Abstract
Retinal dopaminergic amacrine neurons (DA neurons) play a central role in reconfiguring retinal function according to prevailing illumination conditions, yet the mechanisms by which light regulates their activity are poorly understood. We investigated the means by which sustained light responses are evoked in DA neurons. Sustained light responses were driven by cationic currents and persisted in vitro and in vivo in the presence of L-AP4, a blocker of retinal ON-bipolar cells. Several characteristics of these L-AP4-resistant light responses suggested that they were driven by melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs), including long latencies, marked poststimulus persistence, and a peak spectral sensitivity of 478 nm. Furthermore, sustained DA neuron light responses, but not transient DA neuron responses, persisted in rod/cone degenerate retinas, in which ipRGCs account for virtually all remaining retinal phototransduction. Thus, ganglion-cell photoreceptors provide excitatory drive to DA neurons, most likely by way of the coramification of their dendrites and the processes of DA neurons in the inner plexiform layer. This unprecedented centrifugal outflow of ganglion-cell signals within the retina provides a novel basis for the restructuring of retinal circuits by light.
Keywords: adaptation, dopamine, melanopsin, photoreception, vision
Dopaminergic amacrine neurons (DA neurons) comprise the central neuromodulatory system of the retina, forming an intraretinal centrifugal pathway that reconfigures retinal circuits according to prevailing illumination conditions. Through dopaminergic signaling, they restructure retinal function by modulation of chemical and electrical synapses, as well as by modification of the functional properties of retinal neurons, optimizing the encoding of visual stimuli at different levels of illumination (1). DA neurons receive synaptic input in the inner plexiform layer (IPL) of the inner retina and through direct synaptic contacts, or through volume transmission, influence visual signaling by all major classes of retinal neurons, from photoreceptors to ganglion cells (2–6).
Whereas the key role of DA neurons in retinal network modulation is clear, the mechanisms by which illumination regulates DA neuron activity are just beginning to be understood. Dopamine is released in the retina in response to both flickering light and steady background illumination, as well as prolonged darkness (7–9). This functional heterogeneity is reflected at the cellular level in DA neurons as transient, sustained, and null light responses in physiologically distinct neuronal subpopulations (10). The light responses of transient DA neurons are driven by rod or cone photoreceptors through ON-bipolar cells, the neurons that carry excitatory light responses from the photoreceptors to the inner retina. Surprisingly, input from ON-bipolar cells is not essential for the excitatory light responses of sustained DA neurons (10), suggesting the possibility of a novel retinal circuit supporting DA neuron responses to background illumination.
A possible source of the enigmatic light responses recorded in sustained DA neurons may be found among the retinal ganglion cells (RGCs), the glutamatergic projection neurons that transmit visual signals from the retina to the rest of the brain. A small minority of RGCs have recently been shown to express the photopigment melanopsin and to be functional photoreceptors (11, 12). These intrinsically photosensitive ganglion cells (ipRGCs) exhibit sustained excitatory light responses that drive the sustained components of a variety of non-image-forming visual functions, such as circadian photoentrainment and the pupillary light reflex (12–15). Unlike conventional, synaptically mediated ganglion-cell light responses, intrinsic light responses of ipRGCs persist when synapses between photoreceptors and ON-bipolar cells are blocked or when rod and cone photoreceptors have degenerated (11, 16). In addition, the dendrites of ipRGCs and DA neurons costratify within the IPL (17–19) suggesting a possible structural basis for interaction, and melanopsin phototransduction has been implicated in retinal adaptation (20, 21). Here, we report convergent in vitro and in vivo evidence supporting the hypothesis that ipRGCs are the source of sustained DA neuron light responses and thus provide a novel basis for the reconfiguration of retinal circuits by light.
Results
We examined the light input to ON-sustained DA neurons by using in vitro electrophysiology and in vivo light-induced gene expression. Electrophysiological experiments were performed by using TH::RFP transgenic mice that harbor a reporter gene in which the gene promoter for tyrosine hydroxylase (TH) drives the expression of red fluorescent protein (RFP), genetically marking retinal DA neurons for in situ recording (10, 22).
DA Neuron Sustained Light Responses.
