Abstract
Terminally ill insulin-deficient rodents with uncontrolled diabetes due to autoimmune or chemical destruction of β-cells were made hyperleptinemic by adenoviral transfer of the leptin gene. Within ≈10 days their severe hyperglycemia and ketosis were corrected. Despite the lack of insulin, moribund animals resumed linear growth and appeared normal. Normoglycemia persisted 10–80 days without other treatment; normal physiological conditions lasted for ≈175 days despite reappearance of moderate hyperglycemia. Inhibition of gluconeogenesis by suppression of hyperglucagonemia and reduction of hepatic cAMP response element-binding protein, phoshoenolpyruvate carboxykinase, and peroxisome proliferator-activated receptor-γ-coactivator-1α may explain the anticatabolic effect. Up-regulation of insulin-like growth factor 1 (IGF-1) expression and plasma levels and increasing IGF-1 receptor phosphorylation in muscle may explain the increased insulin receptor substrate 1, PI3K, and ERK phosphorylation in skeletal muscle. These findings suggest that leptin reverses the catabolic consequences of total lack of insulin, potentially by suppressing glucagon action on liver and enhancing the insulinomimetic actions of IGF-1 on skeletal muscle, and suggest strategies for making type 1 diabetes insulin-independent.
Keywords: leptin, hyperglucagonemia, glucagon suppression, IGF-1 upregulation, insulinomimetic
Until the discovery of insulin in 1921 by Banting and Best (1) type 1 diabetes (T1D) was a uniformly fatal illness. Ever since the introduction of insulin treatment, it has been assumed that only insulin can reverse this lethal catabolic syndrome, in which virtually all insulin-producing cells are destroyed by an autoimmune process.
The discovery in 1994 of the adipocyte hormone leptin (2) introduced a novel agent with a myriad of physiologic and pharmacologic effects. Among these effects is a robust blood glucose-lowering effect observed in normal rodents (3) and in rodents with partial insulin deficiency induced by streptozotocin (STZ) (4). Leptin is used therapeutically in patients with lipodystrophic diabetes (5). However, because none of these leptin-responsive diabetic models were completely insulin-deficient, it is generally assumed that the antihyperglycemic effects of leptin treatment result from potentiation of residual levels of endogenous insulin. The idea that leptin had actually replaced the therapeutic actions of insulin has never been seriously tested.
In this report we demonstrate that, in three etiologically different rodent models of β-cell destruction, adenovirally induced hyperleptinemia not only corrects the hyperglycemia, but restores terminally ill rodents to full health within 2 weeks of treatment, despite the absence of insulin in their plasma and pancreas.
Results
Hyperleptinemia Normalizes Uncontrolled Diabetes in Nonobese Diabetic (NOD) Mice.
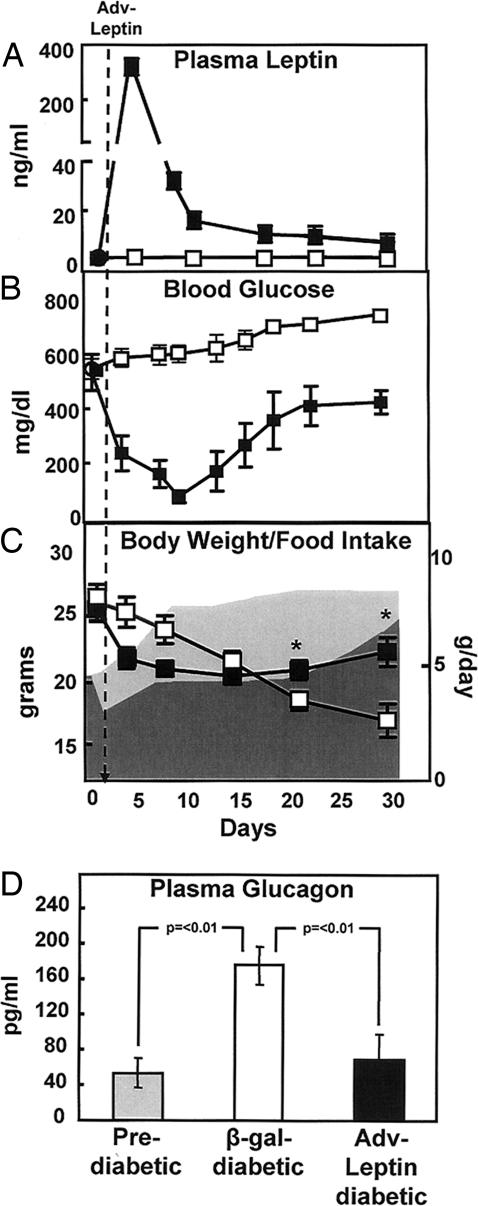
The NOD mouse is the most commonly used rodent model of autoimmune or type 1 diabetes. Seventy-three percent of the females (6) die with the clinical manifestations of insulin deficiency (7), unless treated with insulin. Here, we studied 20 female NOD mice, 15 of which developed diabetes between 12 and 20 weeks of age. Six of the diabetic mice were either untreated or received adenovirus containing the cDNA of β-galactosidase (Adv-β-gal) as an irrelevant control. There were no clinical differences between untreated and Adv-β-gal-treated mice. Nine mice were treated with adenovirus containing the leptin cDNA (Adv-leptin), inducing marked hyperleptinemia averaging 319 ± 76 ng/ml 3 days after the injection (Fig. 1A). Leptin levels decreased rapidly thereafter, measuring 16 ± 4 ng/ml by the 9th postinjection day. For at least 30 days after treatment, they remained >1 ng/ml, the mean leptin level in untreated diabetic animals.
