Abstract
The myelodysplastic syndromes (MDS) comprise a group of premalignant hematologic disorders characterized by ineffective hematopoiesis, dysplasia, and transformation to acute myeloid leukemia (AML). Although it is well established that many malignancies can be transplanted, there is little evidence to demonstrate that a premalignant disease entity, such as MDS or colonic polyps, can be transplanted and subsequently undergo malignant transformation in vivo. Using mice that express a NUP98-HOXD13 (NHD13) transgene in hematopoietic tissues, we show that a MDS can be transplanted to WT recipients. Recipients of the MDS bone marrow displayed all of the critical features of MDS, including peripheral blood cytopenias, dysplasia, and transformation to AML. Even when transplanted with a 10-fold excess of WT cells, the NHD13 cells outcompeted the WT cells over a 38-week period. Limiting-dilution experiments demonstrated that the frequency of the cell that could transmit the disease was ≈1/6,000–1/16,000 and that the MDS was also transferable to secondary recipients as a premalignant condition. Transformation to AML in primary transplant recipients was generally delayed (46–49 weeks after transplant); however, 6 of 10 secondary transplant recipients developed AML. These findings demonstrate that MDS originates in a transplantable, premalignant, long-term repopulating, MDS-initiating cell.
Keywords: hematopoiesis, HOX gene, leukemia, transplant, NUP98
The myelodysplastic syndromes (MDSs) comprise a heterogeneous group of clonal blood disorders characterized by dysplasia, ineffective hematopoiesis, and transformation to acute myeloid leukemia (AML) (1, 2). The ineffective hematopoiesis can be recognized morphologically as a maturation arrest and leads to chronic peripheral blood (PB) cytopenias despite a hypercellular or normocellular bone marrow (1, 2). The annual incidence of MDS varies between 2 and 12 new cases per 100,000 persons and increases with age (1). MDS can arise de novo or as a result of exposure to known genotoxic agents, including occupational exposure to benzene and other solvents. Therapy-related MDS (t-MDS) develops in cancer survivors as a consequence of treatment with anticancer chemotherapy and/or ionizing radiation (3).
Similar to many malignant or premalignant conditions, patients with either de novo MDS or t-MDS have acquired mutations in the MDS cells, both in the form of gross chromosomal rearrangements and deletions and point mutations. Single-nucleotide substitutions leading to missense or nonsense mutations have been identified in the NRAS, KRAS, FLT3, KIT, and RUNX1 genes (4, 5). Chromosomal changes can be recognized by conventional light-microscopic techniques in ≈50% of MDS patients and a higher frequency in t-MDS patients (3). The most common karyotypic abnormalities are chromosomal losses (chromosomes 5, 7, and 20) and gains (chromosome 8). Although rare, a number of balanced chromosomal translocations involving EVI1, RUNX1, MLL, and NUP98 have been associated with MDS (http://cgap.nci.nih.gov/Chromosomes/Mitelman). Of particular interest are the fusions that involve the NUP98 gene (6). Approximately half of the translocations involving NUP98 fuse NUP98 to a clustered homeobox (HOX) gene; the other half fuse NUP98 to a nonhomeobox gene. Both the NUP98-HOX and NUP98-nonHOX fusions have been linked to MDS. Although NUP98-HOXD13 translocations are rare in patients with MDS (7–9), NUP98-HOXD13 expression leads to overexpression of HOXA9 (10), which is frequently overexpressed in patients with MDS (11), suggesting that overexpression of abd-b HOX genes is linked to MDS. In addition, transgenic mice that express a NUP98-HOXD13 (NHD13) transgene in the hematopoietic compartment develop a MDS that recapitulates all of the features of MDS, including PB cytopenias, bone marrow (BM) dysplasia, apoptosis and transformation to acute leukemia (12).
Although MDS has been recognized as a disease entity for 50 years (13) and is considered to be a clonal stem cell disorder, efforts to verify this hypothesis using xenograft mouse transplantation models have failed, because the MDS cells either engraft poorly or not at all (14–16), and the mice do not develop clinical evidence of MDS. These results stand in contrast to those obtained with AML, in which the disease can be readily transplanted into immunodeficient mice (17); these xenotransplant assays have been crucial for the identification of a subset of cells that can transfer leukemia to immunodeficient recipients; referred to as leukemia-initiating cells (L-ICs) (17, 18). In an analogous fashion, we wished to determine whether MDS could be transplanted as a premalignant condition, before transformation to AML, and identify a cell that can initiate MDS (hereafter referred to as an MDS-initiating cell or M-IC).
