Abstract
Exposure measurements from several countries indicate that humans are routinely exposed to low levels of bisphenol A (BPA), a synthetic xenoestrogen widely used in the production of polycarbonate plastics. There is considerable debate about whether this exposure represents an environmental risk, based on reports that BPA interferes with the development of many organs and that it may alter cognitive functions and mood. Consistent with these reports, we have previously demonstrated that BPA antagonizes spine synapse formation induced by estrogens and testosterone in limbic brain areas of gonadectomized female and male rats. An important limitation of these studies, however, is that they were based on rodent animal models, which may not be representative of the effects of human BPA exposure. To address this issue, we examined the influence of continuous BPA administration, at a daily dose equal to the current U.S. Environmental Protection Agency's reference safe daily limit, on estradiol-induced spine synapse formation in the hippocampus and prefrontal cortex of a nonhuman primate model. Our data indicate that even at this relatively low exposure level, BPA completely abolishes the synaptogenic response to estradiol. Because remodeling of spine synapses may play a critical role in cognition and mood, the ability of BPA to interfere with spine synapse formation has profound implications. This study is the first to demonstrate an adverse effect of BPA on the brain in a nonhuman primate model and further amplifies concerns about the widespread use of BPA in medical equipment, and in food preparation and storage.
Keywords: monkey, stereology, synaptic plasticity
Since the 1950s, the synthetic xenoestrogen bisphenol A (BPA) has been used in the manufacture of plastics with a broad range of uses, including dental prostheses and sealants (1), the polycarbonate lining of metal cans used to preserve foods (2), baby bottles (3) and the clear plastic cages used to house laboratory animals (4). BPA also is used as an additive in many products. Its global production rate is >6 billion pounds per year. Polycarbonate is less durable than commonly believed, because the ester bond linking BPA molecules to the plastic can be hydrolyzed. With the rate of hydrolysis increasing dramatically under both acidic and basic conditions, and at elevated temperatures, BPA leaches out of polycarbonate containers into food and beverages under normal conditions of use (3–5). As a result, exposure measurement data from several countries, including the United States, indicate that humans are widely exposed to low levels of BPA on a continuous basis (6).
There is considerable debate about whether this exposure represents an environmental risk, based mostly on the fact that some hormonally active chemicals exhibit radically different potencies in different bioassay systems, making it difficult to assess their potential adverse effects (7). For example, in a two-generation trial in rats, BPA was reported to not induce significant reproductive abnormalities at doses up to 200 μg/kg (8), consistent with the relatively low affinity of BPA for the nuclear estrogen receptors (ERs) ERα and ERβ, and its weak bioactivity in standard tests of estrogenicity (9). Nonetheless, some reports have indicated that in mice, BPA doses of 2–100 μg/kg interfere with the development of prostate, preputial, and mammary glands (10), and the central nervous system (11, 12). BPA administration at doses below the U.S. Environmental Protection Agency's (EPA) reference safe daily limit (50 μg/kg) for human exposure also interferes with the development of nonreproductive behaviors, such as play and maze learning, in both female and male rodents (13–17). These developmental actions of BPA are particularly worrisome, because relatively high levels of BPA have been detected in human amniotic fluid and placenta (18, 19). Recent experiments also have revealed that BPA is capable of influencing other receptor mechanisms besides the nuclear ERs that play important roles in the brain, including the membrane ER (20), thyroid hormone receptor (21), and androgen receptor (22–25).
Our laboratory has demonstrated that BPA antagonizes spine synapse formation induced by estrogens and testosterone in limbic brain areas of gonadectomized female and male rats (26, 27). Because sex steroids are widely thought to play critical roles in higher brain activities, such as cognition and mood, through modulating structural and functional synaptic plasticity (28–30), our findings suggest that exposure to low-dose BPA may have widespread effects on brain structure and function.
An important limitation of previous studies of BPA is that the studies were based on rodent animal models. One argument defending the safety of BPA use is that the clinical predictive power and experimental utility of rodent studies is limited, due to dissimilarities between rodent and human endocrine systems and brains. Thus, it is conceivable that the adverse effects of BPA observed in rodents might not occur in primates, including humans, within the dose range expected from normal environmental exposure. To address this issue, we examined the influence of continuous exposure to BPA, at a daily dose representing the EPA's current reference safe daily limit, on estradiol-induced spine synapse formation in hippocampus and prefrontal cortex (PFC) of a nonhuman primate model. Our data indicate that the primate response to estradiol is quite similar to that of rodents, and that BPA completely abolishes the synaptogenic effect of estradiol even at relatively low exposure levels.
Results
Effects of Estradiol and BPA on the Number of Spine Synapses.