To isolate putative melanopsin-driven light responses of DA neurons, we blocked excitatory neurotransmission from photoreceptors to the inner retina by bath application of L-(+)-2–4-amino-4-phosphonobutyric acid (L-AP4), an agonist of the mGluR6 receptor, which blocks transmission of visual signals between photoreceptors and ON-bipolar cells (23). DA neuron light responses were recorded by using extracellular loose patch or whole cell voltage clamp from in vitro retinal whole mounts. Fig. 1A shows a spike recording (Upper) and the corresponding peri-stimulus time histogram (PSTH, Lower) of a light response from a typical transient DA neuron. This neuron exhibited an increase in spike frequency at the onset of a 3 s light pulse that rapidly declined back to baseline within ≈1 s during the light pulse, and then remained at baseline following lights off (left panels). Application of L-AP4 completely abolished the light response (50 μM, right panels). In contrast, the light response of a typical sustained DA neuron also exhibited an initial peak of excitation which decayed to some degree during the light pulse but which maintained an elevated spike rate throughout the entire 3 s light stimulus as well as for some time after lights-off and which persisted in the presence of L-AP4 (Fig. 1B).
Fig. 1.
Transient and sustained light responses in dopaminergic amacrine neurons. (A and B) Extracellular loose patch recordings from a representative transient DA neuron (A) and representative sustained DA neuron (B). (Upper) Spike recordings made before and during L-AP4 application (50 μM). (Lower) Corresponding peri-stimulus time histograms (PSTH, bin width: 300 ms in A, 500 ms in B) before and during L-AP4 application. Bars indicate light on (open bar, duration 3 s, intensity −2 log I) and light off (filled bar). Dashed lines indicate prestimulus baseline. (C–E) Whole-cell voltage clamp recordings of light responses from transient and sustained DA neurons. Light-evoked excitatory inward currents in a transient DA neuron were blocked (C), whereas those in a sustained DA neuron were resistant to 75 μM L-AP4 (D), and L-AP4-resistant currents in a sustained DA neuron were blocked by 40 μM DNQX (E). Stimulus duration 3 s, intensity −1 log I in C and −2 log I in D and E.
Whole cell voltage clamp recordings were performed to investigate the ionic basis of the light input to DA neurons. Light stimulation evoked excitatory inward currents in both the L-AP4 sensitive transient DA neurons (Fig. 1C) and in the L-AP4 resistant sustained DA neurons (Fig. 1D). The light-evoked inward currents in transient neurons exhibited rapid onset and inactivation during light, as well as block by L-AP4, whereas the light-evoked inward currents in sustained neurons exhibited slower decline kinetics and persisted in L-AP4. In sustained neurons, L-AP4 abolished an initial rapid component of light-evoked inward currents, indicating that this rapid component is likely a reflection of the previously described ON-bipolar cell input to ipRGCs (24), whereas the L-AP4 resistant sustained component may be driven by the intrinsic photoresponse of ipRGCs. The brief epi-fluorescence light that was used to localize DA neurons (10) likely produced partial light adaptation of the retinas and may have also reduced the apparent cone input to DA neurons through ipRGCs. Of 41 light-responsive cells, 23 transient cells exhibited L-AP4 sensitive light-induced currents and 18 sustained cells exhibited L-AP4 resistant currents (75–100 μM). Recordings were performed in the presence of intracellular Cs+ and QX-314 to block K+ and Na+ channels, and at a holding potential (−70 mV) that minimized chloride currents. Under these conditions, the light-evoked inward currents observed were cationic, consistent with an excitatory glutamatergic synaptic circuit to sustained DA neurons mediated through AMPA/kainate-type glutamate receptors and consistent with a lack of involvement of chloride-dependent GABAergic and glycinergic OFF-channel signals (10). Indeed, L-AP4-resistant sustained light responses were blocked by the further addition of the AMPA/kainate receptor blocker DNQX (n = 2) (Fig. 1E).
Sustained Light Responses Have Characteristics of Melanopsin Photo-Responses.