Results
Hematopoietic Progenitors Isolated from NHD13 BM Demonstrate Ineffective Hematopoiesis in Vitro.
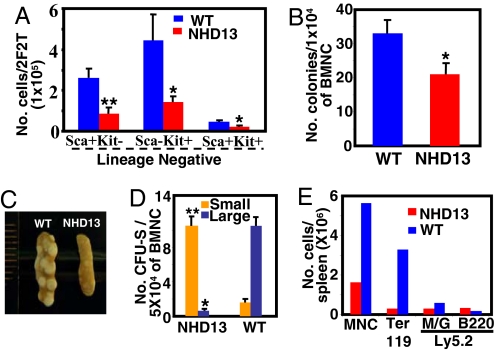
BM from the NHD13 mice showed modestly reduced numbers of LinnegSca+Kit+ cells and LinnegSca−Kit+ cells [which include the common myeloid progenitor (CMP), granulocyte monocyte progenitor (GMP), and megakaryocyte erythroid progenitor (MEP) populations] (Fig. 1A). This finding was consistent with results from a colony-forming cell (CFC) assay that also showed a modestly reduced number of progenitor cells in the NHD13 BM (Fig. 1B).
Fig. 1.
Aberrant primitive hematopoietic progenitors from NHD13 BM. (A) Distribution of the lineage-negative population in terms of Sca-1 and c-Kit. 2F2T refers to cells harvested from two femora and two tibiae per mouse. (B) CFC assay. Total colony counts were determined 12 days after plating unfractionated BMNC (n = 6). (C) CFU-S were evaluated 10 days after injection of 5 × 104 BMNC into lethally irradiated recipients. (D) Day 10 CFU-S (n = 4). (E) Cell number and lineage analysis of CFU-S. Error bars represent SEM. M/G, Mac-1/Gr-1; MNC, mononuclear cell. *, P < 0.05; **, P < 0.01.
To detect changes of more immature progenitor cells, we performed a CFU in-spleen (CFU-S) assay using BM nucleated cells (BMNC) from NHD13 or WT mice. The CFU-S results showed a remarkable difference in the sizes of the colonies between the two groups; the vast majority of WT CFU-S colonies were large, whereas the overwhelming majority of NHD13 spleen colonies were small (Fig. 1 C and D). This observation was confirmed by FACS analysis that showed a marked decrease in the number of Ter119-positive erythroid cells in CFU-S from the NHD13 mice (Fig. 1E). These findings suggest that BM from NHD13 mice displays either decreased numbers of hematopoietic progenitor cells or impaired differentiation of progenitors and is consistent with the PB cytopenias observed.
MDS from NHD13 Mice Is Transplantable.
To determine whether a premalignant MDS could be transferred to WT recipients, we harvested BMNC from NHD13 mice and WT controls that were positive for the Ly5.2 allotype of CD45 and injected the cells into lethally irradiated WT congenic recipients that expressed the Ly5.1 allotype of CD45. The NHD13 mice that served as BM transplant (BMT) donors for this study showed evidence of MDS, with a macrocytic anemia and leukopenia, despite a normocellular BM [supporting information (SI) Table S1). There were no signs of leukemic transformation in the donor mouse BM, because the mean percentage of blasts in the BM was 11.9%, and there was no evidence of parenchymal organ infiltration (Table S1). Immunophenotype analysis of NHD13 BMNC demonstrated a decrease in Ter119+ erythroid cells, a decrease in B220+ lymphoid cells, an increase in Mac1+/Gr1+ myeloid cells, and the appearance of a recently described Mac1+/B220+ population of cells (19, 20) (Fig. S1 A and B). The myeloid series showed an increase number of immature cells, as evidenced by an increase in Gr1dim cells, as well as the morphology of May–Grunwald–Giemsa (MGG)-stained cells (Fig. S1 B and C). Dysplastic binucleate cells and apoptotic cells were also detected in the BM of the NHD13 donor mice (Fig. S1C).
