα, platelets lacking PIP5KIβ exhibited impaired aggregation accompanied by disaggregation. Although platelets lacking PIP5KIβ had only a moderate deficiency of PIP2 under basal conditions, they had a striking deficiency in PIP2 synthesis and IP3 formation after thrombin stimulation. We have also observed that platelets lacking both PIP5KIα and PIP5KIβ have a complete loss of thrombin-induced IP3 synthesis even though they still contain PIP5KIγ, the predominant PIP5KI isoform in platelets. These results demonstrate that PIP5KIβ, like PIP5KIα, contributes to the rapid synthesis of a pool of PIP2 that is required for second-messenger formation, whereas the pool of PIP2 synthesized by PIP5KIγ does not contribute to this process. Additionally, we found that PIP5KIβ-null platelets failed to form arterial thrombi properly in vivo. Together, these data demonstrate that PIP5KIβ is required for rapid PIP2 synthesis, second-messenger production, and stable platelet adhesion under shear in vivo. These results also demonstrate that after stimulation of a G protein-coupled receptor, IP3 is completely derived from a rapidly synthesized discrete pool of PIP2 synthesized by PIP5KIα and PIP5KIβ.
Keywords: phosphatidylinositol 4,5-bisphosphate; second messenger; platelet; thrombosis; inositol triphosphate
Phosphatidylinositol 4,5-bisphosphate, also known as PIP2, plays an important role in several cellular events, including focal adhesion formation and actin reorganization. PIP2 contributes to these events by two distinct signaling pathways. First, PIP2 is a substrate of enzymes such as phospholipase C or PI3-kinase, which form second messengers such as IP3, diacylglycerol (DAG), and phosphatidylinositol 3,4,5-trisphosphate (PIP3). These second messengers then activate a variety of signaling pathways that affect actin dynamics. Additionally, PIP2 can directly associate with and thereby regulate the activity of several actin-binding proteins, including talin, vinculin, and filamin (1, 2). Therefore, PIP2 is a major regulator of cytoskeletal dynamics in all eukaryotic cells.
PIP2 is synthesized by either the phosphorylation of phosphatidylinositol 4-phosphate (PI4P) by the lipid kinase phosphatidylinositol 4-phosphate 5-kinase type I (PIP5KI) or by the phosphorylation of PI5P by phosphatidylinositol 5-phosphate 4-kinase type II (PIP5KII or PIP4K). Radiolabeled phosphate pulse–chase experiments that analyzed the relative labeling rate of the D-4 and D-5 positions of the inositol ring suggest that PIP5KI-mediated phosphorylation of PI4P is the predominant pathway for PIP2 synthesis (3, 4). Independent murine genes encode the three isoforms (α, β, and γ) of PIP5KI.** These isoenzymes are all capable of phosphorylating the same substrate, PI4P, at the 5-position of the inositol ring to generate the same product, PIP2 (5–7). In addition, all three isoforms can be stimulated by small GTPases (Rho, Rac, Cdc42, and Arf6) and by phosphatidic acid. Despite this apparent biochemical redundancy, curiously, all cells contain more than one isoform. Notably, the three isoforms of PIP5KI have significantly dissimilar primary structures and different expression levels in different tissues, and they appear to localize within different compartments within some cells (3, 8–12). For example, PIP5KIα localizes in membrane ruffles (8), whereas PIP5KIβ localizes near endosomes (9), and PIP5KIγ is targeted to focal adhesions and nerve terminals (10–12).
Work by Majerus et al. (13) demonstrated that thrombin stimulation induces PIP2 synthesis in platelets. Grondin et al. (14) subsequently linked the increase in PIP2 synthesis after thrombin stimulation of platelets to the increase in PIP5KI activity at the cytoskeleton. PIP5KIα was also shown to alter its localization to the cell membrane upon stimulation of the thrombin receptor, PAR1 in COS-7 cells and in platelets (15, 16). Studies by Hartwig et al. (17) and Carpenter and coworkers (18) demonstrated that PIP5KIα stimulation led to actin filament assembly in response to thrombin and Rac1 in permeabilized platelets. In contrast, Gratacap et al. (19) showed that stimulation of the thromboxane A2 stimulated PIP5K by the sequential activation of Rho and Rho kinase. The critical role of Rho and its effector Rho kinase on PIP5KI activation in intact human platelets was subsequently confirmed (16). Together, these results show that in platelets, Rho and its effector Rho kinase are the predominant regulators of platelet PIP5KI and PIP2 synthesis.
That all cells possess more than one isoform of PIP5KI suggests that these isoforms do not have completely identical functions. Supporting this hypothesis, we have found that megakaryocytes lacking PIP5KIγ fail to anchor their cell membrane to the underlying cytoskeleton (20). In addition, Sasaki et al. (21) have shown that a murine line lacking PIP5KIα has a selective signaling defect in mast cells. These murine models suggest that individual PIP5KI isoforms are fulfilling specific niches within discrete domains of the cell. Of the three PIP5KI isoforms, PIP5KIα and PIP5KIβ have the most homologous primary sequence. This finding may imply that these two isoforms have functional redundancy, leaving PIP5KIγ to fulfill other specialized functions.
