Abstract
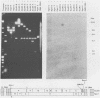
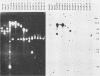
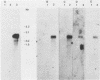
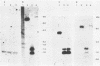
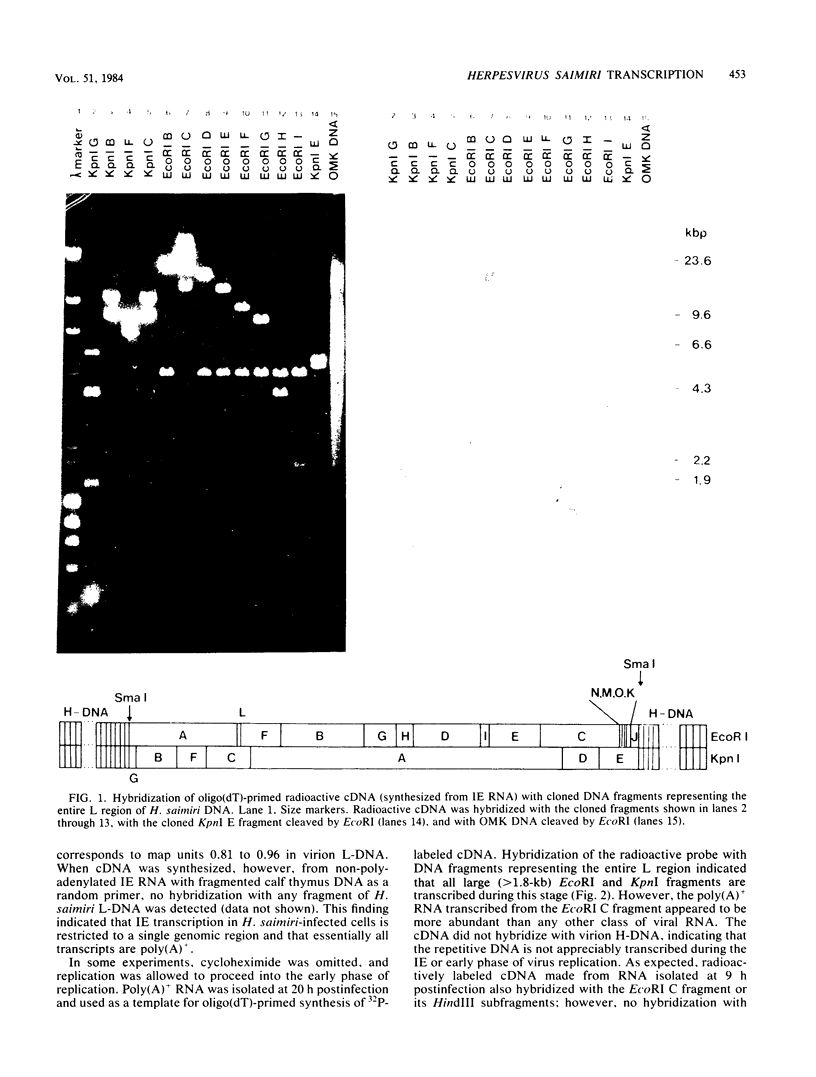
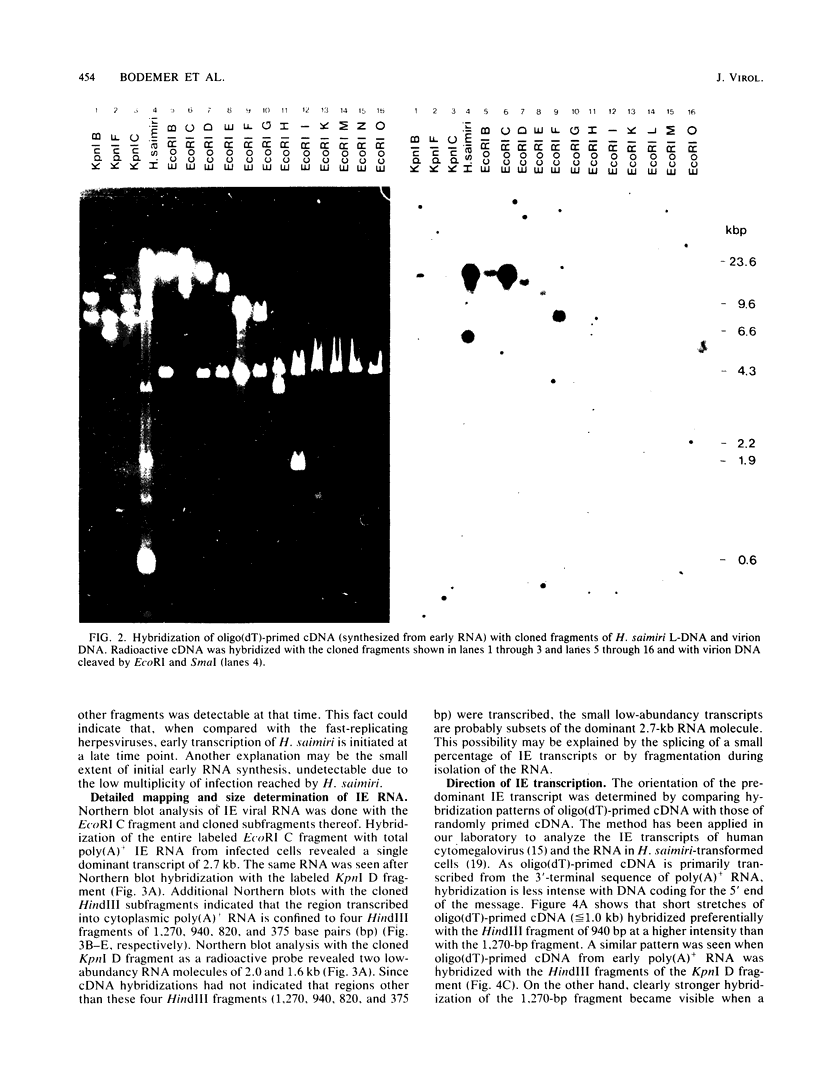
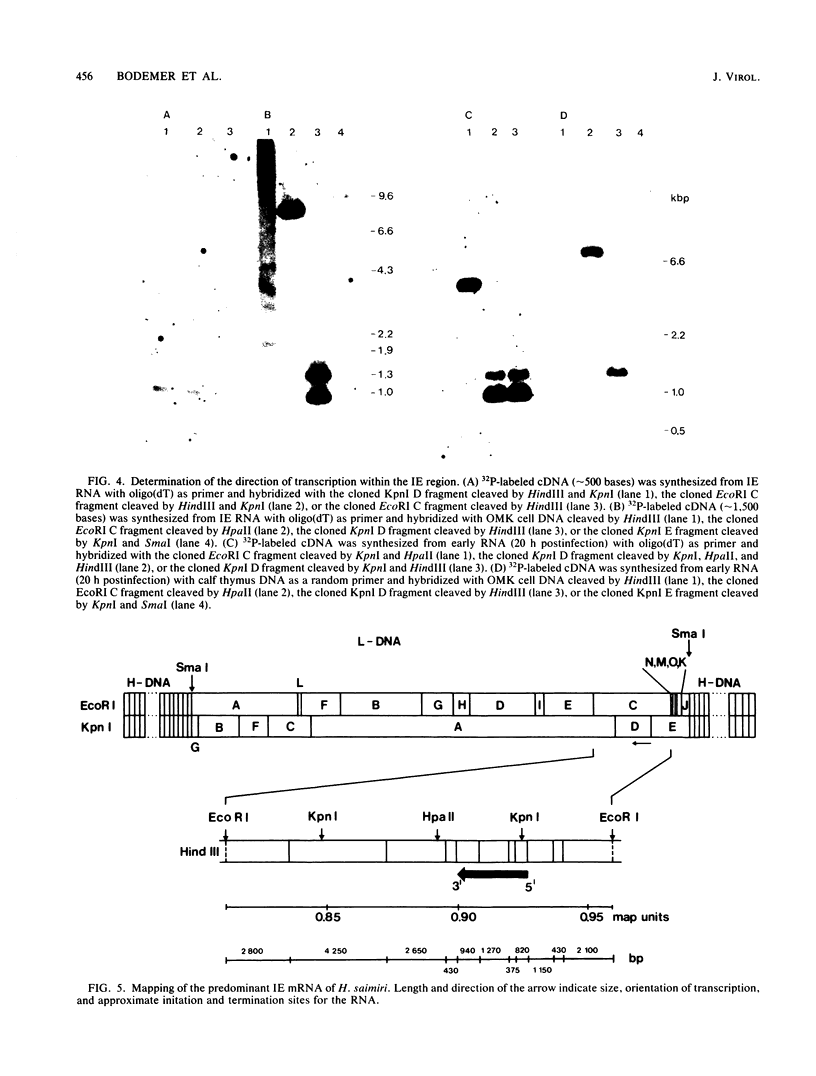
Transcription of Herpesvirus saimiri was characterized during the initial phases of productive infection by Northern blot analyses and hybridizations of radioactive cDNA with cloned fragments of virion L-DNA. Under conditions of immediate-early transcription, e.g., blocking of viral protein synthesis by cycloheximide, a single cytoplasmic polyadenylated viral RNA of 2.7 kilobases was found in infected cells. The sequence coding for this RNA was between map units 0.89 and 0.93; it was transcribed from right to left in prototype arrangement of M-DNA. The immediate-early mRNA of lytically infected cells appeared to be very similar, if not identical, to the single viral RNA species found in lymphoid cells transformed by H. saimiri.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Jean J. H., Kaplan A. S. Early functions of the genome of herpesvirus. IV. Fate and translation of immediate-early viral RNA. Virology. 1974 Jun;59(2):524–531. doi: 10.1016/0042-6822(74)90462-0. [DOI] [PubMed] [Google Scholar]