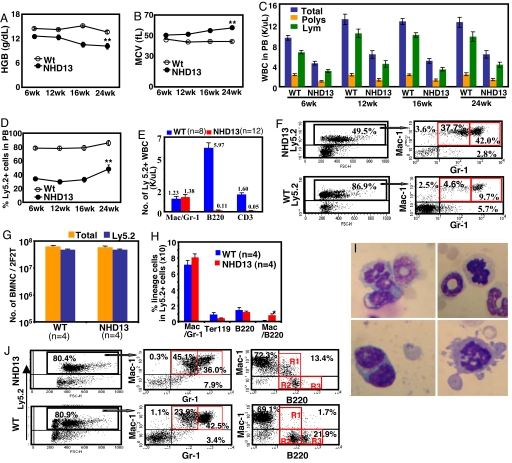
In the initial experiments, 1 × 106 BMNC from NHD13 or WT donors (Ly5.2+) together with a life-sparing dose of 1 × 105 WT BMNC (Ly5.1+) were transplanted into Ly5.1+ recipient mice. Four independent experiments, using independent NHD13 and WT donor mice, were performed in this fashion. The recipient mice showed macrocytic anemia and leukopenia as early as week 6 after transplantation (Fig. 2 A–C). The severity of the anemia gradually increased with time, because the hemoglobins of NHD13 recipients and WT recipients at week 24 after transplantation were 10.1 ± 0.7 g/dl and 13.6 ± 0.4 g/dl, respectively. Total WBC counts were 6.3 × 103 ± 0.7 × 103 per microliter for the NHD13 recipients and 12.5 × 103 ± 1.0 × 103 per microliter for the WT recipients at week 24 after transplantation. Given that a 10-fold excess of Ly5.2+ NHD13 or WT BMNC were cotransplanted with Ly5.1+ life-sparing WT BMNC, it is not surprising that 80% of the PB nucleated cells were Ly5.2+ in recipients of WT BMNC (Fig. 2D). In contrast, only 40% of the PB nucleated cells from NHD13 BMNC recipients were Ly5.2+, less than half of expected, supporting the hypothesis that the BMNC from NHD13 mice were impaired in their ability to differentiate and mature into circulating blood cells (Fig. 2 D and E).
Fig. 2.
Primary recipient mice transplanted with NHD13 or WT BMNC. (A–C) Hemoglobin (Hgb) (A), mean corpuscular volume (MCV) (B) values, and total WBC (C), absolute polymorphonuclear neutrophil (Poly), and absolute lymphocyte (Lym) counts at the indicated week after transplantation. (D) Percent engraftment of donor origin (Ly5.2+). (A–D) n = 12 WT BM recipients, n = 16 NHD13 recipients, except for the 24-week time point, n = 7 WT and n = 10 NHD13. (E) Lineage distributions of donor origin peripheral blood nucleated cells (PBNC) at 16 weeks after transplantation. (F) Representative FACS profiles of PBNC from the recipients. (G) Total number of BMNC and BMNC of donor origin (Ly5.2+) per mouse, two femora and two tibiae (2F2T) at 16 weeks after transplantation. (H) Lineage distribution of BMNC. (I) Dysplastic BMNC from NHD13 recipients stained with MGG. (J) Representative FACS profiles of BMNC from recipients. R1, Mac1+/B220dim; R2, Mac1−/B220dim; R3, Mac1−/B220bright. n, number of mice. Error bars represent SEM; *, P < 0.05; **, P < 0.01.
Similar to the NHD13 donors, there was an increased percentage of immature Mac1+Gr1dim myeloid cells (Fig. 2 F and J) in the PB and BM of the NHD13 recipients, suggesting ineffective myelopoiesis or premature release of myeloid precursors. Despite the decreased percentage of Ly5.2+ cells in the PB, the percent chimerism in the BM of NHD13 and WT recipients was almost identical (74.6% vs. 75.8%), and the number of total BMNC was not different between the two groups (Fig. 2 G and H). Although the numbers of B220+ cells in the BM were comparable in the NHD13 and WT recipients, the B220+ cells in the NHD13 recipients were abnormal in terms of staining intensity and coexpression of Mac1 (Fig. 2J), suggesting that these were not normal B cell precursors. Dysplasia was manifested as binucleated cells, cells with nucleocytoplasmic asynchrony, and bizarre mitosis (Fig. 2I). Finally, we demonstrated that the NHD13 MDS phenotype could be successfully transferred to normal recipients in the absence of WT life-sparing BMNC, by transplanting 1 × 106 BMNC without WT life-sparing BMNC into lethally irradiated mice (Fig. S2).
Competitive Repopulation Assays Demonstrate a Growth Advantage for the NHD13 MDS Cells.
A competitive repopulation assay using equal numbers (1 × 105 cells) of BMNC from NHD13 or WT donors (Ly5.2+) and WT competitor BMNC (Ly5.1+) showed similar BM engraftment kinetics for the two groups (Fig. S3A). However, although the ratio of donor PB nucleated cells (PBNC) to donor BMNC was close to 1.0 for the WT control group, as expected for this type of competitive repopulation assay (Fig. S3B), the ratio of donor PBNC to donor BMNC was only 0.25 for the NHD13 group at 6 weeks after transplant. These results again demonstrate that although the NHD13 BMNC engraft well, they do not readily differentiate into mature circulating blood cells, consistent with the ineffective hematopoiesis associated with MDS.