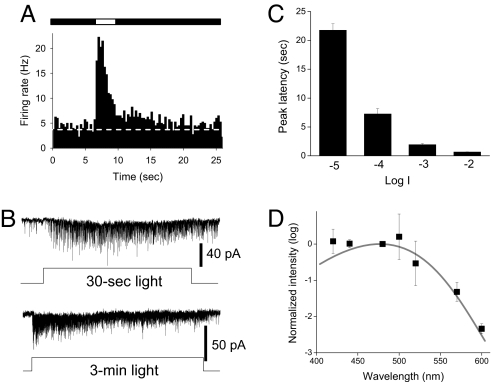
Melanopsin-mediated photo-responses can be distinguished from those originating in rods and cones by their highly prolonged time course, persistence following light termination, long-latency at low light intensities and distinct spectral sensitivity peaking at ≈480 nm (11) [supporting information (SI) Fig. S1]. We thus further characterized the kinetics and spectral sensitivity of ON-sustained DA neuron light responses. Averaged PSTH responses obtained from DA neurons in the presence of L-AP4 showed that spike rates remained at significantly elevated levels over the entire duration of the 3 s light pulses and that spike rates remained elevated for approximately ≈10 s following termination of the light pulse (n = 6) (Fig. 2A). As previously described, there was no significant increase in spike rate at lights-off (i.e., no OFF response), just a persistence of the already elevated rate (10). Similar features, including poststimulus persistence, were evident in whole-cell voltage clamp recordings of sustained DA neurons made in the presence of L-AP4. Light invariably drove inward synaptic currents throughout a 30 s stimulus (n = 6) (Fig. 2B, upper trace) and sustained responses could last at least 3 min (n = 4) (Fig. 2B, lower trace). Response latency was prolonged and intensity-dependent, with time-to-peak of L-AP4 resistant light responses averaging more than 20 s at threshold light intensity and progressively shortening to several hundred milliseconds at higher intensities (n = 6) (Fig. 2C). The action spectrum for L-AP4 resistant light responses in DA neurons exhibited an average peak spectral sensitivity of 478 ± 1.0 nm (n = 4) (Fig. 2D), virtually identical to that of a variety of melanopsin-driven responses (11, 15, 25, 26). By contrast, ON-transient L-AP4 sensitive DA neurons had a peak spectral sensitivity of 500 ± 3.3 nm (n = 5), closer to that of opsins in rods (498 nm) and M-cones (508 nm) (27). Thus, light responses of ON-sustained DA neurons in the presence of L-AP4 exhibit the sustained nature, persistence, long-latency, and spectral sensitivity characteristic of melanopsin-based photo-transduction in ipRGCs (11).
Fig. 2.
L-AP4 resistant DA neuron light responses have characteristics of melanopsin phototransduction. All experiments were performed in the presence of L-AP4. (A) Average PSTH of DA neuron discharge before, during, and after light stimulus recorded by loose patch (bin width: 300 ms, n = 6, intensity −2 log I). (B) Typical whole-cell recordings show light-induced inward current that persisted throughout a 30-s light pulse (upper traces, −4 log I) and a 3-min light pulse (lower traces, −4 log I). (C) The latency of the peak light-induced current was long in duration and decreased as light intensity increased. (D) Lambda-max of the L-AP4-resistant light responses as determined by the method of ref. 26. For additional details see SI Methods. Mean data from 4 DA neurons were fit with Lamb's nomogram.
Sustained Light Responses Persist in Photoreceptor Degenerate Retinas.
If light input to sustained DA neurons is from ipRGCs, as the preceding results suggest, then these responses should also persist in photoreceptor degenerate retinas, in which transduction and transmission by rods and cones is severely compromised, but in which melanopsin phototransduction persists. To test this proposition, we recorded from DA neurons in TH::RFP mice homozygous for the rd1 photoreceptor degeneration mutation (28) (Fig. S2). These experiments used rd1 mice 4–13 months in age in which all rods and at least 98% of cones have degenerated and in which rod/cone-driven light responses are absent in retinal ganglion cells (29, 30). Robust light responses persisted in DA neurons of rd1 retinas. All recorded responses were sustained DA neuron light responses (34 of 34) (Fig. 3A Left); no transient DA neuron responses were observed (0 of 34). In addition, in all cases tested, DA neuron light responses in rd1 retinas were resistant to L-AP4 (19 of 19) (Fig. 3A Right). DA neuron light responses in rd1 retinas also exhibited the poststimulus persistence, highly prolonged time course, and long latency characteristic of ipRGC photo-responses and of L-AP4 resistant DA neuron responses in wild type (WT) retinas. Fig. 3B shows average PSTH responses for DA neurons in rd1 retinas in the absence (Fig. 3B Left) and presence (Fig. 3B Right) of L-AP4 (50 μM), illustrating the persistence of elevated spiking for at least 10 s following termination of a 3 s light pulse (n = 6). DA neuron light responses in rd1 retinas were sustained throughout 1 min light stimuli (n = 4) (Fig. 3C) and exhibited response latencies to low intensity 470 nm light of several seconds that decreased with increasing intensity (n = 4) (Fig. 3D). The degree of poststimulus persistence was more modest following responses to 1 min light pulses, in accordance with the known decline in ipRGC intrinsic photoresponse during prolonged light stimuli (31) and the corresponding decrease in synaptic drive to DA neurons (Fig. 2B). DA neuron light responses in rd1 retinas were blocked by the AMPA/kainate receptor blocker CNQX (n = 4) (Fig. 3E).
