Abstract
New neurons are continuously generated in restricted regions of the adult mammalian brain. Although these adult-born neurons have been shown to receive synaptic inputs, little is known about their synaptic outputs. Using retrovirus-mediated birth-dating and labeling in combination with serial section electron microscopic reconstruction, we report that mossy fiber en passant boutons of adult-born dentate granule cells form initial synaptic contacts with CA3 pyramidal cells within 2 weeks after their birth and reach morphologic maturity within 8 weeks in the adult hippocampus. Knockdown of Disrupted-in-Schizophrenia-1 (DISC1) in newborn granule cells leads to defects in axonal targeting and development of synaptic outputs in the adult brain. Together with previous reports of synaptic inputs, these results demonstrate that adult-born neurons are fully integrated into the existing neuronal circuitry. Our results also indicate a role for DISC1 in presynaptic development and may have implications for the etiology of schizophrenia and related mental disorders.
Keywords: adult neurogenesis, synaptogenesis, DISC1, hippocampus, axon guidance
New neurons generated in the adult mammalian brain are believed to contribute to specific brain functions (1–4). Although the existence of synaptic inputs to the dendrites of adult-born neurons has been well documented (5–12), there is a lack of knowledge regarding whether and when the axons of new neurons form synaptic outputs. This makes the claim of their full integration into existing neural circuitry premature. Newborn dentate granule cells (DGCs) in the adult hippocampus are known to extend their axons into the CA3 subfield (8, 13, 14), but it is unclear whether typical synaptic structures are formed by these new neurons in vivo. Using a retrovirus expressing EGFP to birth-date and label adult-born neurons (5, 8, 15), we examined the development of their axons and synaptic outputs by confocal and quantitative immuno-electron microscopy (EM) with 3D reconstruction of labeled boutons (15–17). We show that the axons of adult-born DGCs form typical mossy fiber bouton synaptic complexes with CA3 pyramidal cells within 8 weeks.
To explore the molecular mechanisms regulating axonal outputs by new neurons in the adult brain, we examined the role of disc1, a susceptibility gene for schizophrenia and other major mental illness (18–20). DISC1 (Disrupted-in-Schizophrenia-1) is highly expressed in the developing and adult hippocampus (21), and its expression pattern, together with the recent identification of a large number of DISC1 interacting proteins (22), strongly suggests potential roles for DISC1 in regulating neuronal development (18–20). The in vivo cellular functions of DISC1, however, are not completely understood. Interfering with DISC1 function in utero leads to defects in embryonic cortical neuronal migration and dendritic orientation (23). Perturbation of DISC1 functions in genetically modified mice by deleting a subset of DISC1 isoforms leads to some defects in migration and dendritic orientation of DGCs in the developing brain (24, 25). Interestingly, newborn DGCs with reduced DISC1 expression in the adult hippocampus exhibit neuronal positioning defects and accelerated development of dendrites and formation of synaptic inputs (15). Although a number of in vitro studies have implicated DISC1 in regulating neurite outgrowth of primary neurons and PC12 cells (26, 27), nothing is known about DISC1 function in axonal development, targeting, and presynaptic differentiation in vivo. In this study, we use retrovirus-mediated expression of an shRNA against mouse disc1 (15) to knock down DISC1 expression in newborn DGCs in the adult hippocampus. We here show that knockdown of DISC1 in newborn DGCs results in mistargeting of mossy fibers and accelerated formation of synaptic outputs, pointing to an important role of DISC1 in regulating the development of axons and synaptic outputs of newborn neurons in the adult brain.
Results
Axonal Targeting of Newborn DGCs in the Adult Hippocampus.
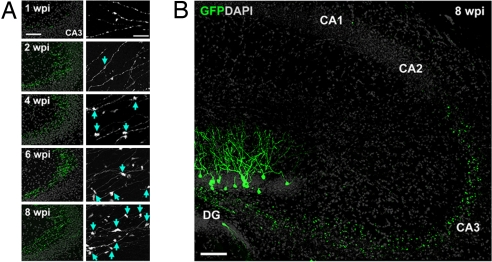
An oncoretrovirus-mediated approach was used to express GFP for birth-dating and genetic labeling of newborn DGCs in the adult mouse hippocampus (see Methods) (5, 7, 8, 15). We first characterized the axonal targeting of GFP-expressing (GFP+) DGCs with confocal microscopy. Consistent with early findings (8, 13, 14), GFP+ axons reached the stratum lucidum of CA3 within 1 week postinjection (wpi; Fig. 1A), although these axons were restricted to proximal CA3 and were relatively few in number. By 1.5 wpi, GFP+ axons were at the curve of CA3, and en passant expansions began to form (data not shown). GFP+ axons did not extend beyond the CA3 border to other subfields at later stages (Fig. 1B). Thus, mossy fibers of adult-born DGCs seem to extend along the same trajectory as preexisting, mature mossy fibers. The presence of bouton-like expansions, which grew visibly larger over time (Fig. 1A), suggests the formation of synaptic complexes by new neurons.
Fig. 1.