Platelets, like other hematopoietic cells, undergo rapid bursts of both PIP2 synthesis and actin cytoskeletal reorganization after agonist stimulation (16, 18). We have shown that platelets contain PIP5KIβ and PIP5KIγ but very little PIP5KIα (16). To begin to understand the unique contribution of PIP5KIβ to cell biology and development, we generated a murine line lacking this isoform of PIP5KI and studied the effect of this mutation on platelet biology. Loss of PIP5KIβ induced biochemical and cellular defects within platelets, demonstrating its unique function within these cells.
Results
Loss of PIP5KIβ Leads to Partial Intrauterine Loss.
To elucidate the functions of PIP5KIβ, we used an ES cell line that contained a β-geo gene trap within the first intron of the PIP5KIβ gene [supporting information (SI) Fig. S1A] (22). The gene trap strategy used to create this ES cell line was designed to create an abnormal mRNA transcript from the trapped allele that would produce a fusion protein corresponding to the first 28 aa of PIP5KIβ fused to β-galactosidase. This ES cell line was used to create chimeric founders that gave rise to germ-line heterozygotes harboring the targeted PIP5KIβ-null mutation.
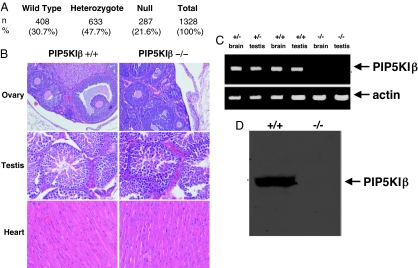
Heterozygous PIP5KIβ+/− mice were intercrossed, and viable PIP5KIβ mice were identified. Genotyping offspring of PIP5KIβ+/− crossings demonstrated that both PIP5KIβ−/− mice and PIP5KIβ+/− mice were born at slightly less than the anticipated frequency (Fig. 1A). Compared with the number of PIP5KIβ+/+ mice produced from these pairings, slightly 30% fewer PIP5KIβ−/− mice and 22% fewer PIP5KIβ+/− mice were born than anticipated by unbiased Mendelian Genetics. Mice lacking PIP5KIβ who survived to birth appeared normal and survived to adulthood. However, both PIP5KIβ+/− and PIP5KIβ−/− mice bred poorly and produced few offspring. This finding demonstrates that loss of PIP5KIβ impairs conception and leads to mild fetal loss. Histologic examination demonstrated that the PIP5KIβ-null mutation had no influence on the morphologic appearance of the gonads or the heart (Fig. 1B). The gross development of the brain, thymus, lung, liver, stomach, kidney, skeletal muscle, spleen, uterus, and bone marrow was also normal in mice lacking PIP5KIβ (data not shown). PIP5KIβ-null mice had complete blood counts, including platelet counts that were similar to their wild-type littermates. Furthermore, PIP5KIβ-null mice exhibited no spontaneous hemorrhage.
Fig. 1.
Targeting of PIP5KIβ produces complete null muation. (A) Genotypes of offspring derived from matings of PIP5KIβ+/− mice. Compared with the number of wild-type embryos generated, the PIP5KIβ−/− mice were born at slightly less than the expected frequency. (B) Micrographs demonstrating the normal morphology of organs lacking PIP5KIβ. H&E stain was used on all organs. (Magnification: ovary, ×100; testis, ×200; heart, ×200.) (C) RT-PCR using a sense primer from exon 1 and an antisense primer from exon 3. The absence of the PCR product demonstrates the loss of PIP5KIβ message beyond the gene trap. In contrast, using primers specific for β-actin demonstrated that targeting of PIP5KIβ had no effect on expression of an unrelated mRNA. (D) Anti-PIP5KIβ immunoblot shows complete loss of protein derived from PIP5KIβ−/− murine muscle.
Sequence analysis of genomic DNA from targeted mice confirmed that the site of gene trap insertion was 5′ to exons 2–14, including the entire kinase domain (data not shown). Therefore, the predicted fusion protein lacks all phosphoinositol kinase activity. We confirmed that the gene trap insertion resulted in loss of wild-type PIP5KIβ expression by RT-PCR and by anti-PIP5KIβ immunoblotting (Fig. 1 C and D). These findings show that although a complete null mutation within the PIP5KIβ gene leads to partial embryonic lethality, some PIP5KIβ-null mice can survive to adulthood without this enzyme.
Platelets Lacking PIP5KIβ Exhibit Disaggregation.