Fig. 3.
L-AP4 resistant light responses of DA neurons persist in rod/cone degenerate retinas. (A) DA neuron loose patch recording from a 13-month-old rd1 mouse retina showing light response and persistence in the presence of 50 μM L-AP4. Representative examples of recordings made before (Left) and during (Right) L-AP4 application. (B) Average PSTH before and during L-AP4 application (bin width: 300 ms, n = 6, intensity −2 log I). (C) Average PSTH of DA neuron discharge to light stimuli (duration: 60 s, intensity −2 log I) in the presence of L-AP4 (bin width: 1000 ms, n = 4). (D) Latency of the peak of the light response was long in duration and decreased as light intensity increased (n = 5, 10 s duration). (Inset) Example Gaussian fit of spike frequency to determine latency (see SI Methods). (E) Blockade of light responses with CNQX (100 μM, intensity −2 log I).
In Vivo DA Neuron Photic Responses.
To confirm L-AP4 and photoreceptor degeneration resistant light responses in DA neurons by independent means, light was delivered in vivo to mice and photic activation of DA neurons was subsequently detected by double-label immunocytochemistry for c-Fos and TH. In the absence of light stimulation, expression of the immediate early gene c-fos in the inner retina is virtually undetectable, thus making it possible to map functional circuits in the retina using light-induced c-Fos protein expression. Light-induced c-Fos has been described in the ganglion cell layer, in the inner nuclear layer of rd1 mice (32), in DA amacrine neurons in retinas of both WT animals and in mutant mice possessing deficits in specific retinal circuits (33, 34).
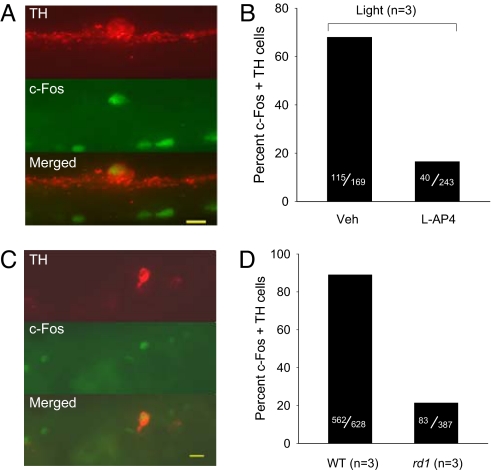
In one experiment, one eye of each mouse was injected with L-AP4 (100 μM) and the other a vehicle control. Light elicited c-Fos induction in 68% of DA neurons in vehicle control eyes and light responses persisted in 16% of DA neurons in L-AP4-injected eyes (Fig. 4 A and B). No c-Fos induction was evident in eyes that did not receive light stimulation (Fig. S3). The proportion of DA neurons exhibiting in vivo L-AP4 resistant light-induced c-Fos responses (16%) was similar to the proportion of DA neurons exhibiting L-AP4 resistant light responses previously measured by loose patch spike recording in vitro (21%) (10) as were the proportions of light responsive cells in the absence of L-AP4 (68% vs. 60%) (10). Similar in vivo light stimulation of untreated rd1 photoreceptor degenerate mice in a second experiment resulted in c-Fos induction in 21% of DA neurons (Fig. 4 C and D), a subset essentially identical in proportion to L-AP4 resistant ON-sustained DA neurons in WT retina. In both experiments, photic activation of ipRGCs was confirmed by double-label immunocytochemistry for melanopsin and c-Fos (data not shown).