Fig. 1.
Hyperleptinemia reverses abnormalities of uncontrolled autoimmune diabetes in the absence of insulin. Comparison of mean (± SEM) leptin levels (A), blood glucose levels (B), body weight and food intake (shaded dark area = Adv-leptin) (C), and plasma glucagon levels (D) in diabetic NOD mice after either treatment with Adv-leptin (■) (n = 9) or injection of Adv-β-gal (□) as a control (n = 6). Plasma glucagon levels of diabetic NOD mice were obtained 30 days after treatment with Adv-leptin (■) (n = 9) or injection of Adv-β-gal (□) (n = 6). Glucagon levels of prediabetic NOD mice (n = 6) are also displayed ( ). *, P < 0.01.
). *, P < 0.01.
The nonfasting glucose levels of the controls averaged 534 ± 199 mg/dl before Adv-leptin treatment (Fig. 1B). Urine was strongly positive for glucose and ketones (data not shown). Plasma insulin levels in the fed state were all <0.1 ng/ml by 18 weeks of age, compared with 1.4 ± 0.1 ng/ml in normal nondiabetic controls. Glucose levels fell to normal in every Adv-leptin-treated mouse from >534 ± 199 mg/dl to 77 ± 67 mg/dl at 9 days after infection (Fig. 1B). Subsequently, however, they gradually increased and by 22 days after Adv-leptin injection glucose levels had risen to 410 ± 146 mg/dl At this point they seemed to reach a plateau, never approaching the ≈600 mg/dl range of untreated animals during 4 weeks of observation. Moreover, there was no weight loss or apparent deterioration in their health. In addition to lack of measurable plasma insulin, pancreatic preproinsulin mRNA was undetectable (CT ≈34) in both the leptinized diabetic mice and in the untreated controls, using primer sequences that do not differentiate between preproinsulins 1 and 2, whereas preproglucagon mRNA was increased (CT ≈23). Thus, the metabolic improvement in the former group of NOD mice appears to have occurred independently of insulin.
Hyperleptinemia reduced food intake to 51% of the control diabetic NOD mice given Adv-β-gal (data not shown). Despite their hyperphagia, body weight of the control mice declined, whereas in hyperleptinemic mice the weight loss halted at ≈7 days after Adv-leptin injection and body weight rose slightly thereafter (Fig. 1C). Pair-feeding of untreated controls to the hyperleptinemic mice proved to be lethal within 4 days of diet restriction and was discontinued. Remarkably, the leptinized group appeared to thrive and was without abnormalities in appearance or behavior.
Suppression of Diabetic Hyperglucagonemia in NOD Mice by Hyperleptinemia.
Hyperglucagonemia is present in insulin deficiency states (8) and is essential for the hepatic overproduction of glucose and ketones of uncontrolled diabetes (9). To determine whether suppression of hyperglucagonemia by leptin accounted for the reversal of the extreme catabolic state, we compared glucagon levels in untreated and treated diabetic mice. Plasma glucagon measured 175 ± 21 pg/ml in untreated mice, significantly higher than levels in prediabetic NOD mice (53 ± 17 pg/ml) (P < 0.01) (Fig. 1D). Thirty days after Adv-leptin treatment glucagon levels averaged 69 ± 28 pg/ml, significantly below the Adv-β-gal-treated mice (P < 0.01). The latter value was not significantly different from plasma glucagon levels in the prediabetic mice. Thus, leptin-mediated suppression of diabetic hyperglucagonemia may contribute to the reversal of the diabetic state.
Hyperleptinemia Normalizes the Uncontrolled Diabetes of STZ and Alloxan Diabetic Rats.
To determine whether hyperleptinemia would be as effective in other forms of diabetes in another species, we studied its effects in rats with chemically induced β-cell destruction. Six normal, lean wild-type Zucker Diabetic rats received 80 mg/kg of STZ, their maximal sublethal dose, and 11 rats received 100 mg/kg of alloxan. All untreated animals died in <3 months with severe hyperglycemia and ketosis.
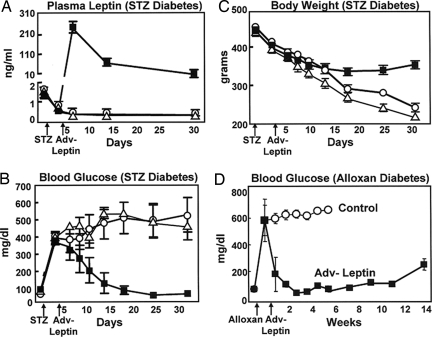
A single i.v. injection of 1012 plaque-forming units of Adv-leptin induced hyperleptinemia of ≈300 ng/ml at 3 days, after which levels declined slowly to 20 ng/ml by the 30th day (Fig. 2A). Glucose levels averaging 400 ± 96 mg/dl were restored to normal within 18 days in all six STZ-diabetic rats and normoglycemia persisted throughout a 30-day observation period (Fig. 2B). During this period the progressive weight loss of uncontrolled diabetes was halted and, remarkably, body weight increased despite the hyperleptinemia (Fig. 2C). Treatment with Adv-β-gal had no effect on the diabetes. The effects of hyperleptinemia on other relevant clinical and laboratory manifestations of uncontrolled diabetes are recorded in supporting information (SI) Table S1. Similar normalization of hyperglycemia was observed in all 11 alloxan-diabetic rats (Fig. 2D), and the improvement persisted for ≈80 days without any other therapy.