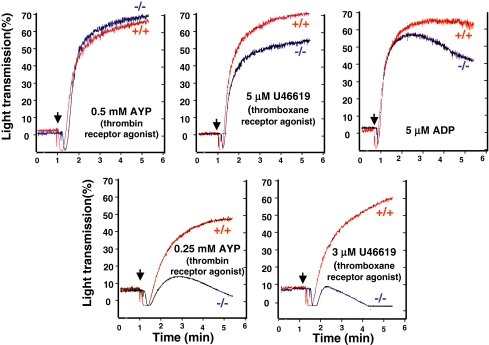
Because second messengers generated from PIP2 by PLC and PI3K contribute to the signaling responsible for platelet aggregation (23–27), we analyzed whether platelets with defective PIP2 production exhibited an ex vivo aggregation defect. We found small decreases in the extent of aggregation of platelets from PIP5KIβ-null mice that were stimulated by the PAR4 thrombin receptor agonist peptide (AYPGQV), the thromboxane A2 receptor agonist (U46619), or ADP (Fig. 2). The impaired aggregation was most apparent at lower agonist concentrations and became less apparent as the concentration of agonist was increased. When the aggregation tracings were observed over time, disaggregation was notable particularly when submaximal doses of agonists were used as the stimulant. These data show that PIP5KIβ-derived second messengers are required for maximal and stable platelet aggregation.
Fig. 2.
Platelets lacking PIP5KIβ have an aggregation defect. Murine platelets lacking PIP5KIβ were analyzed after agonist stimulation in a Lumi-Dual aggregometer. Compared with platelets derived from wild-type littermates, platelets derived from PIP5KIβ-knockout mice have a defect in aggregation in response to low doses of all analyzed agonists and exhibit disaggregation. Higher doses of agonists are able to overcome this defect. Results are representative of six experiments.
It is possible that PIP2 synthesis by other PIP5KI isoforms compensates for the loss of PIP5KIβ in platelets. Quantitative PCR demonstrates that the expression of neither PIP5KIα nor PIP5KIγ mRNA is up-regulated in PIP5KIβ-null cells. We and others have recently reported the phenotype of a murine line lacking PIP5KIγ (28, 29). However, the early lethality caused by the lack of this PIP5KI isoform precludes studies of platelet aggregation. Mice lacking PIP5KIα have also been recently described, and these mice are viable (21). Even though platelets contain very little PIP5KIα, we analyzed whether PIP5KIα contributes to platelet aggregation. In cells lacking PIP5KIα, quantitative PCR indicates that there is no up-regulation of the mRNA of either PIP5KIβ or PIP5KIγ. As shown in Fig. S2, platelets lacking PIP5KIα had no defect in platelet aggregation at all analyzed concentrations of agonists. This finding confirms that the PIP5KIβ isoform but not the PIP5KIα isoform is required for ex vivo platelet activation.
Contribution of PIP5KIβ to the Synthesis of PIP2.
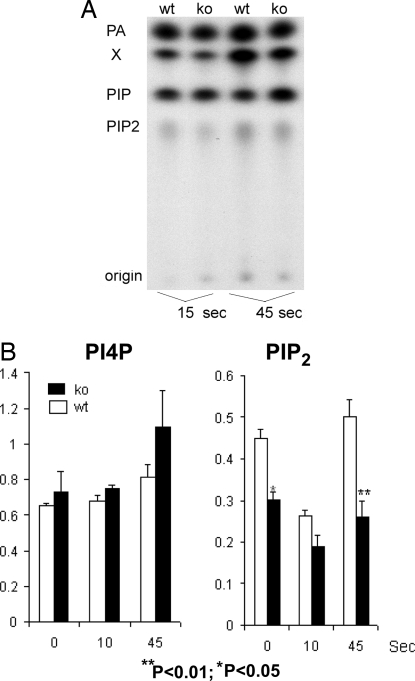
After thrombin stimulation, PIP5KI synthesizes PIP2, and this product can be hydrolyzed by phospholipase C to generate second messengers such as IP3. Therefore, we analyzed PIP2 concentrations in platelets lacking PIP5KIβ-knockout platelets. Platelets were loaded with radioactive orthophosphate, and cells were stimulated, lysed, and fractionated by thin-layer chromatography as demonstrated by a typical experiment shown in Fig. 3A.
Fig. 3.
Loss of PIP5KIβ induces a defect in polyphosphoinositide synthesis. Thin-layer chromatography of extracts of radiolabeled platelets derived from wild-type (wt) and PIP5KIβ-knockout (ko) mice is shown. (A) Results of a representative experiment show effects of thrombin stimulation. (B) Pooled analysis of six experiments show the effect of loss of PIP5KIβ mutation on PIP and PIP2 concentrations after thrombin stimulation.
Results from pooled analysis of six experiments are shown in Fig. 3B. As reported, PIP2 levels normally decline within seconds after agonist stimulation, presumably because PIP2 is consumed during the formation of second messengers. However, the concentration of PIP2 is rapidly restored within 1 min by PIP5KI-mediated phosphorylation of PI4P. Platelets lacking PIP5KIβ had only moderately decreased concentrations of PIP2 before agonist stimulation. In contrast, after agonist stimulation, PIP5KIβ-null platelets had marked impaired PIP2 synthesis. This impaired synthesis resulted in significantly decreased concentrations of PIP2 within 45 s after exposure of the platelets to agonists, although there was enough synthesis to bring the concentration of PIP2 up to basal levels. It is notable that the concentration of the PIP2 precursor, PI4P, in the PIP5KIβ-null platelets was not significantly different from the concentration found in wild-type platelets. These data show that PIP5KIβ is required for the rapid synthesis of PIP2 in platelets after stimulation of G protein-coupled receptors.