Fig. 4.
Light-induced c-Fos gene expression of DA neurons in vivo. (A and B) Light-induced c-Fos induction in the presence and absence of L-AP4. (A) Fluorescence micrographs for immunolocalization of TH (red), c-Fos (green), and colocalization (yellow), calibration bar = 10 μm. Veh, vehicle. (B) Percent c-Fos/TH double-labeled cells in the absence and presence of 100 μM L-AP4. (C and D) Light induced c-Fos gene induction in DA neurons of rd1 mice. (C) Fluorescence micrographs for immunolocalization of TH (red), c-Fos (green), and colocalization (yellow), calibration bar = 12 μm. (D) Percent c-Fos/TH double-labeled cells in WT and rd1 retinas.
Discussion
Sustained DA Neurons are Driven by Melanopsin Phototransduction.
The main finding of this study is that melanopsin-based phototransduction in ipRGCs apparently drives sustained light responses in retinal DA neurons, the principal neuromodulatory system of the vertebrate retina. Sustained drive from ipRGCs to DA neurons is likely responsible for dopaminergic signaling elicited by steady illumination (9, 35) that mediates reconfiguration of the retinal circuits to background light through modulation of retinal networks, neurons, and synapses. Indeed, melanopsin photopigment has been shown to contribute to the modulation of second order neurons in human and mouse retinas (20, 21).
In support of this finding, sustained DA neurons retain their light responses in the face of pharmacological blockade of the known excitatory pathway from photoreceptors to the inner retina and exhibit light responses with all of the hallmarks of melanopsin-based photo-responses in ipRGCs. DA neuron sustained light responses are maintained for tens of seconds to minutes, have latencies on the order of seconds, persist for several seconds after termination of light stimuli, and have peak spectral sensitivity near 480 nm (11). In contrast, retinal ON responses driven by photoreceptors, such as transient DA neuron light responses, exhibit rapid onset and adaptation, and have peak spectral sensitivities at distinct wavelengths of 359, 498, 508 nm corresponding to the absorbance spectra of mouse rod and cone visual pigments (27). The input to sustained DA neurons from ipRGCs was also shown to carry the known input of ON-bipolar cells to ipRGCs (24), reflected as a transient light-induced synaptic current which was blocked by L-AP4 (Fig. 1D).
As additional support we found that light responses of sustained DA neurons persist, both in vitro and in vivo, in photoreceptor degenerate mouse retinas. This, too, implicates a melanopsin drive to DA neurons, because in such mice the great majority of rods and cones have degenerated, the function of ON-bipolar cells is compromised by loss of mGluR6 receptor expression, and there is loss of rod/cone driven light responses in the inner retina (29, 36). Moreover, the persisting ON-sustained DA neuron light responses also exhibit the latency, poststimulus persistence and highly prolonged time-course of melanopsin-based photo-responses. Melanopsin phototransduction in ipRGCs has been shown previously to persist in photoreceptor degenerate retinas and to be the sole remaining photopigment mediating circadian and pupillary photic responses in rd1 retinas (37). In addition to the persistence of sustained responses, we also found that the photoreceptor-driven transient DA neuron light responses were absent from photoreceptor degenerate retinas, providing confirmation of loss of rod and cone function. Taken together, these results provide strong evidence that ipRGCs are the source of light input to sustained DA neurons and present a likely explanation for the persistence of light responsiveness of retinal dopamine levels and rhythms in rod/cone degenerate retinas (38–40). Although Vugler et al., (40) concluded that this light responsiveness was because of residual cones, based on a lack of morphologically identified ipRGC to DA neuron synapses; we now provide positive functional evidence for ipRGC drive to DA neurons and for a lack of cone-driven responses in DA neurons of photoreceptor degenerate mouse retinas.
Circuit for Centrifugal Influence of ipRGCs on DA Neurons.