Axonal development of newborn granule cells in the adult hippocampus. (A) Sample confocal projection images of axons from newborn DGCs in the CA3 subfield at different time points after retroviral labeling. Shown on the left is a low-magnification view of mossy fiber axons (green) and DAPI staining (gray). (Scale bar, 100 μm.) Shown on the right is an enlarged view of axons with expansions (arrows). (Scale bar, 20 μm.) (B) Sample confocal projection image of the hippocampus at 8 wpi. Note that GFP+ mossy fiber axons did not extend beyond the CA3 subfield. (Scale bar, 100 μm.)
Development of Mossy Fiber Boutons by Newborn DGCs in the Adult Brain.
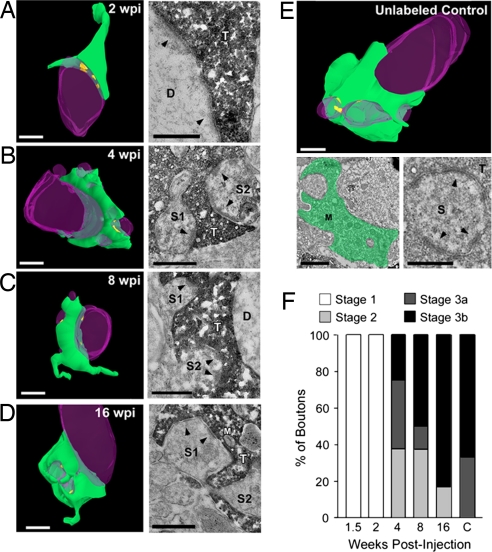
To further characterize synaptic outputs, we performed quantitative immuno-EM with a focus on the large boutons that form synaptic complexes with the apical dendrites of CA3 pyramidal cells (see Methods). We focused our analysis on these boutons because they are one of the major stops for information transfer through the hippocampal trisynaptic circuit. In the hilus and CA3 regions, mossy fibers also target interneurons (28). However, the mossy boutons that contact interneurons are generally small in size and contain one synapse. In contrast, the synaptic complexes analyzed in this study are composed of a large presynaptic mossy fiber bouton and postsynaptic dendritic excrescence from a CA3 pyramidal cell with multiple synaptic contacts forming between the two (16, 17, 29). Given the small percentage of new neurons among all of the existing mature DGCs in the adult hippocampus, unlabeled mossy fiber boutons adjacent to GFP+ boutons were analyzed and used as a control for mature bouton structure.
The changes in bouton structure over development were assessed according to a classic staging paradigm (16, 17) (see Methods). Briefly, stage 1 boutons form either no or few synaptic contacts exclusively onto dendritic shafts (nonspinous synaptic contacts), stage 2 boutons have synaptic contacts onto immature dendritic spines (finger-like structures without discernible organelles), and stage 3 boutons have synaptic contacts onto mature dendritic spines containing organelles, with stage 3a boutons having fewer synaptic contacts onto each dendritic spine (<2 synaptic contacts per spine) than stage 3b boutons (≥2 synaptic contacts per spine).
The unlabeled control boutons exhibited characteristics similar to mature mossy fiber boutons reported previously (16) (Fig. 2E). All of these boutons were assigned to stage 3, with the majority being of the most mature stage of development, stage 3b (Fig. 2F). We next analyzed GFP+ boutons across development. All 1.5-wpi and 2-wpi boutons were immature stage 1 boutons with no invading dendritic spines (Fig. 2F). There were no synaptic contacts in 1.5-wpi boutons, and all synaptic contacts at 2 wpi were formed with dendritic shafts (Fig. 2A and data not shown). By 4 wpi, all boutons were beyond this initial stage of development, and approximately one third of boutons fell into each of the remaining stages (2, 3a, and 3b) (Fig. 2F). These boutons contained invading dendritic spines that varied in maturity level, and all had synaptic contacts onto them (Fig. 2B). At the time points thereafter, boutons continued to mature and increasingly contained mature dendritic spines with a number of synaptic contacts (Fig. 2 C and D). At both 8 and 16 wpi, the morphologic maturity of GFP+ boutons was similar to the mature control boutons that surround them (Fig. 2F).
Fig. 2.
Ultrastructural analysis of adult-born mossy fiber synaptic outputs in the CA3 subfield. (A–D) 3D reconstructions (Left) of serial sections from mossy fiber boutons in the adult mouse with accompanying high-magnification electron micrographs (Right) at 2 wpi (A), 4 wpi (B), 8 wpi (C), and 16 wpi (D). At 2 wpi, synaptic contacts are visible only onto the dendritic shaft (A; arrowheads). Over time, boutons became more mature with synaptic contacts onto dendritic spines (B–D; arrowheads). (E) 3D reconstruction of a mature, unlabeled mossy fiber bouton in the CA3 subfield and accompanying low-magnification (Lower Left) and high-magnification (Lower Right) electron micrographs. The bouton has been pseudocolored in green in the low-magnification image, and the high-magnification image shows synaptic contacts onto an invading dendritic spine (arrowheads). (Scale bars in 3D reconstructions and low-magnification micrograph, 1 μm; in high-magnification micrographs, 0.5 μm.) Green, axonal bouton; purple, dendrite; yellow, synapse. D, dendrite; M, mitochondria; S, dendritic spine; T, axon terminal. (F) Mossy fiber bouton staging paradigm to describe the overall maturation state of boutons across development (see Methods). Shown is the percentage of boutons categorized in each stage for all developmental time points. This suggests that boutons born in the adult brain are qualitatively similar to mature, unlabeled boutons by 8 weeks.