Fig. 2.
Hyperleptinemia reverses abnormalities of uncontrolled chemical diabetes in the absence of insulin. Comparisons of mean (± SEM) leptin levels (A), plasma glucose levels (B), and body weight (C) in untreated streptozotocin (STZ)-diabetic rats on an unrestricted diet (○) (n = 5), or untreated streptozotocin (STZ)-diabetic rats pair-fed with leptinized rats (△) (n = 3), and in streptozotocin (STZ)-diabetic rats treated with Adv-leptin (■) (n = 6). (D) Blood glucose levels in untreated alloxan-diabetic rats (○) (n = 5) or rats treated with Adv-leptin (■) (n = 6).
In a separate longer-term study encompassing 174 days, hyperglycemia slowly reappeared but reached a plateau well below the pretreatment levels and the animals remained in apparent good health (Fig. S1).
Thus, as in the NOD mice, hyperleptinemia reversed the metabolic and clinical manifestations of chemically induced β-cell destruction in the absence of any insulin.
Potentiation of Residual Insulin as the Mechanism of Hyperleptinemic Action.
Although potentiation of residual insulin was excluded as the mechanism of hyperleptinemic reversal of NOD diabetes, it seemed important to confirm this in chemically induced diabetes as well. The nonfasting plasma insulin levels in the streptozotocin-diabetic rats were very low after the Adv-leptin treatment of the diabetic rats (0.2 ± 0.03 ng/ml before and 0.18 ± 0.07 ng/ml after), versus 1.4 ± 0.3 ng/ml level of nonfasting plasma insulin in normal rats. Nevertheless, they were higher than the “zero” reading on the standard curve.
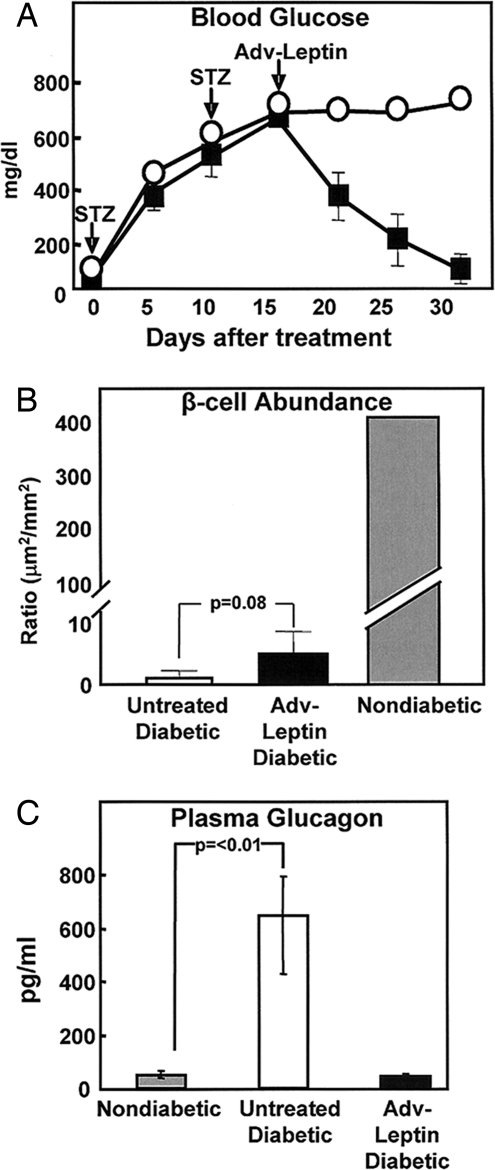
Therefore, to rule out the possibility that hyperleptinemia had potentiated these miniscule insulin levels, we administered the 80 mg/kg dose of streptozotocin twice (2XSTZ) to nine normal rats in an effort to achieve more complete β-cell destruction. These rats exhibited mean blood glucose levels of 674 ± 18 mg/dl without treatment, and their plasma insulin levels were below the detection levels of the assay. The induction of hyperleptinemia in both the 2XSTZ diabetic rats elicited the same progressive decline in glucose levels to normal and complete clinical improvement within 14–20 days (Fig. 3A). Immunostaining of the pancreata of Adv-leptin-treated rats still revealed 1 or 2 insulin-positive cells per 10–15 islets (Fig. 3B), which, although not statistically different, was more than in the untreated controls (P = 0.08). However, once again preproinsulin mRNA could not be detected in the pancreas by quantitative RT-PCR (CT >34), although preproglucagon mRNA was readily detected (CT ≈23). This suggests that these rats were incapable of insulin biosynthesis, and raises the possibility the very rare insulin-positive cells in the pancreas represent insulin granules trapped in badly damaged nonfunctional β-cells undergoing apoptosis and/or macrophages that had engulfed insulin granules.