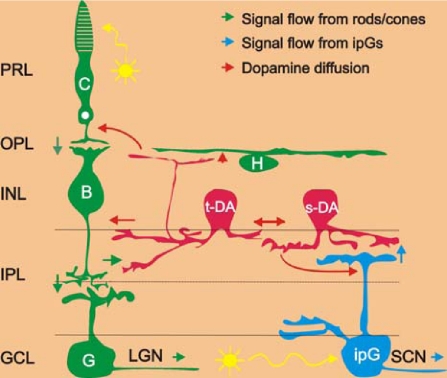
A parsimonious circuit model for the excitatory influence of ipRGCs on sustained DA neurons (Fig. 5) is that glutamate is released from the dendrites of light activated ipRGCs and acts at AMPA/kainate receptors on the costratifying processes of DA neurons in the outermost IPL. This model is consistent with the fact that ipRGCs are glutamatergic (41), that DA neurons express AMPA/kainate receptors (42), that sustained light responses in DA neurons are blocked by AMPA/kainate receptor antagonists (10) (Figs. 1E and 3E), and with preliminary recordings indicating that sustained, L-AP4 resistant DA neuron light responses are absent from melanopsin knockout mouse retinas (D.-Q.Z., P.J.S., G.E.P., and D.G.M., unpublished work). The present data also indicate that the melanopsin-driven excitation of DA neurons is mediated by a cationic conductance in DA neurons, consistent with an ionotropic glutamatergic input.
Fig. 5.
Neuronal circuit diagram of the light input pathways to dopamine cells in the mammalian retina. Blue arrows represent the light signal flow from melanopsin ganglion cells to sustained dopamine cells and the SCN; Green arrows: light signal flow from rods/cones to ganglion cells through ON-bipolar cells to transient dopamine cells and the LGN. Red arrows represent the dopamine diffusion to target cells in all retinal layers. Yellow arrows represent light. C, cones; H, horizontal cells; B, ON-type cone bipolar cells; t-DA, transient dopamine cells; s-DA, sustained dopamine cells; G, ganglion cells; ipG, melanopsin-expressing intrinsically photoreceptive ganglion cells; PRL, photoreceptor layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; LGN, lateral geniculate nuclei, the thalamic visual nuclei of the brain that are innervated by conventional ganglion cells; SCN, suprachiasmatic nuclei, the hypothalamic master biological clock nuclei that are innervated by intrinsically photoreceptive ganglion cells.
RGCs have been shown to be presynaptic to other IPL processes in the catfish retina (43), and a variety of brain neurons have been shown to exhibit dendritic glutamate release, including mitral cells of the olfactory bulb, cerebellar Purkinje cells, and cortical pyramidal cells (44–47), but to our knowledge, there is no precedent for glutamate release from dendrites of mammalian RGCs. To date, no vesicles or other presynaptic specializations have been observed within melanopsin-immunoreactive dendrites where they contact the processes of DA neurons (17–19, 48). This may be because conventional synapses between these cells occur infrequently at sites of mutual contact or they may occur outside the main DA and melanopsin plexus in the outer IPL (OFF sublamina), perhaps in the inner IPL (ON sublamina) in which both ipRGCs and DA neurons have sparse processes and in which ON-bipolar cell input to transient DA neurons is proposed to occur (10, 18, 49). Alternately, ipRGC drive to DA neurons could proceed via an unconventional synaptic arrangement such as nonvesicular transmitter release, as occurs in the outer retina (50) and activation of extrasynaptic glutamate receptors on DA neurons (42).
Alternative explanations for the melanopsin-based responses in DA neurons are at odds with available data. For example, the possibility of intrinsic melanopsin-based phototransduction by DA neurons can be excluded because neither the melanopsin protein nor its mRNA has been detected in these cells (12, 19) and because AMPA/kainate receptor antagonists block the sustained DA neuron photo-response at both the spiking and synaptic levels in the presence of L-AP4 and in photoreceptor degenerate retinas (10) (Figs. 1E and 3E). Although ipRGCs have been suggested to make gap junctional contacts with other inner retinal neurons (16), electrical coupling between ipRGCs and DA cells cannot directly mediate the melanopsin signal analyzed here because it is vulnerable to blockade of ionotropic glutamatergic transmission. Recently, long-latency ON responses, proposed to be carried by the retinal OFF channel, have been described in mouse and zebrafish ganglion cells under circumstances in which the ON channel is genetically or pharmacologically inactivated (51, 52). This mechanism is unlikely to underpin the sustained light responses of DA neurons because the OFF channel-mediated GC ON responses are transient and presumably would carry the spectral signature of rod or cone input, not of melanopsin input as observed here.