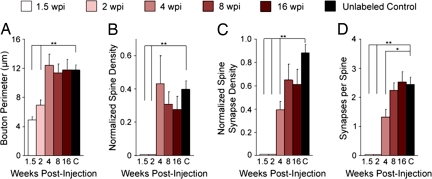
We further quantified the development of the adult-born boutons with several morphologic features that have been used in previous studies (16, 17) [see Methods; all morphologic data are summarized in supporting information (SI) Table S1]. To focus on the synaptic outputs of adult-born mossy fibers, we excluded nonsynaptic puncta adherentia in this analysis (see Methods). At 1.5 wpi, the GFP+ boutons were simple in shape, with no visible mitochondria. They were commonly located apposed to dendritic shafts but did not form any synaptic contacts (n = 4 boutons; Fig. 3 and data not shown). At 2 wpi, the GFP+ boutons were still simple in structure, but they were larger and began to contain mitochondria. Fifty percent of these boutons started to form synaptic contacts, which were exclusively with the dendritic shafts of CA3 pyramidal cells (n = 8 boutons; Figs. 2A and 3). Thus, new DGCs establish nonspinous synaptic outputs as early as 2 weeks after they are born in the adult brain.
Fig. 3.
Quantification of morphologic properties of adult-born mossy fiber boutons. Quantification of average bouton perimeter (A), normalized spine density (B), normalized density of synaptic contacts per bouton onto dendritic spines (C), and number of synaptic contacts per spine (D) at 1.5, 2, 4, 8, and 16 wpi and of unlabeled control boutons (C). This demonstrates that bouton perimeter and spine density are the same as controls by 4 wpi, whereas spinous synaptic contact density and the number of synaptic contacts per spine are the same as controls by 8 wpi. Thus, it takes ≈8 weeks for adult-born mossy fiber boutons to reach morphologic maturity. Values represent mean ± SEM. **P < 0.01; *P < 0.05. See Methods for normalization procedure and refer to Table S1 for raw numbers.
At 4 wpi, the GFP+ boutons had reached a mature size and had a mature number of invading dendritic spines when compared with control boutons (n = 8 boutons; Figs. 2B and 3 A and B). However, even though 80% of their synaptic contacts were localized to dendritic spines, the number of spinous synaptic contacts was significantly smaller than control boutons, indicating that the 4-wpi boutons had not yet reached morphologic maturity (Fig. 3 C and D). At 8 wpi (n = 8 boutons) and 16 wpi (n = 6 boutons), GFP+ boutons were similar to mature control boutons across all parameters (Figs. 2 C and D and 3). Thus, results from both staging and quantitative analyses indicate that by 8 weeks the axons of new DGCs have developed morphologically mature synaptic outputs onto CA3 pyramidal neurons.
Axonal Targeting of Newborn DGCs with DISC1 Knockdown in the Adult Brain.
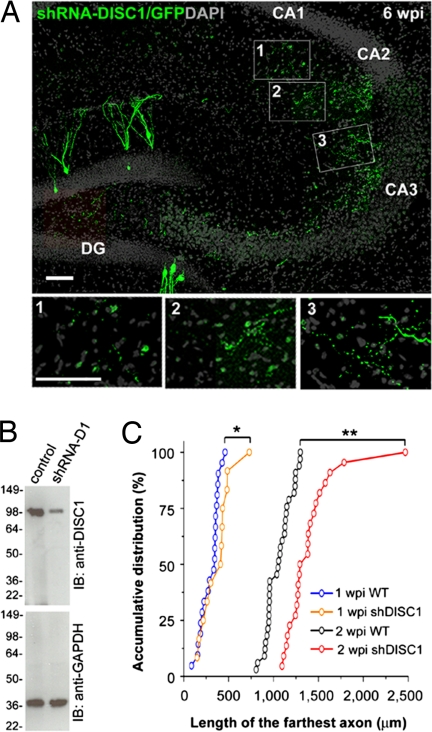
We have recently shown that DISC1 is required for proper dendritic development and formation of synaptic inputs onto newborn DGCs in the adult brain using retrovirus-mediated expression of an shRNA against mouse disc1 (shRNA-D1) (15). Lentivirus-mediated expression of shRNA-D1, but not control lentivirus, led to a significant reduction in the expression of the endogenous full-length DISC1 in primary hippocampal neurons, as shown by Western blot (Fig. 4B). To investigate whether DISC1 is also required for the development of mossy fiber outputs, we used retrovirus-mediated expression of shRNA-D1 to examine axonal targeting and bouton development. A control shRNA against DsRed (shRNA-C1) was used for comparison (15).