Fig. 3.
Hyperleptinemia reverses abnormalities of uncontrolled diabetes induced by a double dose of STZ. (A) Comparison of mean (± SEM) blood glucose levels treated with Adv-leptin (■) (n = 5) or untreated double-dose STZ-diabetic rats (○) (n = 5). (B) Morphometric comparison of insulin-positive cells (mean ± SEM) in pancreata of 4 untreated diabetic rats, 5 Adv-leptin-treated STZ-diabetic rats, and 3 normal nondiabetic controls. (C) Plasma glucagon levels 30 days after treatment (*, P < 0.01).
Finally, the possibility of extrapancreatic insulin production, reported in liver of insulin-deficient rodents (10, 11), was also examined. We were unable to identify in liver any preproinsulin mRNA by quantitative RT-PCR (CT >35), and therefore conclude that the antidiabetic effect of hyperleptinemia in chemically induced β-cell destruction is unlikely to be mediated by potentiation of endogenous pancreatic or extrapancreatic insulin.
Suppression of Diabetic Hyperglucagonemia by Hyperleptinemia in STZ-Induced Diabetic Rats.
To determine whether suppression of hyperglucagonemia by hyperleptinemia contributed to the antidiabetic effect in STZ-diabetic rats, we measured plasma glucagon before and 30 days after injection of the Adv-leptin. Glucagon levels before the STZ induction of diabetes averaged 63 ± 35 pg/ml. Thirty days after the onset of untreated STZ diabetes they measured 649 ± 205 pg/ml. Thirty days after treatment with Adv-leptin plasma glucagon had declined to 46 ± 6 pg/ml (P < 0.0.1) (Fig. 3C). Thus, as in NOD mice, glucagon suppression may have contributed to the reversal of the chemically induced uncontrolled diabetic state in rats.
Inhibition of Hepatic Glucagon Action by Hyperleptinemia in STZ-Induced Diabetic Rats.
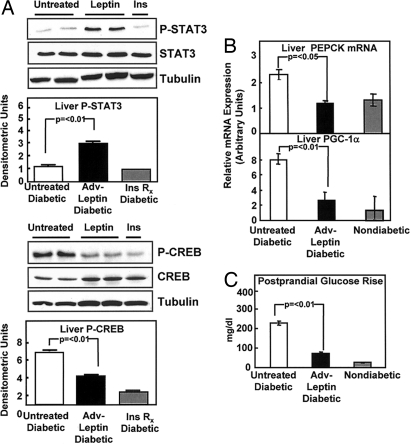
Insulin treatment reverses the excess glycogenolysis, gluconeogenesis, and ketogenesis of insulin deficiency, not only by suppressing the hypersecretion of glucagon, but also by direct action on the liver to inhibit the hepatic effects of any unsuppressed plasma glucagon. To determine whether the hyperleptinemia had acted directly on the liver, we measured hepatic phospho-STAT3, an index of leptin action mediated via the hepatic leptin receptor. P-STAT3 was significantly increased (P < 0.01) (Fig. 4A). This provides evidence for autocrine activity of the liver-derived hyperleptinemia on hepatocytes infected with Adv-leptin.
Fig. 4.
Hyperleptinemia activates liver STAT-3 and down-regulates proteins of gluconeogenesis, while limiting postprandial hyperglycemia. Comparisons (mean ± SEM) of relevant signal transcription factors for leptin and glucagon and their gluconeogenic targets in livers of untreated diabetic rats (□) (n = 4), double-dose STZ-diabetic rats 3 days after Adv-leptin treatment (■) (n = 5), and 3 h after insulin treatment ( ) (n = 3). (A) Immunoblotting for P-STAT-3 and total STAT-3 (Upper), and immunoblotting for P-CREB and total CREB (Lower). Results are in densitometric units. (B) mRNA of phosphoenolpyruvate carboxykinase (PEPCK) and peroxisome proliferator-activated receptor coactivator-1 (PGC-1α). (C) Postprandial rise above fasting levels in blood glucose of untreated STZ rats (□), Adv-leptin-treated STZ rats (■), and nondiabetic rats (
) (n = 3). (A) Immunoblotting for P-STAT-3 and total STAT-3 (Upper), and immunoblotting for P-CREB and total CREB (Lower). Results are in densitometric units. (B) mRNA of phosphoenolpyruvate carboxykinase (PEPCK) and peroxisome proliferator-activated receptor coactivator-1 (PGC-1α). (C) Postprandial rise above fasting levels in blood glucose of untreated STZ rats (□), Adv-leptin-treated STZ rats (■), and nondiabetic rats ( ).
).
Glucagon action on the liver increases the phosphorylation of cAMP response element-binding protein (P-CREB) (12). P-CREB was elevated in the livers of untreated STZ-induced diabetic rats with hyperglucagonemia. Both insulin treatment and treatment with Adv-leptin significantly reduced P-CREB (P < 0.01). At 30 days after treatment, P-CREB in the hyperleptinemic rats was significantly lower than in untreated rats (P < 0.01), although not as low as 3 h after insulin treatment (Fig. 4A).