Ganglion Cell Efferent Input to DA Neurons: BiDirectional Visual Signaling in the Retina.
These findings expand the functional contributions of ipRGCs beyond those already established for circadian, pupillary, and hormonal regulation to a novel role in intraretinal adaptation. Retinal dopamine, released by DA neurons, modulates the function of all major classes of retinal neurons, including photoreceptors and horizontal and bipolar cells in the outer retina (3, 5, 6). Thus, the excitatory influence of ipRGCs on DA neurons provides a basis for sustained photic signals originating in the innermost layer of the retina to feed back centrifugally to the outer retina, reversing the canonical direction of visual signaling (Fig. 5). In contrast to the fast, spatially discrete feed forward pathway, this feedback signaling is slow, modulatory, and, as a consequence of the wide ranging cell processes of both ipRGCs and DA neurons, spatially diffuse. These results establish that information flow in the retina is truly bi-directional and that ganglion cell photoreceptors act as both interneurons for intraretinal visual signaling, as well as projection neurons transmitting visual signals to central visual nuclei.
Methods
Animals.
Transgenic mice expressing RFP under the control of the TH promoter were generated at Vanderbilt University (22). Mice homozygous for the retinal degeneration allele Pde6brdl (C3H/HeJ, rd1) and WT C57BL/6J were obtained from Jackson Laboratory. Animals were maintained under 12-h-light:12-h-dark conditions. All procedures conformed to National Institutes of Health guidelines for work with laboratory animals and were approved by the Institutional Animal Care and Use Committees at Vanderbilt University, Brown University, and Colorado State University.
Loose Patch Voltage-Clamp Recordings.
Tissue preparation, electrophysiological recordings, and data analysis were performed as described previously (10). Light stimuli for loose patch recordings were 470-nm light at an unattenuated intensity of 1.35 × 1015 photons cm−2 s−1. For additional details see SI Methods.
Whole-Cell Voltage Clamp Recordings.
Whole-cell patch recordings were performed as described previously (24, 53). Light stimuli for whole cell recordings were 480-nm light at an unattenuated intensity of 8.4 × 1014 photons cm−2 s−1. For additional details see SI Methods.
Light-Induced c-Fos and Intraocular Injection and Immunocytochemistry.
See SI Methods.
Supplementary Material
Acknowledgments.
We thank Tongrong Zhou, Allison Evans, Connie King, and Anne Simpson for excellent technical assistance. Supported by National Institutes of Health Grants R03 EY0107032 (to D.-Q.Z.), F32 EY16678 and K99 EY18863 (to K.Y.W.), R01 EY12793 and R01 EY17137 (to D.M.B.), NS 035615 and EY 17809 (to G.E.P. and P.J.S.), and R01 EY15815 and EY9256 (to D.G.M.) and the Vanderbilt Vision Core Grant P30-EY008126.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803893105/DCSupplemental.