- Bodemer W. W., Bodemer M. Partial characterization of herpes simplex virus type 2 (HSV-2)-specific poly(A)+ RNA by hybridization to EcoRI-generated HSV-2 DNA fragments. Virology. 1979 Jan 30;92(2):507–517. doi: 10.1016/0042-6822(79)90153-3. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Da Silva M. P. Cuidados de enfermagem nos pacientes com "shunt" e fistula artério-venosa. Rev Enferm Nov Dimens. 1976 Nov;2(5):290–294. [PubMed] [Google Scholar]
- Deinhardt F. W., Falk L. A., Wolfe L. G. Simian herpesviruses and neoplasia. Adv Cancer Res. 1974;19(0):167–205. doi: 10.1016/s0065-230x(08)60054-8. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Deinhardt F. Isolation of Herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus). J Natl Cancer Inst. 1972 May;48(5):1499–1505. [PubMed] [Google Scholar]
- Feldman L. T., Demarchi J. M., Ben-Porat T., Kaplan A. S. Control of abundance of immediate-early mRNA in herpesvirus (pseudorabies)-infected cells. Virology. 1982 Jan 15;116(1):250–263. doi: 10.1016/0042-6822(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Feldman L., Rixon F. J., Jean J. H., Ben-Porat T., Kaplan A. S. Transcription of the genome of pseudorabies virus (A herpesvirus) is strictly controlled. Virology. 1979 Sep;97(2):316–327. doi: 10.1016/0042-6822(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. C., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA VII. alpha-RNA is homologous to noncontiguous sites in both the L and S components of viral DNA. J Virol. 1977 Jan;21(1):268–276. doi: 10.1128/jvi.21.1.268-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Dietrich W., Fleckenstein B., Bodemer W. Virus-specific transcription in a Herpesvirus saimiri-transformed lymphoid tumor cell line. J Virol. 1983 Nov;48(2):377–383. doi: 10.1128/jvi.48.2.377-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Schirm S., Dietrich W., Bodemer W., Kolb E., Fleckenstein B. Cloning of Herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983 Nov;25(2-3):281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Sullivan M., Vande Woude G. F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNA's which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981 Jan;37(1):431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]