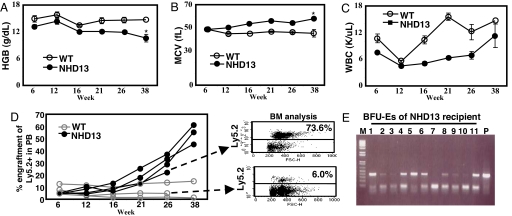
To test the ability of NHD13 MDS BMNC to expand and replace normal BM elements in the presence of excess WT hematopoietic stem cells (HSC), we transplanted 1 × 105 NHD13 BMNC together with 1 × 106 WT BMNC into lethally irradiated recipient mice. Despite receiving 10-fold fewer NHD13 BMNC, these mice gradually developed a progressive macrocytic anemia and a variable degree of leukopenia (Fig. 3 A–C), suggesting that the MDS clone gradually outcompeted the WT stem/progenitor cells in the BM. This prediction was confirmed by serial analysis of PB chimerism, which demonstrated that 50% of the PB cells were Ly5.2+ NHD13 cells by 38 weeks after transplant. In contrast, mice transplanted with a 1:10 ratio of WT Ly5.2+ BMNC continued to show engraftment levels of 2–14% (Fig. 3D). Interestingly, at 21 weeks after transplant, the NHD13 mice showed a progressive macrocytic anemia, with a normal relative distribution width (RDW), suggesting that the recipient's erythropoiesis was almost entirely due to the NHD13 cells, even though the percentage of nucleated PB cells that were of donor (NHD13) origin was only 22–36% (Fig. 3 B and D). To resolve this apparent paradox, we harvested BM from NHD13 and WT recipients and analyzed donor contribution by both FACS and genotype of Burst Forming Units-Erythroid (BFU-E) colonies grown in vitro (Fig. 3E). These results confirmed that the majority of BMNC were derived from NHD13 cells.
Fig. 3.
Clonal expansion of NHD13 BMNC. NHD13 or WT BMNC (Ly5.2+) (1 × 105) were transplanted with 1 × 106 WT BMNC (Ly5.1+). PB samples were analyzed at the indicated time points. (A) Hemoglobin level of the recipients. (B) MCV values. (C) Total WBC counts. (D) Percent engraftment of donor origin in PB of individual recipients and FACS profile of BM at week 26 after transplantation. (E) Detection of the NHD13 transgene in BFU-E colonies isolated from BMNC of an NHD13 recipient 26 weeks after transplantation. Error bars, SEM; *, P < 0.05.
Transplantation of WT HSC into nonirradiated, immunocompetent syngeneic hosts is generally inefficient, unless the hematopoietic “niches,” occupied by recipient HSCs, are cleared by either cytotoxic conditioning or antibodies (21). However, because the NHD13 BMNC demonstrated a competitive advantage compared with WT BMNC in reconstituting the hematopoietic system of lethally irradiated mice, we attempted to transplant NHD13 MDS cells into mice that had not been irradiated. Despite transfer of 1 × 106 NHD13 BMNC, there was no evidence of NHD13 engraftment in nonirradiated recipients (Table S2) during the 17-week study period, a time frame in which NHD13 cells engrafted in irradiated mice when 10-fold fewer NHD13 BMNC were used (Fig. 3, Fig. S3). Furthermore, two of the three mice injected with NHD13 BMNC were assayed for engraftment at 58 weeks after transplant and showed no evidence of engraftment at this time point. Although it remains possible that engraftment may have been detectable at later time points, these findings suggest that clearance of hematopoietic niches is important for engraftment of the NHD13 M-IC.
Transplantable M-IC Resides in the Lineage-Negative (Linneg) Compartment.
To determine whether a M-IC, similar to HSCs, resided in the Linneg compartment, lethally irradiated mice were injected with 5 × 104 Linneg BMNC from NHD13 or WT donor mice, together with a life-sparing dose of 1 × 105 WT BMNC. Recipients of Linneg NHD13 BMNC developed a macrocytic anemia (Fig. S4) and leukopenia and showed a decreased percentage of Ly5.2+ cells in the PB compared with WT controls, indicating engraftment of MDS. To assess the frequency of M-ICs, three independent experiments with three different donors were performed (Table 1); the estimated frequency of the M-IC was between 1/6,213 and 1/16,244, a number comparable with the estimated frequency of normal murine HSC (≈1/20,000).
Table 1.