Based on these findings, one would predict a reversal of the increased hepatic gluconeogenesis of uncontrolled diabetes. Such a reversal would be reflected by decreased expression of phoshoenolpyruvate carboxykinase (PEPCK), a key gluconeogenic enzyme that is stimulated by glucagon (13), which is elevated in uncontrolled diabetes. In untreated STZ-diabetic rats PEPCK mRNA was 48% above normal and declined by 43% at 30 days after the Adv-leptin treatment (Fig. 4B). In addition, Adv-leptin treatment reduced peroxisome proliferator-activated receptor-γ-coactivator-1α (PGC-1α) mRNA, which is also implicated in activation of gluconeogenesis (14) (Fig. 4B). The reduction by hyperleptinemia of these gluconeogenic proteins may well contribute importantly to the reversal of the catabolic state.
The suppression of glucagon and its activity by hyperleptinemia would also be expected to inhibit ketogenesis (15), although leptin itself causes nonketotic fatty acid oxidation of lipids (16). In untreated insulin-deficient rats hepatic TAG content had declined from a normal value of 6.8 ± 0.8 mg/g of tissue weight to 1 ± 0.5 mg/g. Thirty days after the induction of hyperleptinemia it measured 4.7 ± 0.8 mg/g of liver. Remarkably, plasma triacylglycerol (TAG), which averaged >1,000 mg/dl in untreated diabetic rats, was <9 mg/dl in hyperleptinemic rats (Table S1), suggesting that a profound reduction in the secretion of very low density lipoproteins (VLDLs) coincided with the increase in hepatic TAG content. Plasma free fatty acids (FFA) in untreated STZ rats averaged 2.2 ± 1.2 mEq/liter, almost 8 times the value in lean nondiabetic rats (P < 0.004); one month after Adv-leptin treatment they measured 0.19 ± 0.06 mEq/liter (Table S1). This most probably reflected the loss of adipocyte fat because of the lipolytic consequences of insulin deficiency coupled with the lipo-oxidative action of the hyperleptinemia (17).
Effect of Hyperleptinemia on Postprandial Glucose in STZ-Induced Diabetic Rats.
To determine whether hyperleptinemia mimics the extrahepatic actions of insulin, we compared fasting and nonfasting glucose levels in normal, untreated STZ-diabetic, and Adv-leptin-treated STZ-diabetic rats (Fig. 4C). In normal rats the fasting and postprandial glucose levels differed by only 26 ± 1.2 mg/dl. In untreated diabetic rats, they differed by 227 ± 11 mg/dl, whereas in the leptinized group the difference was 74 ± 6 mg/dl at 30 days after treatment. Thus, it appears that leptin action reduces postprandial hyperglycemia in insulin-deficient rats.
Activation of the Insulin Signal Transduction Pathway by Hyperleptinemia.
To determine whether the insulin-like actions of hyperleptinemia involved the activation of elements of the insulin signal transduction pathway, we compared the phosphorylation of insulin receptor substrate (IRS)-1, phosphotidylinositol-3-kinase (PI3K), protein kinase B (Akt)-1, and extracellular signal-regulated kinase (ERK) in the livers and skeletal muscles of untreated insulin-deficient rats, insulin-treated and Adv-leptin-treated diabetic rats. Unlike insulin, leptin had no significant effect on any of these four insulin targets in liver, despite an almost 3-fold increase in hepatic phosho-STAT-3 (P < 0.0001) (data not shown). Because, in the liver of normal rodents, leptin induces a 6.8-fold activation of mitogen-activated protein kinase (MAPK) (18), we suspect that this difference reflects leptin-mediated potentiation of insulin's hepatic action, which was lacking in our insulin-deficient rodents.
However, in skeletal muscle the effects of hyperleptinemia were more insulin-like. P-IRS-1, which was undetectable in untreated diabetic rats, was increased in the hyperleptinemic rats. At 3 days after Adv-leptin injection it measured >60% of the value observed 3 h after insulin injection (Fig. S2). PI3K in skeletal muscle of leptinized rats was also significantly greater than in untreated diabetics, measuring >60% of the value noted 3 h after insulin administration (P < 0.001). P-ERK was increased 9-fold above the level in muscle of untreated diabetic rats to 44% of the insulin-induced increment. However, there was no increase in P-Akt in either liver or muscle of rats 30 days after induction of hyperleptinemia. These results suggest that, although in insulin-deficient rodents the hepatic effects of hyperleptinemia do not involve the insulin-signaling pathway, those in skeletal muscle may be mediated by certain components of the pathway.
Leptin Induction/Potentiation of Insulinomimetic Hormones.
The activation by hyperleptinemia of insulin-signaling molecules in skeletal muscle, but not in liver, raised the possibility of potentiation of an insulinomimetic hormone. Fibroblast growth factor (FGF)-21 is reported to have insulinomimetic properties that could play a regulating role in metabolism (19). However, FGF21 mRNA in livers of STZ rats was not up-regulated by hyperleptinemia (data not shown).