Fig. 4.
Axonal development of newborn granule cells with DISC1 knockdown in the adult hippocampus. (A) A sample confocal projection image of mossy fiber axons (green) of newborn neurons with DISC1 knockdown at 6 wpi and DAPI staining (gray). Note the presence of GFP+ axons in the CA1 subfield. (Scale bars, 50 μm.) (B) Knockdown of endogenous full-length DISC1 by shRNA-D1. Primary hippocampal neurons were infected with lentivirus expressing shRNA-D1 and GFP (shRNA-D1), or GFP alone (control), at 2 days in vitro and analyzed at 9 days in vitro by Western blot (IB) using anti-DISC1 antibodies. The membrane was reblotted for GAPDH as a loading control. (C) Quantitative comparison of the length of the farthest axon from WT and shDISC1 mossy fibers at 1 and 2 wpi. Axons with DISC1 knockdown exhibit accelerated outgrowth compared with WT. **P < 0.01; *P < 0.05, Kolmogorov–Smirnov test.
We first examined the growth of adult-born mossy fibers with DISC1 knockdown under the fluorescent microscope. At 1 wpi, the axons with DISC1 knockdown were significantly longer than control axons and had begun to form bouton-like expansions in CA3 (Fig. 4C and data not shown). At 1.5 wpi and all later time points, these axons extended beyond the CA3 border and projected into the CA1 subfield (Fig. 4A and data not shown), which was never observed in control axons. In addition, the axons with DISC1 knockdown seemed to be less tightly restricted to the stratum lucidum of CA3 (Fig. 4A). Thus, DISC1 is required for proper axonal targeting of newborn neurons in the adult brain. It is interesting to note that the aberrant axons with DISC1 knockdown found in CA1 did not form discernible bouton-like expansions until 2 wpi. We characterized these aberrant 2-wpi boutons in more detail using immuno-EM (n = 3 boutons; Fig. 5A′) and found that they made no clear synaptic contacts and were apparently less developed than the 2-wpi boutons with DISC1 knockdown localized within CA3 (Fig. 5A). These observations may imply that, while DISC1 is required for proper axonal targeting of adult-born neurons, its knockdown does not result in the formation of synaptic outputs by aberrant axons with incorrect targets.
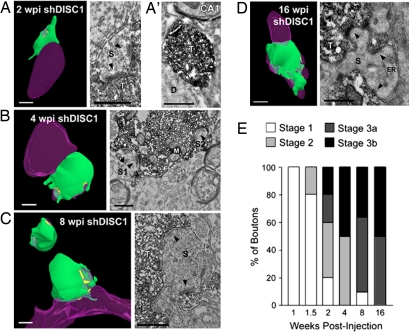
Fig. 5.
DISC1 knockdown accelerates adult-born mossy fiber bouton development. (A–D) 3D reconstructions (Left) of serial sections from mossy fiber boutons with accompanying high-magnification electron micrographs (Right) at 2 wpi (A), 4 wpi (B), 8 wpi (C), and 16 wpi (D). At 2 wpi, synaptic contacts (arrowheads) were found on dendritic spines that invaded the mossy fiber bouton (A). Thus, spine invasion and the formation of synaptic contacts were both accelerated with DISC1 knockdown. A low-magnification electron micrograph (A′) shows that in contrast to 2 wpi boutons in CA3 (A), an aberrant bouton in CA1 at 2 wpi lacks synaptic contacts. Boutons appear more mature at later time points (B–D). (Scale bars in 3D reconstructions and low-magnification micrograph, 1 μm; in high-magnification micrographs, 0.5 μm.) Green, axonal bouton; purple, dendrite; yellow, synapse. D, dendrite; ER, endoplasmic reticulum; M, mitochondria; S, dendritic spine; T, axon terminal. (E) Mossy fiber bouton staging paradigm to describe the overall maturation state of boutons across development (see Methods). Shown is the percentage of boutons categorized in each stage for all developmental time points. This demonstrates that, in comparison with WT boutons, the maturation process is accelerated in boutons with DISC1 knockdown, but fewer boutons reach stage 3b at later stages of development.
Development of Mossy Fiber Boutons by Newborn DGCs with DISC1 Knockdown in the Adult Brain.
We used immuno-EM to analyze the development of the adult-born mossy fiber boutons with DISC1 knockdown that were appropriately localized to the stratum lucidum of CA3. The staging paradigm revealed that the development of boutons with DISC1 knockdown was accelerated compared with WT boutons. At both 1 and 1.5 wpi, the boutons with DISC1 knockdown were at stage 1 (Fig. 5E and data not shown). By 2 wpi, 80% of the boutons with knockdown had developed into stage 2, 3a, or 3b (Fig. 5E), which was significantly accelerated when compared with 2-wpi WT boutons (Fig. 2F). At 4 wpi, 50% of the boutons with knockdown were mature stage 3b boutons (Fig. 5E), which was almost identical to the mature 8-wpi WT boutons (Fig. 2F). At 8 and 16 wpi, there was no further increase in the number of stage 3b boutons with DISC1 knockdown, even though a larger percentage of boutons with knockdown became stage 3a. This suggests that, although DISC1 knockdown accelerates the overall maturation process of adult-born boutons, some of these boutons may not reach the most mature stage of development.