Limiting-dilution transplantation
| Cell dose(per mouse) | Mouse ID | Ly5.2 engraftment in PB, % |
||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | ||
| 2 × 104 | #1 | 8.7 | n/a | 10.2 |
| #2 | 0.5 | n/a | 12.4 | |
| #3 | n/a | n/a | 34.9 | |
| 1 × 104 | #1 | 8.0 | 46.3 | 6.8 |
| #2 | n/a | 69.6 | 12.4 | |
| #3 | n/a | 50.6 | 7.5 | |
| #4 | n/a | 57.6 | 12.8 | |
| #5 | n/a | 77.0 | 0.4 | |
| 5 × 103 | #1 | n/a | 28.6 | n/a |
| #2 | n/a | 23.9 | n/a | |
| Estimated frequency (95% C.I.) | 1/16,244 (1/73,185 ≈ 1/3,606) | >1/7,213 (1/53,273 ≈ 1/977) | 1/6,213 (1/18,466 ≈ 1/2,091) | |
Data were acquired from recipient mice 16 weeks after transplantation. C.I., 95% confidence interval; n/a, not applicable.
M-IC Is a Long-Term Repopulating Cell.
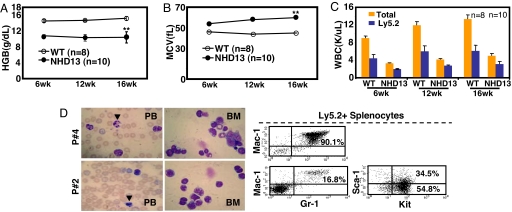
Serial transplantation was performed by using BMNC from primary NHD13 recipients with MDS that were 16 weeks after transplant to determine whether the transplantable MDS was derived from a long-term repopulating cell. Primary recipients from two independent donors were used as donors for the secondary transplants. Results from the two independent experiments were similar, and the results are shown in Fig. 4. Secondary recipients of NHD13 BMNC again developed a macrocytic anemia and leukopenia (Fig. 4 A–C).
Fig. 4.
Serial transplantation of MDS. Lethally irradiated secondary recipient mice were injected with 5 × 106 BMNC from primary NHD13 recipients together with a life-sparing dose of WT BMNC (1 × 105). (A) Hemoglobin levels of the secondary recipients. (B) MCV values. (C) Total WBC counts and donor engraftment cell counts. (D) MGG staining of PB and BM and FACS profiles of leukemic cells from secondary recipients that developed AML. Arrowheads indicate circulating apoptotic cells. Results represent pooled data from two independent experiments. Error bars, SEM; **, P < 0.01.
Transformation to Acute Leukemia.
Although most experiments were ended at 16–24 weeks after transplantation with no signs of acute leukemia in the recipient mice, mice from one experiment were followed for 49 weeks. Of seven NHD13 BM recipients, three were found dead of unknown causes between 13 and 24 weeks after transplant, two were euthanized at 17 weeks without signs of AML, and two developed AML at 46 and 49 weeks after transplantation (Mice nos. 2Pe65.1 and 2Pe65.4; Fig. S5 and S6). Interestingly, 6 of 10 secondary transplant recipients developed AML at a median of 17 (range 16–32) weeks after transplant, characterized by hunched posture, lethargy, splenomegaly, anemia, thrombocytopenia, leukocytosis, and aberrant circulating apoptotic cells, and the other four were found dead of unknown causes 14–32 weeks after transplant (Fig. 4D and Table S3). These AMLs had distinct immunophenotypic characteristics (Fig. 4D); some were typical Mac1+/Gr1+ AML, whereas others were Mac1−/Gr1−, but strongly Kit+, suggesting that different cell types had undergone secondary events leading to AML transformation.
Discussion
MDS is a heterogeneous disease, and the characteristics, and even the existence of an MDS “stem,” or initiating cell, has been the topic of much discussion (2). In some cases, clonal marking studies using specific cytogenetic abnormalities (trisomy 8), have suggested that MDS originated in a committed myeloid progenitor (22). In contrast, other investigators studied cases of MDS with del(5q), observed that 25–90% of the pro-B cells contained the del(5q) marker and concluded that MDS originated in a multipotential HSC (23, 24). Abnormal in vitro hematopoietic colony formation has been observed (24, 25), but interpretation of in vitro colony assays has been complicated by the growth of contaminating normal hematopoietic progenitors that are not part of the malignant clone. In this study, we identified decreased numbers of progenitor cells in NHD13 BM, as evidenced by both immunophenotype (Fig. 1A) and functional assays (CFC and CFU-S) (Fig. 1B). The reduced number of CFC in NHD13 BM is consistent with reports that the colony-forming capacities of hematopoietic progenitors are decreased in the majority of MDS patients (26).