Both PIP5KIα and PIP5KIβ, but Not PIP5KIγ, Contribute to the Production of IP3.
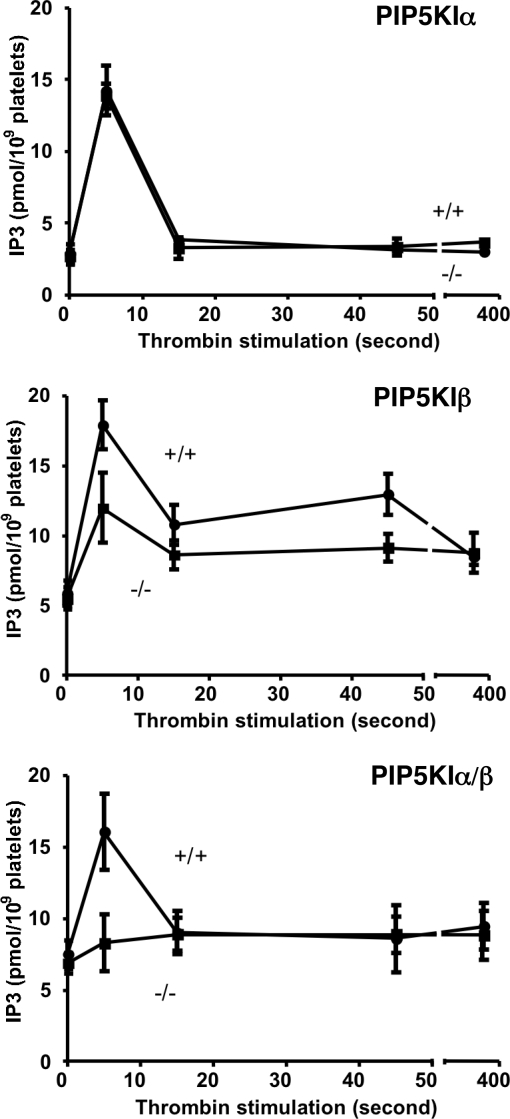
After thrombin stimulation, PIP2 synthesized by PIP5KI can be hydrolyzed by phospholipase C to generate DAG and IP3. To test the hypothesis that the rapid production of PIP2 by PIP5KI is required for production of IP3, we analyzed the concentration of IP3 in cells lacking specific PIP5KI isoforms. We observed that after thrombin stimulation of wild-type control platelets, the concentration of IP3 had a predicted rapid rise followed by a quick decline toward baseline (Fig. 4). Platelets lacking PIP5KIα exhibited no overt defect in IP3 formation after thrombin stimulation (Fig. 4 Top).
Fig. 4.
PIP5KIα and PIP5KIβ are required for IP3 formation. The effect on IP3 production of loss-of-function mutations within the PIP5KIα and PIP5KIβ genes was analyzed. After stimulation by thrombin, the concentration of 1,4,5-IP3 was determined by using methods similar to those described by Rittenhouse and Sasson (37). Although loss of PIP5KIα had no effect on thrombin-induced IP3 production, loss of PIP5KIβ impaired production of IP3 even at the earliest analyzed time point. Loss of both PIP5KIα and PIP5KIβ completely ablated thrombin-stimulated IP3 production in platelets.
Although platelets derived from PIP5KIβ-null littermates had normal basal IP3 levels, they had an ≈50% blunted increase in IP3 that was apparent even 5 s after agonist stimulation (Fig. 4 Middle). These data indicate that PIP5KIβ synthesis of PIP2 contributes to immediate IP3 formation and that PIP5KIα is not required for the formation of this second messenger within thrombin-stimulated platelets. The data also show that loss of PIP5KIβ does not completely eliminate the production of IP3 after thrombin stimulation.
Because PIP5KIα and PIP5KIβ have a high degree of homology, we had expected that both of these isoforms would have been capable of rapidly synthesizing the pool of PIP2 that is used for IP3 formation. Yet our data shown in Fig. 4 Top and Middle indicate that loss of PIP5KIα has no apparent effect on IP3 synthesis, whereas the loss of PIP5KIβ does impair IP3 formation. To account for these observations, we hypothesized that because PIP5KIα is much less abundant in platelets than PIP5KIβ, under normal circumstances, PIP5KIα only makes a minor contribution to platelet second-messenger formation that is not detectable by our assay system. We further hypothesized that in the absence of PIP5KIβ, the contribution of PIP5KIα to platelet second-messenger formation would become apparent.