References
- 1.Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge, MA: Harvard Univ Press; 1987. [Google Scholar]
- 2.Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- 3.Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashida Y, Ishida AT. Dopamine receptor activation can reduce voltage-gated Na+ current by modulating both entry into and recovery from inactivation. J Neurophysiol. 2004;92:3134–3141. doi: 10.1152/jn.00526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nir I, et al. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichinose T, Lukasiewicz PD. Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci. 2007;27:4756–4764. doi: 10.1523/JNEUROSCI.0183-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer B, Ehinger B, Aberg L. [3H]-dopamine release from the rabbit retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215:71–78. doi: 10.1007/BF00414464. [DOI] [PubMed] [Google Scholar]
- 8.Mangel SC, Dowling JE. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985;229:1107–1109. doi: 10.1126/science.4035351. [DOI] [PubMed] [Google Scholar]
- 9.Godley BF, Wurtman RJ. Release of endogenous dopamine from the superfused rabbit retina in vitro: Effect of light stimulation. Brain Res. 1988;452:393–395. doi: 10.1016/0006-8993(88)90046-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DQ, Zhou TR, McMahon DG. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci. 2007;27:692–699. doi: 10.1523/JNEUROSCI.4478-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 12.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 14.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 15.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 17.Viney TJ, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 19.Vugler AA, et al. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205:26–35. doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DQ, Stone JF, Zhou T, Ohta H, McMahon DG. Characterization of genetically labeled catecholamine neurons in the mouse retina. NeuroReport. 2004;15:1761–1765. doi: 10.1097/01.wnr.0000135699.75775.41. [DOI] [PubMed] [Google Scholar]
- 23.Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: A new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- 24.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 26.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs GH, Neitz J, Deegan JF II. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353:655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- 28.Bowes C, et al. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 29.Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol. 2008;99:1408–1421. doi: 10.1152/jn.00144.2007. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez AJ, Garcia-Fernandez JM, Gonzalez B, Foster RG. The spatio-temporal pattern of photoreceptor degeneration in the aged rd/rd mouse retina. Cell Tissue Res. 1996;284:193–202. doi: 10.1007/s004410050579. [DOI] [PubMed] [Google Scholar]
- 31.Hartwick AT, et al. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huerta JJ, Llamosas MM, Cernuda-Cernuda R, Garcia-Fernandez JM. Fos expression in the retina of rd/rd mice during the light/dark cycle. Neurosci Lett. 1997;232:143–146. doi: 10.1016/s0304-3940(97)00595-8. [DOI] [PubMed] [Google Scholar]
- 33.Koistinaho J, Sagar SM. Light-induced c-fos expression in amacrine cells in the rabbit retina. Brain Res Mol Brain Res. 1995;29:53–63. doi: 10.1016/0169-328x(94)00218-4. [DOI] [PubMed] [Google Scholar]
- 34.Hanzlicek BW, Peachey NS, Grimm C, Hagstrom SA, Ball SL. Probing inner retinal circuits in the rod pathway: A comparison of c-fos activation in mutant mice. Vis Neurosci. 2004;21:873–881. doi: 10.1017/S0952523804216078. [DOI] [PubMed] [Google Scholar]
- 35.Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: A dose-response relationship. Brain Res. 1987;424:199–203. doi: 10.1016/0006-8993(87)91211-x. [DOI] [PubMed] [Google Scholar]
- 36.Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 38.Morgan WW, Kamp CW. Dopaminergic amacrine neurons of rat retinas with photoreceptor degeneration continue to respond to light. Life Sci. 1980;26:1619–1626. doi: 10.1016/0024-3205(80)90365-3. [DOI] [PubMed] [Google Scholar]
- 39.Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: Role of the photoreceptors. J Neurochem. 2002;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 40.Vugler AA, Redgrave P, Hewson-Stoate NJ, Greenwood J, Coffey PJ. Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. J Chem Neuroanat. 2007;33:9–22. doi: 10.1016/j.jchemneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Wong KY, Graham DM, Berson DM. The retina-attached SCN slice preparation: An in vitro mammalian circadian visual system. J Biol Rhythms. 2007;22:400–410. doi: 10.1177/0748730407305376. [DOI] [PubMed] [Google Scholar]
- 42.Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E. Extrasynaptic release of dopamine in a retinal neuron: Activity dependence and transmitter modulation. Neuron. 2001;30:211–225. doi: 10.1016/s0896-6273(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 43.Sakai HM, Naka K, Dowling JE. Ganglion cell dendrites are presynaptic in catfish retina. Nature. 1986;319:495–497. doi: 10.1038/319495a0. [DOI] [PubMed] [Google Scholar]
- 44.Duguid IC, Pankratov Y, Moss GW, Smart TG. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci. 2007;27:12464–12474. doi: 10.1523/JNEUROSCI.0178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harkany T, et al. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: Involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trombley PQ, Shepherd GM. Synaptic transmission and modulation in the olfactory bulb. Curr Opin Neurobiol. 1993;3:540–547. doi: 10.1016/0959-4388(93)90053-2. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto K, et al. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 49.Kolb H, Cuenca N, Wang HH, Dekorver L. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol. 1990;19:343–366. doi: 10.1007/BF01188404. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- 51.Renteria RC, et al. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emran F, et al. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci USA. 2007;104:19126–19131. doi: 10.1073/pnas.0709337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.