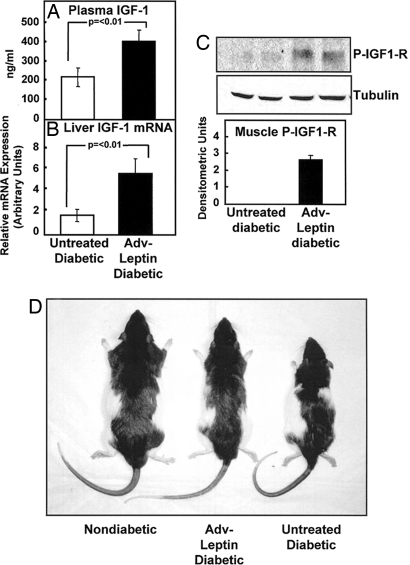
A role for insulin-like growth factor (IGF)-1, which can activate ERK (20), was also considered. A comparison of three untreated and four Adv-leptin-treated STZ-diabetic rats revealed plasma IGF-1 to be significantly increased in the hyperleptinemic rats 30 days after treatment (P < 0.01) (Fig. 5A). Hepatic IGF-1 mRNA was up-regulated (P < 0.01) in the Adv-leptin-treated rats at this time (Fig. 5B). To determine whether the plasma IGF-1 elevation was acting on target tissues, we measured phosphorylation of IGF-1 receptor in liver and muscle. At 3 days posttreatment, but not at 30 days, we found a significant increase in P-IGF1-R in skeletal muscle of hyperleptinemic rats (Fig. 5C), the tissue in which components of the insulin-signaling pathway had been activated. We found no such increase in liver, in which they had not been activated. These findings are consistent with IGF-1 mediation of the insulin-like action of hyperleptinemia in skeletal muscle. They may also be relevant to the impressive increase in body weight and linear growth observed in Adv-leptin-treated diabetic animals (Fig. 5D).
Fig. 5.
Hyperleptinemia increases plasma IGF-1 and IGF-1 action on skeletal muscle, while restoring linear growth in severely insulin-deficient rats. Comparisons of plasma IGF-1 (A) and liver IGF-1 mRNA (B) 30 days after treatment and phosphorylated IGF-1 receptor (P-IGF-1R) in skeletal muscle (C) 3 days after treatment (densitometric units) in untreated (□) (n = 4) and Adv-leptin-treated (■) (n = 4) double-dose-STZ-diabetic rats. (D) Appearance of a nondiabetic normal lean wild-type Zucker Diabetic Fatty (+/+) rat, a double-dose-STZ-diabetic littermate treated with Adv-leptin, and an untreated diabetic littermate. Note that, although both the leptinized and the untreated diabetic rats are slimmer than the nondiabetic wild-type control, the length of the leptinized rat is almost normal. Thus, the growth inhibition caused by insulin deficiency was corrected without insulin replacement.
Discussion
Hyperleptinemia reduces hyperglycemia in streptozotocin diabetic rats with partial insulin deficiency (21, 22), and can directly activate certain components of the insulin signal transduction pathway in normal rats (18). However, the possibility that leptin could rescue insulin-deficient animals from ketoacidosis and death had never been tested. Here, we show that sustained, supraphysiologic hyperleptinemia, induced by a single injection of Adv-leptin, reverses all of the measurable consequences of insulin deficiency, whether autoimmune or chemically induced, and restores health in rodents without insulin preproinsulin mRNA in pancreas or in ectopic sites.
The single, most striking feature of this study was the dramatic clinical improvement achieved without insulin in terminally ill rodents. Without exception every animal exhibited significant daily improvement, becoming normoglycemic and in a normal state of health within 5–12 days. The results were similar in mice with autoimmune destruction and in rats with chemical destruction of β-cells, although the timing differed. In all models weight loss was arrested and weight gain resumed despite the virtual absence of body fat. Although the antihyperglycemic effect of hyperleptinemia in STZ rats wanes 2–3 weeks thereafter, the hyperglycemia remains well below the extremely elevated pretreatment levels—even at 25 weeks after Adv-leptin treatment (Fig. S1), perhaps because the adenovirally induced hyperleptinemia persists at low levels for many weeks (23).
Earlier work from our laboratory had demonstrated that hyperglucagonemia is present in insulin deficiency states (8) and that its suppression by somatostatin prevents the hepatic overproduction of glucose and ketones in uncontrolled diabetes for several hours (9, 24, 25). In this study we found that sustained hyperleptinemia can also profoundly suppress diabetic hyperglucagonemia to normal for extended periods of time. In addition, hyperleptinemia can inhibit glucagon's gluconeogenic action on the liver, as manifested by a reduction in P-CREB, PEPCK, and PGC1α.
Although leptin stimulates MAPK phosphorylation almost 4-fold in the livers of normal rats (18, 26), in the insulin-deprived animals studied here, hyperleptinemia had no such effect. There was, however, evidence of insulin-like effects in an extrahepatic target of insulin, the skeletal muscle, where P-STAT3 increased almost 10-fold. In confirmation of the report of Kim et al. (18), there was an increase in skeletal muscle P-IRS-1, P-ERK, and P13K to ≈65%, 44%, and 30%, respectively, of the insulin-induced increase. These effects could have been mediated by IGF-1, which was up-regulated in the liver of leptinized rats and which was increased in plasma. There was increased phosphorylation of IGF-1 receptor in skeletal muscle, which is consistent with this IGF-1 action, as is the striking restoration of linear growth in the absence of any insulin (Fig. 5C).