We then performed quantitative analysis on the boutons with DISC1 knockdown. At 1 wpi, boutons were commonly apposed to dendritic shafts and had begun to form synaptic contacts (n = 1 of 5 boutons; data not shown). By 1.5 wpi, the percentage of boutons with knockdown that had synaptic contacts on apposed dendritic shafts had increased to 71% (n = 7 boutons; data not shown). In addition, 29% of boutons had synaptic contacts onto abutting dendritic spines that had yet to invade the mossy fiber boutons (Fig. 6C). Compared with WT boutons, which did not form synaptic contacts until 2 wpi, these results indicate that boutons with DISC1 knockdown form synaptic contacts 4–7 days earlier than WT boutons. At 2 wpi, boutons with DISC1 knockdown already had several invading spines with synaptic contacts, although they were similar in size to 2-wpi WT boutons, which all lacked invading dendritic spines (n = 7 boutons; Figs. 5A and 6). By 4 wpi, the boutons with knockdown were comparable in all respects to mature control boutons (n = 4 boutons; Figs. 5B and 6). Thus, knockdown of DISC1 leads to accelerated synaptic integration and precocious maturation of mossy fiber boutons by newborn DGCs in the adult brain. In accordance with this, we previously found that the formation of synaptic inputs onto the dendrites of adult-born DGCs with DISC1 knockdown was also accelerated (15).
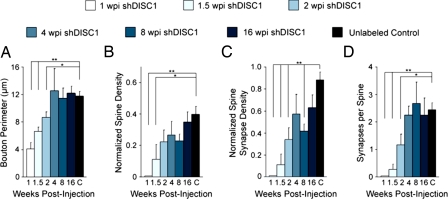
Fig. 6.
Quantification of morphologic properties of adult-born mossy fiber boutons with DISC1 knockdown. Quantification of average bouton perimeter (A), normalized spine density (B), normalized density of synaptic contacts per bouton onto dendritic spines (C), and number of synaptic contacts per spine (D) at 1, 1.5, 2, 4, 8, and 16 wpi and of unlabeled control boutons (C). Control data are repeated from Fig. 3. This demonstrates that bouton perimeter is the same as control by 4 wpi, but the invasion of dendritic spines is accelerated and spine density reaches maturity by 2 wpi. Furthermore, spinous synaptic contact density and the number of synaptic contacts per spine are the same as control by 4 wpi, demonstrating that they reach morphologic maturity faster than WT. Note that boutons at 8 wpi have a significantly smaller spine synapse density than control boutons. Values represent mean ± SEM. **P < 0.01; *P < 0.05. See Methods for normalization procedure and refer to Table S1 for raw numbers.
Finally, we quantified the shRNA-D1/GFP+ boutons at 8 and 16 wpi to explore the effects of DISC1 knockdown after these boutons reached maturity (Fig. 5 C and D). Although the average size of boutons with DISC1 knockdown at 8 wpi was the same as control boutons (n = 11 boutons; Fig. 6A), they had significantly fewer spinous synaptic contacts than control boutons (Fig. 6C). However, when we quantified boutons with DISC1 knockdown at 16 wpi (n = 10 boutons; Fig. 5D), they were very similar to control boutons in all respects, even though more stage 3a boutons were noted in boutons with knockdown (Fig. 5E). Taken together, these findings demonstrate that DISC1 knockdown accelerates the development of adult-born mossy fiber boutons, and implies that at least some of the boutons are unable to fully develop without DISC1.
Discussion
Adult neurogenesis poses a unique challenge to new neurons in that they have to incorporate into a fully functional and active circuit. Integration of a new neuron into a functional neuronal circuit requires proper development of synaptic inputs onto its dendrites and synaptic outputs from its axon. Together with recent findings on dendritic synaptic inputs (8, 9, 15), our study demonstrates that new neurons in the adult hippocampus become fully incorporated into the existing neuronal circuit through a coordinated maturation process of their synaptic inputs and outputs. Our results also indicate that DISC1 is a key player in regulating the maturation rate of newborn neurons in the adult brain in vivo.
Our snapshot ultrastructural analysis of the time course of mossy fiber bouton development suggests the following model (Fig. S1): (i) axons of new DGCs reach the CA3 subfield by 1 to 2 weeks; (ii) presynaptic differentiation occurs and initial synaptic contacts are formed on dendritic shafts by 2 weeks; (iii) mossy fiber boutons grow in size, spine invasion occurs, and spinous synaptic contacts are formed between 2 and 4 weeks; (iv) mossy fiber boutons reach a mature size and contain a mature number of invading dendritic spines at 4 weeks while the number of synaptic contacts within each bouton continues to increase; (v) mossy fiber boutons reach morphologic maturity by 8 weeks and remain stable at 16 wpi. Such a time course is very similar to what has been reported for synaptic inputs onto the dendrites of adult-born DGCs (4, 8, 9): both glutamatergic synaptic inputs onto dendrites (4) and synaptic outputs by axons (this study) are formed at 2 weeks and are not mature until 8 weeks. This indicates that the adult-born neurons coordinate the development of synaptic inputs and outputs to integrate into the existing neuronal circuitry in the adult brain.