To test these hypotheses, we initiated a breeding program to generate mice lacking both PIP5KIα together with PIP5KIβ (PIP5KIα−/− PIP5KIβ−/− mice). Pairing mice that were each homozygous for the null mutation within PIP5KIα and heterozygous for PIP5KIβ (PIP5KIα−/− PIP5KIβ+/− mice) has up to this point produced very few litters. Of these offspring, only five mice lacked both isoforms. The platelets from four of these five mice lacking both PIP5KIα together with PIP5KIβ have been analyzed for IP3 formation. The results of pooled analysis of these studies is shown in Fig. 4 Bottom. This analysis demonstrates that platelets lacking both PIP5KIα together with PIP5KIβ have a total absence of IP3 formation after the stimulation of a G protein-coupled receptor. It is notable that by mRNA analysis, PIP5KIγ is the most abundant isoform of PIP5KI in platelets and megakaryocytes (www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc = GSM160043) (16). Therefore, the data shown in Fig. 4 Bottom show that platelets lacking both PIP5KIα together with PIP5KIβ have an absence of IP3 formation even though they still have PIP5KIγ. This result demonstrates that PIP5KIγ does not synthesize a pool of PIP2 that is used for second-messenger formation after stimulation of the thrombin receptor.
Loss of PIP5KIβ Leads to an in Vivo Thrombosis Defect.
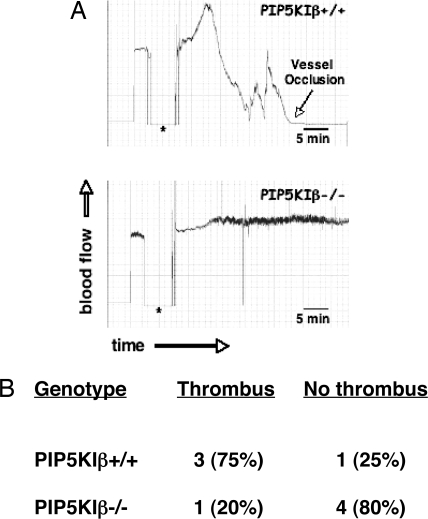
To determine whether loss of PIP2 synthesis and IP3 formation resulted in a defect in vivo, we tested the ability of these mice to form stable occlusions by using a chemical-induced carotid injury model (30, 31). Ferric chloride was applied to an exposed carotid artery of anesthetized mice, and the formation of thrombi was monitored by using a Doppler flow meter. A representative experiment is shown in Fig. 5A. The Doppler tracing of a wild-type mouse shown in the Top demonstrates a completely occlusive thrombus in response to the chemical irritant. In contrast, the PIP5KIβ-null littermate failed to form any vessel occlusion from this injury (Fig. 5A Bottom). The table shown in Fig. 5B shows that loss of PIP5KIβ significantly reduced thrombosis occurrence compared with the wild-type littermates. This indicates that PIP5KIβ plays a critical role in platelet adhesion in vivo, probably through regulating second-messenger production.
Fig. 5.
Lack of PIP5KIβ reduces ferric chloride-induced thrombus formation in the mouse carotid artery. Carotid arteries of PIP5KIβ+/+ and PIP5KIβ−/− mice were subjected to ferric chloride-induced injury, and blood flow was monitored as a measure of thrombus formation. (A) Representative experiment showing the Doppler tracings from a PIP5KIβ+/+ mouse (Upper) and a PIP5KIβ−/− littermate (Lower). The asterisk shows the time that the source of injury was removed from the blood vessel. After ≈1.5 min, an occlusion developed in the artery of the PIP5KIβ+/+ mouse (note the downward reflection of the Doppler tracing). In contrast, there was no disruption of blood flow in the carotid artery of the PIP5KIβ−/− mouse. (B) The percentage of mice that formed thrombi that resulted in greater than a 50% reduction in blood flow is reported (PIP5KIβ+/+, n = 4; PIP5KIβ−/−, n = 5).
Discussion
Our results demonstrate that mice lacking PIP5KIβ have decreased fertility, and platelets lacking PIP5KIβ have defective aggregation, polyphosphoinositide production, and in vivo thrombosis formation. The infertility defect was present in mice lacking PIP5KIβ alone and accentuated in mice lacking both PIP5KIα together with PIP5KIβ. Histological examination failed to identify an anatomic defect in gonads derived from male and female PIP5KIβ-null mice (Fig. 1B). Previous studies have suggested that alterations in IP3 signaling can impede ovulation in other organisms (32, 33). Recently, Lee and coworkers (34) showed that Caenorhabditis elegans lacking a PIP5K homolog, ppk-1, also have a fertility defect. Their work demonstrates that decreased ppk-1 expression does not affect oocyte maturation, but instead causes sterility by reducing contractility of the gonad sheath. Furthermore, they showed that this defect in contractility correlates with defective IP3 formation. At this point, it is not clear whether a similar ovulation defect accounts for the fertility defect observed in PIP5KIβ-null mice.