The existence of an AML leukemia “stem cell,” or L-IC has been established by transfer of human AML to immunodeficient mice (17). Several investigators have used a similar approach in attempts to transfer MDS to immunodeficient mice. However, nonobese diabetic (NOD)/SCID mice transplanted with BM from MDS patients did not develop MDS, because there was no long-term engraftment of the MDS cells (16). In a modified study, modest engraftment (<5%) of cells with clonal markers characteristic of the MDS clone was detected 13–17 weeks after intramedullary transplant of cells into NOD/SCID/β2 microglobulin (NOD/SCID-β2mnull) mice (15). Investigators using NOD/SCID-β2mnull mice that were engineered to express human IL3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and Steel Factor (SF) demonstrated that BM cells from 9 of 11 MDS patients generated detectable levels of human hematopoietic cells (14). However, engraftment was transient in some cases, clonal markers indicated that 15–99% of the cells were derived from normal hematopoietic cells, and there was no evidence that the mice developed peripheral blood cytopenias or other evidence of clinical MDS. Rare case reports of donor-derived MDS in BM transplant recipients have suggested that MDS may be transplantable (27), although in most of these reports, there was no convincing evidence that the donor had MDS.
Transplantation of congenic mice with BM from the NHD13 mice resulted in MDS in the recipient mice. This MDS was characterized by a progressive macrocytic anemia, leukopenia, neutropenia, and dysplasia. Ineffective hematopoiesis was demonstrated by CFU-S assays, by the presence of immature Gr1dim myeloid cells in the PB and BM, and a diminished ratio of NHD13 cells in the PB compared with BM (Figs. 2 and 3, and Fig. S3). Competitive repopulation assays demonstrated an inexorable growth advantage for the NHD13 MDS cells; this was best evidenced by experiments in which a 10-fold excess of WT BM cells were transplanted together with the NHD13 cells (Fig. 3). In these experiments, a gradual increase in the fraction of NHD13-derived cells was evident by 21 weeks after transplant, and by 38 weeks after transplant, >50% of the nucleated cells in the PB were derived from the NHD13 donor.
Normal hematopoiesis is sustained with a small population of HSC having long-term repopulating capacity, and the existence of a long-term stem cell can be documented by serial transplantation. Results from secondary transplantation with the BM of primary MDS recipients clearly demonstrated that the transplantable M-ICs were derived from a long-term repopulating cell. Interestingly, transformation to AML was more common in the secondary transplant recipients rather than primary recipients, suggesting that the additional repopulating stress placed on the M-IC or the increased life span of cells in secondary transplant recipients might predispose these cells to leukemic transformation. Limiting-dilution and cell-fractionation studies demonstrated that the M-IC, similar to normal HSC, was present in the Linneg BM population.
It is not clear why MDS was transplantable as a clinical entity in these experiments, given that attempts to transplant human MDS into immunodeficient mice have been unable to recapitulate the clinical disease, as described above. We think it is unlikely that the NHD13 cells used for transplantation had transformed to AML before transplant, because there was no evidence of leukemia in the donor mice. Furthermore, recipient mice that did develop AML did so only after a lengthy incubation period (17–49 weeks), and leukemias that developed after transplant of cells from the same MDS donor showed distinct features in terms of morphology and immunophenotype (Fig. 4), suggesting that the leukemic transformation had occurred after transplantation. It may be that transplantation of mouse MDS cells into congenic mouse recipients circumvented any xenotransplant barriers or that M-ICs were very rare in the prior studies, and insufficient numbers of M-ICs were transplanted. An additional explanation is that extensive ex vivo manipulation of the NHD13 cells (such as cryopreservation or lengthy selection procedures) was purposely avoided in these studies, because LinnegSca+Kit+ NHD13 cells are prone to undergo apoptosis in vitro (28).
In this study, we have demonstrated that MDS can be transferred to WT recipients, supporting the concept that MDS originates in a transplantable multipotent cell. The NHD13 M-IC is Linneg, has intact self-renewal activity, leads to clonal dominance in competitive repopulation assays, can be serially transplanted, and results in a disease that recapitulates all of the key features of human MDS. This transplantation model should facilitate further elucidation of M-IC properties and provide a platform with which to study the biology of and therapy for this deadly disease.
Experimental Procedures
Transplantation.