Current evidence also indicates that adult-born DGC development is prolonged compared with DGCs born during early postnatal development (12). Growth of both dendrites and axons of adult-born neurons occurs more slowly in the adult hippocampus (8–10). In the developing rodent brain, mossy fiber boutons reach maturity by the beginning of the third postnatal week, when the oldest granule cells in the hippocampus would be ≈4 weeks of age (16). In contrast, the synaptic inputs and outputs of adult-born DGCs are mature by 8 weeks and not 4 weeks as in the neonatal brain. This prolonged time-course of synaptic integration sets a time constraint to when newborn neurons can contribute to neuronal circuitry in the adult brain.
The detailed molecular mechanisms that regulate the synaptic integration of new neurons in the adult brain remain to be determined (12). DISC1 regulates several aspects of synaptic integration of new neurons (15). Our previous in vivo analysis showed that DISC1 knockdown in adult-born DGCs accelerates the development of dendrites, synaptic inputs, and intrinsic neuronal excitability (15) and suggests that DISC1 serves as a regulator of the tempo of neuronal development in the adult brain. In agreement with this view, we find here that DISC1 knockdown accelerates the development of adult-born DGC mossy fibers in vivo (Fig. S1). Axons with DISC1 knockdown exhibit accelerated presynaptic differentiation: initial synaptic contacts are formed by 1 week, synaptic contacts onto dendritic spines are formed by 1.5 weeks, and spine invasion into the bouton occurs by 2 weeks. Thus, synapse formation occurs 4–7 days earlier in boutons with DISC1 knockdown than in WT boutons, and the boutons with DISC1 knockdown precociously reach morphologic maturity by 4 weeks.
We also unexpectedly find that DISC1 regulates axonal targeting of newborn neurons in the adult brain. Adult-born mossy fibers with DISC1 knockdown tend to grow outside the stratum lucidum of CA3 and inappropriately overshoot into the CA1 subfield. Our previous work has shown that DISC1 knockdown has no apparent effect on the targeting of adult-born DGC dendrites (15). Together, this new finding may suggest a differential role for DISC1 in regulating development of axons and dendrites of newborn neurons in the adult brain. However, DISC1 has been shown to play a role in the dendritic orientation of embryonic cortical neurons in vivo (23), and in vitro experiments have shown that DISC1 knockdown in embryonic hippocampal neurons results in a decrease in axonal outgrowth (26, 27). It is therefore likely that the function of DISC1 is diverse and is highly dependent on the context.
After becoming morphologically mature, the adult-born mossy fiber boutons with DISC1 knockdown tend to have fewer total spinous synaptic contacts at 8 wpi. However, this difference is not apparent in boutons with DISC1 knockdown at 16 wpi. Although additional experiments are needed, these observations suggest that DISC1 may regulate the maturation or stability of these boutons. It is interesting to note that a recent EM analysis of mossy fiber boutons from postmortem schizophrenic brains revealed that, although mossy fiber boutons from schizophrenics are the same size as in controls, the boutons in schizophrenic brains have a reduction in both spine and synaptic contact number (30, 31), suggesting that destabilization of the mossy fiber boutons may be important for the pathology of schizophrenia in general.
In summary, the results described here identify new roles of DISC1 in the axonal development of adult-born neurons and support the notion that tempo regulation is critical for integration of new neurons into an active neuronal network. When DISC1 is knocked down, the adult-born DGCs are capable of developing as quickly as DGCs born in the neonatal hippocampus. The mechanisms whereby DISC1 exerts its function in axons remain to be characterized, but the discovery of such novel roles of a susceptibility gene for major mental illness in distinct processes of neuronal development may provide a new perspective on the etiology and pathophysiology of these conditions in humans.
Methods
Construction, Production, and Stereotaxic Injection of Engineered Retroviruses.
Engineered self-inactivating murine oncoretroviruses were used to express EGFP or coexpress GFP and shRNA specifically in proliferating cells and their progeny (5, 15). This virus lacks a nuclear import mechanism and only infects cells that are actively dividing, allowing the specific labeling of newborn granule cells (32). GFP and shRNA were coexpressed from the same viral vector under the control of the EF1α and U6 promoter, respectively (15). The following short-hairpin sequences were used: GGCTACATGAGAAGCACAG (shRNA-D1) and AGTTCCAGTACGGCTCCAA (shRNA-C1). The specificity and efficiency of these shRNAs have been previously characterized both in vitro and in vivo (15). To further confirm the efficacy, lentivirus expressing shRNA-D1/GFP or GFP alone was used to infect P0 primary hippocampal cultures at 2 days in vitro, which was then analyzed for endogenous DISC1 expression at 9 days in vitro by Western blot using anti-DISC1 antibody (goat, Santa Cruz Biotechnology, N-16). High titers of engineered retroviruses (1 × 109 U/ml) were produced as previously described (15). Adult (7- to 8-week-old) female C57BL/6 mice (Charles River) housed under standard conditions were anesthetized, and retroviruses were stereotaxically injected at 4 sites (0.5 μl per site at 0.25 μl/min) with the following coordinates (from bregma in millimeters), as previously described (15): anteroposterior, −2; lateral, ±1.6; ventral, 2.5; and anteroposterior, −3; lateral, ±2.6; ventral, 3.2. All animal procedures were in accordance with institutional guidelines.