This work demonstrates that continuous or rapid synthesis of PIP2 is a critical component of second-messenger formation. Estimates of number of PIP2 molecules within human platelets vary between 7.5 × 105 and 2.4 × 106 (13, 35–38). This is approximately the same number of molecules of total IP3 (1,4,5-P3 plus 1,3,4-P3) synthesized within the first 15 s after thrombin stimulation (37). That PIP2 levels only decrease by ≈25% after thrombin stimulation indicates that the majority of IP3 is derived from a combination of preexisting and newly synthesized PIP2. This result is consistent with our finding that PIP5KIβ-null platelets had markedly decreased IP3 levels immediately after thrombin exposure, and platelets lacking both PIP5KIα and PIP5KIβ had a total absence of thrombin-induced IP3 formation. Our data also suggest that early IP3 production is at least partially derived from a small pool of labile PIP2 that turns over rapidly in response to agonists.
We have recently generated a murine line lacking PIP5KIγ, the other dominant PIP5KI isoform found within platelets and megakaryocytes, and we found that that loss of PIP5KIγ leads to early mortality (28). Consequently, we were not able to study platelets lacking this enzyme. PIP5KIγ-null megakaryocytes derived from yolk sac hematopoietic progenitor cells bleb their cell membrane because of a defective connection between their cytoskeleton and the plasma membrane (20). This failure to anchor the cell membrane to the underlying cytoskeleton was not observed in megakaryocytes lacking either PIP5KIα or PIP5KIβ. In addition, we found that overexpression of PIP5KIβ in PIP5KIγ-null megakaryocytes failed to revert this phenotype. Although the complex biology of megakaryocytes and platelets likely differs, these observations of PIP5KIγ-null megakaryocytes are consistent with what we conclude in this current study of PIP5KIα- and PIP5KIβ-null platelets. Despite the ability of individual PIP5KI isoforms to all synthesize the same phospholipid product, we have found that the isoforms fulfill unique functions within individual cells. Additional analysis of cells lacking specific PIP5KI isoforms should further illuminate the individual and unique roles performed by distinct PIP5KI isoforms.
Immunoblotting of platelet lysates with PIP5KI isoform-specific antibodies (16) along with SAGE analysis (www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc = GSM160043) has demonstrated that PIP5KIγ is the most abundant PIP5KI isoform in murine platelets and megakaryocytes. Therefore, it is notable that platelets lacking both PIP5KIα and PIP5KIβ but still containing PIP5KIγ have no rise in IP3 after thrombin stimulation. This observation suggests three alternative hypotheses as to why PIP5KIγ cannot compensate for the loss of PIP5KIα or PIP5KIβ. First, activation of specific PIP5KI isoforms may be regulated differently. Consistent with this first hypothesis is the observation that outside of the catalytic core domain, there is little amino acid sequence homology between PIP5KIγ and the other PIP5KI isoforms. Work by Yin and coworkers (39) has shown that inhibition of PIP5KIγ expression in HeLa cells by RNAi impairs IP3 formation after stimulation of the histamine receptor. This finding suggests that in this tissue culture cell line, after a specific stimuli, PIP5KIγ can produce a pool of PIP2 that contributes to second-messenger formation. A second hypothesis to explain why PIP5KIγ does not contribute to thrombin-induced second-messenger formation is that different PIP5KI isoforms are localized within different discrete microdomains near the cell membrane. In this situation, individual PIP5KI isoforms contribute to compartmentalized pools of PIP2 that control different aspects of actin dynamics. Finally, it is also possible that in addition to synthesizing PIP2, PIP5KIα and PIP5KIβ bind an unknown accessory protein that is critical for second-messenger formation. In this model, PIP5KIγ would be incapable of interacting with this regulatory protein and therefore unable to contribute to IP3 formation. Regardless of which explanation is predominant, the available data suggest that PIP5KIα and PIP5KIβ synthesize a pool of PIP2 that allows IP3 formation, whereas PIP5KIγ generates PIP2 that is required for the stable association of the membrane with the cytoskeleton (20).
Materials and Methods
Generation of Genetically Altered Mice.
Berkeley Bay Genomics Group provided ES cell lines (XE248) containing disruption of one allele of the PIP5KIβ genes by β-geo random insertion mutagenesis (22). Using a RT-PCR, Southern blotting, and a sequencing-based strategy, we identified the specific site of insertion of the β-geo cassette within the first intron of the gene. Location of the Southern blot probe and PCR primers are shown in Fig. S1. Generation of chimeric mice was performed at the Transgenic Core Facility at the University of Pennsylvania. Mice lacking PIP5KIα have been described in ref. 21.
Immunoblotting.
Tissues derived from murine muscle were lysed in NuPAGE LDS sample buffer (Invitrogen) and then fractionated by electrophoresis on a 7–12% [bis(2-hydroxyethyl)amino]tris(hydroxymethyl) (Bistris) gel. After transfer to nitrocellulose membranes, blots were incubated with anti-mouse PIP5KIβ antibody according to the manufacturer's instructions (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated anti-mouse IgG was used as the secondary antibody, and signals were detected by using ECL (Amersham Biosciences).
Platelet Aggregation and Secretion.