Based on this evidence, we speculate that the reversal by hyperleptinemia of the protein catabolism of total insulin deficiency was the result of suppression of hyperglucagonemia and its protein-catabolic hepatic actions on liver, combined with up-regulation of IGF-1, a protein-anabolic hormone.
Whatever their mechanisms, these results constitute the first report of successful treatment of total insulin deficiency without insulin. They suggest that uncontrolled diabetes can be rescued without insulin by agents that eliminate glucagon-mediated hepatic overproduction of glucose and ketones and that improve glucose utilization in skeletal muscle. Consequently, it is not inconceivable that these findings will lead to the development of glucagon-suppressing/blocking agents that might supplement or even replace insulin treatment in the management of T1D.
Materials and Methods
Animals.
Eight-week-old nonobese diabetic (NOD/LtJ) mice from the Jackson Lab were used for studies of autoimmune diabetes. Ten-week-old lean wild-type (+/+) Zucker Diabetic Fatty (ZDF) rats weighing ≈300 g and fed Teklad 6% fat mouse/rat chow (Teklad) were used for studies of chemical diabetes. To exclude reduced food intake in hyperleptinemic rodents as the cause of improved metabolic state, in some experiments the food intake of controls was matched to that consumed by hyperleptinemic animals on the previous day. This is referred to as “diet-matching.”
All mice and rats were housed in individual cages with constant temperature, and a standard light/dark cycle (6 a.m. to 6 p.m./6 p.m. to 6 a.m.). All were fed 6% mouse/rat chow diet (Teklad) and had free access to water. All animal protocols were approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center.
Induction of Diabetes by STZ.
Normal lean wild-type ZDF (+/+) rats were fasted overnight. A single i.v. injection of 80 mg/kg of body weight of STZ (Sigma) in 300 μl of 0.9% saline solution was administered to one group of rats. In another set of experiments, two doses of 80 mg/kg of body weight were administered with a one-week interval. Blood glucose concentrations were monitored every 3 days after the STZ treatment.
Induction of Diabetes by Alloxan.
After an overnight fast lean wild-type ZDF (+/+) rats received an i.v. injection of alloxan (80 mg/kg of body weight) (Sigma) in 150 μl of 0.9% saline. Blood glucose concentrations were monitored after the alloxan treatment.
Adenovirus-Induced Hyperleptinemia.
Beginning at an age of 14 weeks, most NOD mice developed severe hyperglycemia (nonfasting blood glucose >400 mg/dl) accompanied by marked hypoinsulinemia, ketonuria, and cachexia and were obviously near death. These diabetic mice received intravenously a total of 0.3 × 1012 plaque-forming units of adenovirus containing either the leptin cDNA (Adv-leptin) or, as a control, the β-galactosidase cDNA (Adv-β-gal). Bodyweight and food intake were monitored daily. Blood glucose concentrations were monitored twice a week.
All STZ- and alloxan-treated rats developed severe diabetes with ketonuria and cachexia. Control rats received Adv-β-gal intravenously and were diet-matched to the leptinized controls. A third group of STZ-diabetic mice received insulin 5 units (Humulin R, Eli Lilly) i.p. twice with a 30-min interval. Animals were killed and their tissues harvested after blood glucose levels fell to 100 mg/dl or less. In the case of insulin treatment, this was 3 h after treatment; in the case of Adv-leptin treatment, it was 3 days.
Tissue Collection and Preparation.
NOD mice were killed under phenobarbital anesthesia at 3 days and at 30 days after treatment and nonfasting blood samples were obtained from the inferior vena cava. Heart, liver, kidney, spleen, muscle, and white adipose tissue were rapidly excised and frozen in liquid nitrogen and stored at −80°C.
STZ- and alloxan-diabetic rats were killed under phenobarbital anesthesia 30 days after treatment. Nonfasting blood samples were obtained from the inferior vena cava and tissues of interest were rapidly excised, frozen in liquid nitrogen, and stored at −80°C for protein and RNA extraction.
Plasma Measurements.
Plasma glucose was measured by PGO glucose kit (Sigma). Plasma leptin and insulin were assayed by using rat leptin ELISA kit and ultrasensitive rat insulin ELISA kit (Crystal Chem). Plasma glucagon was measured by rat glucagon RIA kit (Linco Research). Plasma IGF-1 was measured by rat/mouse Insulin-Like Growth Factor (IGF-1) ELISA Kit (Gropep Limited, IDS Inc.).
Immunocytochemical Studies.
Fragments of the tail of pancreata were fixed in Bouin's solution and processed for insulin and glucagon immunohistochemistry staining as previously described (27). Insulin-positive cells were quantified by using Image-J image analysis software and particle analysis macro (Scion). The area of insulin staining in 12 sections of pancreas from 5 animals, relative to total sectional area examined, was quantified by monochromatic thresholding. Pictures were taken with an Axiophot microscope, objective ×20.
Immunoblotting Analysis.