Immunostaining, Confocal Imaging, and Electron Microscopy.
For confocal analysis, horizontal or coronal brain sections (40 μm) were prepared from injected mice and processed for immunostaining for GFP (rabbit, 1:1,000–2,000, Abcam) as described in ref. 15. Images were acquired on a Zeiss 510 multiphoton confocal system using a multitrack configuration. For EM analysis, the procedures were essentially the same as previously described (33). Briefly, animals were perfused with 4% paraformaldehyde plus 0.5% glutaraldehyde. Brains were postfixed several hours in 4% paraformaldehyde at 4°C and sectioned at 40–50 μm with a vibratome (Leica VT1000 S). For 1-wpi and 1.5-wpi shRNA-D1/GFP, only one animal was examined for EM analysis. For all other experimental groups, a minimum of two animals was examined. Unlabeled control boutons were selected at random from six animals. Each experimental set, including shRNA-D1/GFP, shRNA-C1/GFP, or GFP-only expressing samples, was processed simultaneously for immunohistochemistry with rabbit anti-GFP antibodies (Invitrogen, 1:2000) and was visualized by a DAB reaction. Sections were slightly permeabilized with 0.02% Triton X-100 because the antibody was unable to penetrate the tissue in the absence of some permeabilization. Using these conditions, we expect that the antibody will penetrate 8 to 9 μm on either surface (34). This results in only partial detection of the GFP+ cells and axons in a given section. In addition, the use of triton results in a slight loss of membrane integrity. Sections containing densely labeled cells and boutons were first evaluated in a light microscope, and sections with large boutons were selected for electron microscopy. Sections were osmicated in 2% OsO4 and dehydrated in gradient ethanol and acetone, then flat-embedded in Araldite. Serial ultrathin sections (70–80 nm) were cut on an ultramicrotome (Leica Microsystems), and sections at least 1 to 2 μm from the surface were collected on Formva-coated single slot grids (EMS). Sections were lightly stained with uranyl acetate and lead citrate and examined under an electron microscope (Philips CM120) at 80 kV. Electron microscopic images were acquired by a 2K × 2K charge-coupled device camera (Gatan). Images were processed using an EM software program (DigitalMicrograph, Gatan). For 3D reconstruction, serial EM images were traced and reconstructed in Reconstruct 2.4 (Boston University) and rendered using 3D Studio Max (Discreet).
Quantification and Statistical Analysis.
For axonal growth analysis, 40-μm horizontal sections from retroviral-injected animals were immunostained for GFP to enhance the signal. Sections were imaged with confocal microscopy at 1-μm intervals, and projection images were used for analysis of axonal growth as previously described (8). Measurements were carried out in a blind fashion to the genetic manipulation. Data are presented in a distribution plot, and statistical significance was assessed using the Kolmogorov–Smirnov test as previously described (15).
For EM, all boutons analyzed were from serial ultrathin section reconstructions. In selecting boutons of newborn neurons for analysis, we preferentially selected large GFP+ boutons that were visible at the light level, because larger boutons tend to exhibit more mature characteristics (16) and our aim was to describe the structure of the most mature boutons at each developmental time point. This selection was done consistently across experimental groups. Given the small percentage of new neurons among all DGCs in the adult hippocampus, the unlabeled boutons adjacent to GFP+ boutons were likely from mature DGCs. Thus, we analyzed neighboring unlabeled mature boutons as controls. These unlabeled boutons were all of similar size and complexity and we did not preferentially select the largest ones.