Heparin-anticoagulated platelet-rich plasma (PRP) was isolated as described above. The concentration of platelets in the PRP was adjusted to 2.5 × 108 platelets per ml by using Hepes–Tyrode's buffer (pH 7.4) (134 mM NaCl, 3 mm KCl, 0.3 mM NaH2PO4, 12 mM NaHCO3, 2 mM MgCl2, 5 mM Hepes, 5 mM glucose, 0.35% BSA). Platelet aggregation was measured by the turbidometric method at 37°C in a Lumi-Dual aggregometer (Chrono-log).
Polyphosphoinositide Analysis.
Quantitation of PIP2 and PIP.
Blood from the vena cava of anesthetized mice was anticoagulated with 15 units/ml heparin and centrifuged at 200 × g for 7 min at room temperature to obtain PRP and again at 800 × g for 10 min to isolate platelets. Platelets were washed once with modified Tyrode's buffer (pH 6.5) (134 mM NaCl, 3 mm KCl, 0.3 mM NaH2PO4, 12 mM NaHCO3, 2 mM MgC12, 1 mM EGTA, 1 μM PGE1, 5 mM Hepes, 5 mM glucose, 0.35% BSA). The density of platelets was adjusted to 1 × 109/ml by using modified Tyrode's buffer (pH 7.4) without PGE1, EGTA, and NaH2PO4 but supplemented with 1 mM CaC12. Platelet suspensions (0.5 ml) were incubated (“phosphate-starved”) at 37°C for 30 min and then supplemented with 0.5 mCi of 32P for 90 min. The incorporation of 32P was halted by the addition of 50 μl of ice-cold hydrochloric acid and 1 volume of methanol:chloroform (1:1) on ice. Mixtures were vortexed for 1 min to extract phosphoinositides and then centrifuged at 2,000 × g for 3 min. The chloroform phase was isolated, and the remaining aqueous phase was extracted again with chloroform. The two extracted chloroform phases were combined and evaporated under N2 flow. The dried residual was resuspended in 30 μl of ice-cold chloroform. Phosphoinositides in the chloroform solution were fractionated by TLC and analyzed as described in ref. 16.
Quantitation of IP3.
This procedure was performed by using modifications of the protocol described by Rittenhouse and Sasson (37). Briefly, washed mouse platelets were prepared as described above, and their density was adjusted to 7–10 × 108/ml before dividing into 0.2-ml aliquots. One unit/ml thrombin (Chrono-log) was added for indicated times before terminating the reaction by adding 0.2 vol of ice-cold 20% HClO4. After a 20-min incubation on ice, the reaction was adjusted to pH 7.5 by the addition of 1.5 M KOH and then centrifuged at 2,000 × g for 15 min at 4°C. The supernatants were collected and stored at −80°C. Quantitation of IP3 was performed by using a radioreceptor IP3 kit (PerkinElmer) according to the manufacturer's instructions.
Carotid Artery Injury Induced by FeCl3.
Mice weighing 20–30 g at the age of 8 weeks were anesthetized with 80 mg/kg i.p. Nembutal (30, 31). A midline incision was made in the neck, and the right carotid artery was exposed by blunt dissection. A 1 × 1-mm patch of No. 1 Whatman filter paper, soaked in 10% FeCl3, was applied to the exposed artery for 2 min. After removal of the filter paper, the artery was washed with PBS, and blood flow was recorded by using a small-animal blood-flow meter (model T106; Transonic Systems) for 30 min. The percentage of mice that formed thrombi that resulted in greater than a 50% occlusion of blood flow is reported.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants HL073289, HL083392, and HL40387 (to C.S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
It should be noted that the nomenclatures used for the murine and human isoforms are not consistent. Murine PIP5KIβ is the ortholog of human PIP5KIα, and murine PIP5KIα is the ortholog of human PIP5KIβ. In this manuscript, only the murine nomenclature is used.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804139105/DCSupplemental.