Total protein extracts prepared from tissues of lean +/+ rats were resolved by SDS/PAGE and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences). The blotted membrane was blocked in 1× TBS containing 0.1% Tween and 5% nonfat dry milk (TBST-MLK) for 1 h at room temperature with gentle, constant agitation. After incubation with primary antibodies anti-phospho-AKT, anti-phospho-STAT3 (Tyr-705) (Cell Signaling Technology), anti-phospho-CREB (Ser-133) (Cell Signaling Technology), anti-phospho-IGF-1 receptor, anti-phospho-MAP kinase (ERK1/2) (Cell Signaling Technology), or anti-tubulin (Sigma) in freshly prepared TBST-MLK at 4°C overnight with agitation, the membrane was washed two times with TBST buffer. This was followed by incubating with secondary anti-rabbit, -mouse, or -sheep horseradish peroxidase-conjugated IgG antibodies in TBST-MLK for 1 h at room temperature with agitation. The membrane was then washed three times with TBST buffer, and the proteins of interest on immunoblots were detected by an enhanced chemiluminescence detection system (Amersham Biosciences). The corresponding bands were quantified by using National Institutes of Health Image J software (version 1.6).
Immunoprecipitation of Phospho-IRS-1 and Phospho-AKT.
Immunoprecipitation was carried out by incubating 2 mg of protein with IRS-1 antibody (Cell Signaling Technology) overnight; after precipitation of the immunocomplexes with 20 μl of protein A-Sepharose, the beads were washed 5 times in the cell lysis buffer as described above, resolved by SDS/PAGE gel, and analyzed by Western blotting.
Quantitative Real-time RT-PCR.
Total RNA was extracted from pancreas and liver by the TRIzol isolation method according to the manufacturer's protocol (Life Technologies). All reactions were done in triplicate. The real-time amount of all mRNA was calculated by using standard curve method. 18S mRNA was used as the invariant control for all studies. Primer sequences of genes used for quantification of mRNA by real-time PCR appear in Table S2. The primers for preproinsulin mRNA do not distinguish between the 1 and 2 isoforms.
Statistical analysis.
All results are expressed as mean ± SEM. The statistical significance of differences in mean values was assessed by Student's t test for two groups.
Supplementary Material
Acknowledgments.
We thank A. Rossini, C. Li, and M. Brownlee for critical review of the manuscript, D. Foster, J. Horton, P. Scherer, and G. Clark for advice and discussion, K. McCorkle for technical assistance with the art work, and P. McCravy for administrative assistance. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5-R01-DK002700, the VA North Texas Health Care System Merit Review, and Juvenile Diabetes Research Foundation Grant 1-2004-763 (to R.H.U.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806993105/DCSupplemental.
References
- 1.Banting FG, Best CH. Pancreatic extracts. 1922. J Lab Clin Med. 1990;115(2):254–272. [PubMed] [Google Scholar]
- 2.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Koyama K, et al. Beta-cell function in normal rats made chronically hyperleptinemic by adenovirus-leptin gene therapy. Diabetes. 1997;46(8):1276–1280. doi: 10.2337/diab.46.8.1276. [DOI] [PubMed] [Google Scholar]
- 4.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48(7):1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 5.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 6.Oge A, et al. In utero undernutrition reduces diabetes incidence in non-obese diabetic mice. Diabetologia. 2007;50(5):1099–1108. doi: 10.1007/s00125-007-0617-0. [DOI] [PubMed] [Google Scholar]
- 7.Oldstone MB, Ahmed R, Salvato M. Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J Exp Med. 1990;171(6):2091–2100. doi: 10.1084/jem.171.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller WA, Faloona GR, Unger RH. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbs R, et al. Glucagon: Role in the hyperglycemia of diabetes mellitus. Science. 1975;187(4176):544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 10.Kojima H, et al. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci USA. 2004;101(8):2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapir T, et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci USA. 2005;102(22):7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalle S, et al. Glucagon promotes cAMP-response element-binding protein phosphorylation via activation of ERK1/2 in MIN6 cell line and isolated islets of Langerhans. J Biol Chem. 2004;279(19):20345–20355. doi: 10.1074/jbc.M312483200. [DOI] [PubMed] [Google Scholar]
- 13.Christ B, Nath A, Bastian H, Jungermann K. Regulation of the expression of the phosphoenolpyruvate carboxykinase gene in cultured rat hepatocytes by glucagon and insulin. Eur J Biochem. 1988;178(2):373–379. doi: 10.1111/j.1432-1033.1988.tb14460.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Exton JH, Corbin JG, Park CR. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969;244(15):4095–4102. [PubMed] [Google Scholar]
- 16.Lee Y, et al. PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc Natl Acad Sci USA. 2002;99(18):11848–11853. doi: 10.1073/pnas.182420899. (Translated from eng) (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orci L, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci USA. 2004;101(7):2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10(7):727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 19.Kharitonenkov A, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215(1):1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 20.Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56(7):791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hidaka S, et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 2002;16(6):509–518. doi: 10.1096/fj.01-0164com. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol. 2002;282(5):E1084–E1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 23.Higa M, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1999;96(20):11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerich JE, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- 25.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 26.Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci USA. 2000;97(5):2355–2360. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orci L, et al. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci USA. 1976;73(4):1338–1342. doi: 10.1073/pnas.73.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.