Reconstructions consisted of anywhere from 3 to 41 serial ultrathin sections and from ≈13 serial sections on average. The perimeter of each bouton was measured from the serial section in which the bouton was largest using Image J (National Institutes of Health). Because the serial sections from analyzed boutons did not represent complete reconstructions and the number of serial sections in reconstructions varied, synaptic contact and spine densities are reported normalized for reconstruction size (unit volume). We calculated the volume for each reconstructed bouton by multiplying bouton perimeter by bouton thickness (the number of serial sections in a reconstruction multiplied by the thickness of each serial section [70 nm]). Synaptic contact and spine densities were normalized for this calculated bouton volume. Synaptic contacts were defined by a postsynaptic density present in at least two serial sections, presynaptically clustered vesicles, and a synaptic cleft. The electron-dense DAB reaction product is generally stronger at early time points examined. In a few cases in which the DAB reaction signal obstructed the view of the presynaptic terminal, postsynaptic density and synaptic cleft alone were used to identify synaptic contacts. Puncta adherentia are excluded from these criteria because they do not associate with synaptic vesicles, and the electron-dense materials associated with puncta adherentia cannot be traced in adjacent sections (35). We report here the normalized number of synaptic contacts localized to dendritic shafts (shaft or nonspinous synaptic contacts) and the normalized number of synaptic contacts localized to dendritic spines (spinous synaptic contacts). The number of synaptic contacts per spine was calculated by dividing the total number of spinous synaptic contacts per bouton by the total number of spines per bouton. Each tissue section was assigned a number for quantitative analysis; however, quantification was not always performed in a blind manner. To ensure that the quantification was not biased, the quantification was validated blindly by an independent party. There were no significant differences between data from shRNA-C1/GFP+ and GFP+ samples, thus data were pooled. All parameters for each time point were then compared with the control in pairs using a two-tailed Student's t test. We have used a Benjamini and Hochberg correction for multiple comparisons. All statistical analyses were performed in Microsoft Excel.
Bouton Staging.
Bouton staging was assessed essentially as described (16, 17), with some modifications. Classically, bouton perimeter was used as a parameter for determining bouton stage. However, in this study, the labeled boutons that were selected for EM analysis tended to be larger ones within each developmental time point; we therefore did not use bouton perimeter as part of the criteria for staging. As previously described (16, 17), boutons classified as stage 1 had synaptic contacts localized only to the dendritic shaft and had no spines or one spine that lacked organelles. Boutons classified as stage 2 contained immature invading dendritic spines that lacked organelles, and these dendritic spines had synaptic contacts onto them. Boutons classified as stage 3 contained invading dendritic spines with organelles and synaptic contacts. The second modification to bouton staging in our study was as follows. We found that stage 3 boutons could be further divided into two subcategories according to the number of synaptic contacts per spine. When boutons reached stage 3, the parameters of the bouton remained relatively constant except for an apparent increase in the number of synaptic contacts per spine. For mature boutons, there was an average of just over two synaptic contacts per spine, which is consistent with a previous report (16). We divided stage 3 boutons into stages 3a and 3b using this value of two synaptic contacts per spine to reflect this developmental change. Stage 3a boutons had fewer than two synaptic contacts per spine, and stage 3b boutons had two or more synaptic contacts per spine.
Supplementary Material
Acknowledgments.
This work was supported by the National Institutes of Health Grants NS047344 (to H.S.), AG024984 (to H.S.), MH084018 (to H.S.), NS048271 (to G.-I.M.), and HD045757 (to H.-J.C.); a McKnight Scholar Award, the Robert Packard Center for ALS Research, the Muscular Dystrophy Association, the Rett Syndrome Research Foundation, and the Simons Foundation (H.S.); Sloan, a Klingenstein Fellowship Award in the Neurosciences, the March of Dimes Foundation, and the Adelson Medical Research Foundation (G.-I.M.); the Whitehall Foundation, a Klingenstein Fellowship Award in the Neuroscience, the Autism Speaks/National Alliance for Autism Research, and the March of Dimes Foundation (H.-J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806658105/DCSupplemental.
References
- 1.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 2.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 3.Kitabatake Y, Sailor KA, Ming GL, Song H. Adult neurogenesis and hippocampal memory function: New cells, more plasticity, new memories? Neurosurg Clin N Am. 2007;18:105–113. doi: 10.1016/j.nec.2006.10.008. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 10.Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 15.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 17.Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): Neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 19.Mackie S, Millar JK, Porteous DJ. Role of DISC1 in neural development and schizophrenia. Curr Opin Neurobiol. 2007;17:95–102. doi: 10.1016/j.conb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Camargo LM, et al. Disrupted in Schizophrenia 1 Interactome: Evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 23.Kamiya A, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka K, et al. Evidence that many of the DISC1 isoforms in C57BL/6J mice are also expressed in 129S6/SvEv mice. Mol Psychiatry. 2007;12:897–899. doi: 10.1038/sj.mp.4002024. [DOI] [PubMed] [Google Scholar]
- 25.Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taya S, et al. DISC1 regulates the transport of the NUDEL/LIS1/14–3–3epsilon complex through kinesin-1. J Neurosci. 2007;27:15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinoda T, et al. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollenhagen A, et al. Structural determinants of transmission at large hippocampal mossy fiber synapses. J Neurosci. 2007;27:10434–10444. doi: 10.1523/JNEUROSCI.1946-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: A postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 31.Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- 32.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XB, Jones EG. Fine structural localization of connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- 34.Piekut DT, Casey SM. Penetration of immunoreagents in Vibratome-sectioned brain: A light and electron microscopic study. J Histochem Cytochem. 1983;31:669–674. doi: 10.1177/31.5.6341457. [DOI] [PubMed] [Google Scholar]
- 35.Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System: The Neurons and Supporting Cells. New York: Oxford Univ Press; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.