Eight- to 10-week-old C57Bl6 recipient mice, which expressed the Ly5.1 allotype of CD45, were purchased from Charles River. Congenic C57Bl6 NHD13 mice that expressed the Ly5.2 allotype of CD45, were used as donors. BMNC were obtained freshly from the femora and tibiae of donor mice. BMNC (1 × 105 to 1 × 106 per mouse) were injected into lethally irradiated (1,000 cGy) recipient mice by tail vein injection, typically with a life-sparing dose of 1 × 105 WT BMNC. For secondary transplantation, 5 × 106 primary recipient BMNC were transplanted into lethally irradiated recipients along with a life-sparing dose of 1 × 105 WT BMNC. Lineage depletion of BMNC was performed by using StemSep (Stem Cell Technologies) for the Linneg BMNC transplantation. Animal experiments were approved by the National Cancer Institute Animal Care and Use Committee.
Evaluation of Engraftment.
PB was examined at fixed intervals after transplantation, most commonly 6, 12, and 16 weeks. Each PB sample was divided into samples for flow cytometry (FACS) and complete blood count (CBC). CBCs were determined by using a HEMAVET Multispecies Hematology Analyzer (CDC Technologies). To evaluate BM engraftment, mice were killed at 16 weeks after transplantation and BMNC harvested from femora and tibiae. Morphology of the PB and BM was evaluated by using MGG-stained cytospins.
Flow Cytometry.
Staining was performed by using conjugated antibodies obtained from either BD Pharmingen (BD) or eBioscience (eB). Cells were resuspended with Hanks' balanced salt solution (HBSS) (Invitrogen) containing 2% FBS (HF2) and then incubated for 30 min on ice with one or more of the following antibodies, Mac1 (Cd11b)-PE (BD), Gr1-FITC (BD), CD45.2 (Ly5.2)–APC (eB), B220 (CD45R)-FITC (BD), CD3e-PE (eB), Ter119-FITC (BD), cKit (CD117)-FITC (BD), Sca-1-PE (BD). To stain lineage-positive cells, a biotinylated antibody mixture (Stem Cell Technologies) containing antibodies that recognize CD5, CD19, Ter119, B220, Gr-1, Mac-1, and a monoclonal antibody (7–4) against neutrophils was used with Streptavidin-APC (BD). After staining, cells were washed twice with PBS, resuspended with HF2 buffer containing 1 μg/ml propidium iodide (Sigma), and analyzed with a dual-laser FACScan (BD Bioscience).
CFU-S Assay.
To generate CFU-S10, recipient mice (n = 5) were lethally irradiated and injected via the tail vein with 5 × 104 to 1 × 105 fresh BMNC. Spleens were harvested 10 days after injection and fixed in Tellesniczky's solution (5% formalin, 5% glacial acetic acid, 70% ethanol) Macroscopic colonies were counted manually. Background CFU-S10 was determined on irradiated mice not injected with BMNC. To determine the immunophenotype, a single cell suspension was prepared from whole spleens harvested from CFU-S10 recipient mice and stained as described above for FACS analysis.
CFC Assay.
BMNC (3 × 104) were plated on 35-mm Petri dishes in Methocult M3434 medium (Stem Cell Technologies). Methocult M3434 is 2% methylcellulose supplemented with cytokines [50 ng/ml recombinant mouse stem cell factor (SCF), 10 ng/ml rmIL-3, 10 ng/ml rhIL-6, and 3 units/ml rhEpo]. CFC plates were incubated at 37°C in a 5% CO2 incubator and the number of colonies determined 12 days after plating. BFU-E colonies aspirated from CFC culture plates were genotyped by PCR (primers: NUP98, 5′-TGGAGGGCCTCTTGGTACAGG-3′; HoxD13-L1, 5′-GGCTTCTAAGCTGTCTGTGGCC-3′). Cells harvested from BFU-E colonies were washed once with PBS and DNA prepared by alkaline hydrolysis (50 mM NaOH solution). The amplification profile consisted of incubation at 94°C for 3 min, followed by 35 cycles of 94°C for 30 seconds, 62°C for 30 seconds, and 72°C for 1 min.
Statistics.
Data are expressed as the mean ± SEM or SD, where applicable. Differences between groups were analyzed by Student's t test. P values < 0.05 were considered to be significant.
Supplementary Material
Acknowledgments.
We thank Eli Estey, R. Keith Humphries, David Caudell, and Helge Hartung for discussion; Bobby Smith and Leslie Johnston for technical assistance; and John Dennis and Danielle O'Mard for oversight of animal care. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804507105/DCSupplemental.