References
- 1.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidylinositol 4,5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 2.Furuhashi K, et al. Inositol phospholipid-induced suppression of F-actin-gelating activity of smooth muscle filamin. Biochem Biophys Res Commun. 1992;184:1261–1265. doi: 10.1016/s0006-291x(05)80018-x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 4.Whiteford CC, Brearley CA, Ulug ET. Phosphatidylinositol 3,5-bisphosphate defines a novel PI 3-kinase pathway in resting mouse fibroblasts. Biochem J. 1997;323:597–601. doi: 10.1042/bj3230597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara H, et al. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271:23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- 6.Loijens JC, Anderson RA. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J Biol Chem. 1996;271:32937–32943. doi: 10.1074/jbc.271.51.32937. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, et al. Type I phosphatidylinositol-4-phosphate 5-kinases: Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J Biol Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- 8.Doughman RL, et al. Membrane ruffling requires coordination between type Iα phosphatidylinositol phosphate kinase and Rac signaling. J Biol Chem. 2003;278:23036–23045. doi: 10.1074/jbc.M211397200. [DOI] [PubMed] [Google Scholar]
- 9.Padron D, et al. Phosphatidylinositol phosphate 5-kinase Iβ recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J Cell Biol. 2003;162:693–701. doi: 10.1083/jcb.200302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling K, et al. Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 11.Di Paolo G, et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 12.Wenk MR, et al. PIP kinase Iγ is the major PI(4,5)P(2)-synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DB, Neufeld EJ, Majerus PW. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985;260:1046–1051. [PubMed] [Google Scholar]
- 14.Grondin P, et al. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacylglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem. 1991;266:15705–15709. [PubMed] [Google Scholar]
- 15.Chatah NE, Abrams CS. G protein-coupled receptor activation induces the membrane translocation and activation of phosphatidylinositol-4-phosphate 5-kinase Iα by a Rac- and Rho-dependent pathway. J Biol Chem. 2001;276:34059–34065. doi: 10.1074/jbc.M104917200. [DOI] [PubMed] [Google Scholar]
- 16.Yang SA, Carpenter CL, Abrams CS. Rho and Rho kinase mediate thrombin induced PIP5K trafficking in platelets. J Biol Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- 17.Hartwig JH, et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 18.Tolias KF, et al. Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 19.Gratacap MP, Payrastre B, Nieswandt B, Offermanns S. Differential regulation of Rho and Rac through heterotrimeric G proteins and cyclic nucleotides. J Biol Chem. 2001;276:47906–47913. doi: 10.1074/jbc.M104442200. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, et al. Loss of PIP5KIγ, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki J, et al. Regulation of anaphylactic responses by phosphatidylinositol phosphate kinase type Iα. J Exp Med. 2005;201:859–870. doi: 10.1084/jem.20041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skarnes WC. Gene trapping methods for the identification and functional analysis of cell surface proteins in mice. Methods Enzymol. 2000;328:592–615. doi: 10.1016/s0076-6879(00)28420-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee SB, et al. Decreased expression of phospholipase Cβ2 isozyme in human platelets with impaired function. Blood. 1996;88:1684–1691. [PubMed] [Google Scholar]
- 24.Yang X, Sun L, Ghosh S, Rao AK. Human platelet signaling defect characterized by impaired production of inositol 1,4,5-triphosphate and phosphatidic acid and diminished Pleckstrin phosphorylation: Evidence for defective phospholipase C activation. Blood. 1996;88:1676–1683. [PubMed] [Google Scholar]
- 25.Hartwig JH, et al. D3 phosphoinositides and outside-in integrin signaling by glycoprotein IIb-IIIa mediate platelet actin assembly and filopodial extension induced by phorbol 12-myristate 13-acetate. J Biol Chem. 1996;271:32986–32993. doi: 10.1074/jbc.271.51.32986. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch E, et al. Resistance to thromboembolism in PI3Kγ-deficient mice. FASEB J. 2001;15:2019–2021. doi: 10.1096/fj.00-0810fje. [DOI] [PubMed] [Google Scholar]
- 27.Lian L, et al. The relative role of PLCβ and PI3Kγ in platelet activation. Blood. 2005;106:110–117. doi: 10.1182/blood-2004-05-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. PIP5Kγ is required for cardiovascular and neuronal development. Proc Natl Acad Sci USA. 2007;104:11748–11753. doi: 10.1073/pnas.0700019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Paolo G, et al. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 30.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 31.Kufrin D, et al. Antithrombotic thrombocytes: Ectopic expression of urokinase-type plasminogen activator in platelets. Blood. 2003;102:926–933. doi: 10.1182/blood-2003-01-0054. [DOI] [PubMed] [Google Scholar]
- 32.Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, et al. Linking integrin to IP(3) signaling is important for ovulation in Caenorhabditis elegans. FEBS Lett. 2005;579:549–553. doi: 10.1016/j.febslet.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Guo H, Wycuff DL, Lee M. Role of phosphatidylinositol-4-phosphate 5′-kinase (ppk-1) in ovulation of Caenorhabditis elegans. Exp Cell Res. 2007;313:2465–2475. doi: 10.1016/j.yexcr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettitt TR, et al. Analysis of intact phosphoinositides in biological samples. J Lipid Res. 2006;47:1588–1596. doi: 10.1194/jlr.D600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Vickers JD, Kinlough-Rathbone RL, Packham MA, Mustard JF. Inositol phospholipid metabolism in human platelets stimulated by ADP. Eur J Biochem. 1990;193:521–528. doi: 10.1111/j.1432-1033.1990.tb19367.x. [DOI] [PubMed] [Google Scholar]
- 37.Rittenhouse SE, Sasson JP. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin: Inhibitory effects of phorbol ester. J Biol Chem. 1985;260:8657–8660. [PubMed] [Google Scholar]
- 38.Rittenhouse SE. Human platelets contain phospholipase C that hydrolyzes polyphosphoinositides. Proc Natl Acad Sci USA. 1983;80:5417–5420. doi: 10.1073/pnas.80.17.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YJ, et al. Critical role of PIP5KIγ87 in InsP3-mediated Ca2+ signaling. J Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.