References
- 1.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649–1660. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 2.Corey SJ, et al. Myelodysplastic syndromes: The complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 4.Bacher U, et al. A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica. 2007;92:744–752. doi: 10.3324/haematol.10869. [DOI] [PubMed] [Google Scholar]
- 5.Harada H, Harada Y, Kimura A. Implications of somatic mutations in the AML1/RUNX1 gene in myelodysplastic syndrome (MDS): Future molecular therapeutic directions for MDS. Curr Cancer Drug Targets. 2006;6:553–565. doi: 10.2174/156800906778194595. [DOI] [PubMed] [Google Scholar]
- 6.Slape C, Aplan PD. The role of NUP98 gene fusions in hematologic malignancy. Leuk Lymphoma. 2004;45:1341–1350. doi: 10.1080/10428190310001659325. [DOI] [PubMed] [Google Scholar]
- 7.Arai Y, et al. Heterogenous fusion transcripts involving the NUP98 gene and HOXD13 gene activation in a case of acute myeloid leukemia with the t(2;11)(q31;p15) translocation. Leukemia. 2000;14:1621–1629. doi: 10.1038/sj.leu.2401881. [DOI] [PubMed] [Google Scholar]
- 8.Raza-Egilmez SZ, et al. NUP98-HOXD13 gene fusion in therapy-related acute myelogenous leukemia. Cancer Res. 1998;58:4269–4273. [PubMed] [Google Scholar]
- 9.Kobzev YN, et al. Analysis of translocations that involve the NUP98 gene in patients with 11p15 chromosomal rearrangements. Genes Chromosomes Cancer. 2004;41:339–352. doi: 10.1002/gcc.20092. [DOI] [PubMed] [Google Scholar]
- 10.Pineault N, et al. Induction of acute myeloid leukemia in mice by the human leukemia-specific fusion gene NUP98-HOXD13 in concert with Meis1. Blood. 2003;101:4529–4538. doi: 10.1182/blood-2002-08-2484. [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs S, et al. CD34+ cell selection is required to assess HOXA9 expression levels in patients with myelodysplastic syndrome. Br J Haematol. 2005;130:83–86. doi: 10.1111/j.1365-2141.2005.05555.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106:287–295. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block M, Jacobson LO, Bethard WF. Preleukemic acute human leukemia. J Am Med Assoc. 1953;152:1018–1028. doi: 10.1001/jama.1953.03690110032010. [DOI] [PubMed] [Google Scholar]
- 14.Thanopoulou E, et al. Engraftment of NOD/SCID-beta2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood. 2004;103:4285–4293. doi: 10.1182/blood-2003-09-3192. [DOI] [PubMed] [Google Scholar]
- 15.Kerbauy DM, et al. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood. 2004;104:2202–2203. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 16.Benito AI, et al. NOD/SCID mice transplanted with marrow from patients with myelodysplastic syndrome (MDS) show long-term propagation of normal but not clonal human precursors. Leuk Res. 2003;27:425–436. doi: 10.1016/s0145-2126(02)00221-7. [DOI] [PubMed] [Google Scholar]
- 17.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 18.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 19.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19− hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 20.Anderson K, et al. Ectopic expression of PAX5 promotes maintenance of biphenotypic myeloid progenitors coexpressing myeloid and B-cell lineage-associated genes. Blood. 2007;109:3697–3705. doi: 10.1182/blood-2006-05-026021. [DOI] [PubMed] [Google Scholar]
- 21.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitoh K, Miura I, Takahashi N, Miura AB. Fluorescence in situ hybridization of progenitor cells obtained by fluorescence-activated cell sorting for the detection of cells affected by chromosome abnormality trisomy 8 in patients with myelodysplastic syndromes. Blood. 1998;92:2886–2892. [PubMed] [Google Scholar]
- 23.Nilsson L, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110:3005–3014. doi: 10.1182/blood-2007-03-079368. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson L, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96:2012–2021. [PubMed] [Google Scholar]
- 25.Juvonen E, Aimolahti A, Volin L, Ruutu T. The prognostic value of in vitro cultures of erythroid and megakaryocyte progenitors in myelodysplastic syndromes. Leuk Res. 1999;23:889–894. doi: 10.1016/s0145-2126(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg PL. Biologic and clinical implications of marrow culture studies in the myelodysplastic syndromes. Semin Hematol. 1996;33:163–175. [PubMed] [Google Scholar]
- 27.Sevilla J, et al. Transient donor cell-derived myelodysplastic syndrome with monosomy 7 after unrelated cord blood transplantation. Eur J Haematol. 2006;77:259–263. doi: 10.1111/j.1600-0609.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi CW, Chung YJ, Slape C, Aplan PD. Impaired differentiation and apoptosis of hematopoietic precursors in a mouse model of myelodysplastic syndrome. Haematologica. 2008 doi: 10.3324/haematol.13042. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.