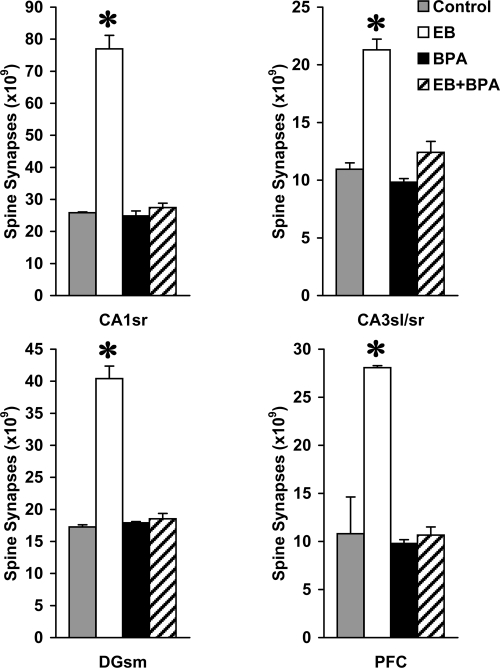
Experimental manipulation of hormone levels and administration of BPA resulted in major changes in the number of spine synapses in all hippocampal and cortical areas examined (Fig. 1). Two-way ANOVA (treatment × sampling area) found significant treatment (F3,32 = 698.815; P < .001) and sampling area effects (F3,32 = 583.567; P < .001), indicating significant differences in the number of spine synapses both among treatment groups and among analyzed areas. In addition, a significant treatment × sampling area interaction effect was seen (F9,32 = 86.304; P < .001), suggesting that the analyzed areas did not respond to treatment in the same way. Compared with vehicle-treated control animals, supplementation with estradiol benzoate (EB) significantly increased the number of spine synapses, by 198.1% in the CA1 stratum radiatum (CA1sr), by 94.7% in the CA3 stratum lucidum and radiatum (CA3sl/sr), by 134.1% in the dentate gyrus stratum moleculare (DGsm), and by 159.9% in the PFC. BPA alone had no effect, whereas administration of BPA along with EB (EB+BPA) completely prevented the strong synaptogenic response to estradiol; both the BPA- and the EB+BPA-treated groups did not differ significantly from the control animals (Fig. 1). These analyses had strong statistical power; with α = 0.05, power was 100% for all of the treatment, sampling area, and treatment × sampling area interaction effects.
Fig. 1.
The number of spine synapses in CA1 stratum radiatum (CA1sr), CA3 stratum lucidum and radiatum (CA3sl/sr), dentate gyrus stratum moleculare (DGsm), and layer II/III of PFC of vehicle-treated (Control), estradiol benzoate-treated (EB), BPA-treated (BPA), and EB+BPA-treated (EB+BPA) monkeys. One-way ANOVA revealed significant treatment effects in all four brain regions examined (CA1sr: F3,8 = 354.2, P < .001; CA3sl/sr: F3,8 = 151.8, P < .001; DGsm: F3,8 = 330.5, P < .001; PFC: F3,8 = 60.7, P < .001). Asterisks above the bars indicate significant differences versus the corresponding sampling area of vehicle-treated controls (Tukey-Kramer test; P < .05). Spine synapse numbers in the BPA and the EB+BPA groups were not significantly different from those of controls.
There were no significant differences among the experimental groups in terms of volumes of brain areas examined (data not shown). For all of the experimental groups combined, mean sampling area volume was 37.51 ± 3.2 mm3 for layer II/III of the PFC, 75.18 ± 10.2 mm3 for the CA1sr, 27.04 ± 2.5 mm3 for the CA3sl/sr, and 39.39 ± 3.4 mm3 for the DGsm.
Serum Estradiol Levels.
A nondetectable level of circulating estradiol was found in the animals that were vehicle- or BPA-treated. In all animals except one that received estradiol treatment (EB- and EB+BPA-treated groups), the serum estradiol concentration was in the range of 80–90 pg/ml, in line with our previous measurements (31). This estradiol level represents the early follicular phase of the monkey menstrual cycle (32). In one animal (EB+BPA-treated), the Silastic capsule was filled with extracellular fluid, indicating that a small hole had developed in the capsule during treatment, leading to a very high estradiol level of 450 pg/ml. This animal was not removed from the study, however, because the number of spine synapses was within the range established for the other animals in the experimental group.
Discussion
This study demonstrates that continuous exposure of ovariectomized nonhuman primates to BPA at a daily dose of 50 μg/kg completely abolishes the synaptogenic effect of estradiol in all hippocampal subregions and layer II/III of the PFC. This finding is in line with our previous results in rat models, demonstrating a strong negative effect of BPA on gonadal hormone–induced spine synapse growth in both gonadectomized females and males (26, 27). The finding of no significant alterations in the volume of brain areas examined indicates that the changes in the number of spine synapses are attributable to varying spine synapse density.
It appears that among the brain regions investigated, the CA1sr and PFC were the most sensitive to estradiol. In both of these regions, the number of spine synapses was about three times higher in the estradiol-treated animals than in all other groups. This increase may seem unusually high compared with our previous study of the same primate species, in which estradiol replacement of ovariectomized monkeys caused only an ≈70% rise in CA1 spine synapse density (31). In this earlier study, however, the animals were maintained on a regular, phytoestrogen-containing diet, whereas in the present experiment, the animals received soybean-free food. Comparing data from the two studies shows that withdrawal of soy caused a ≈50% drop in CA1 spine synapse density, whereas synapse counts after estradiol supplementation were approximately the same in both studies (31). Thus, the difference in the apparent magnitude of estradiol effects between the two studies can be explained by the well established estrogenic action of diets containing crude soy meal (33). However, spine synapses in the CA3sl/sr and DGsm hippocampal areas appear to be less responsive to estradiol. In these regions, the increase in spine synapse numbers was approximately twofold. Many earlier studies have consistently failed to demonstrate morphological cellular plasticity in response to estradiol in these areas (28, 34, 35); however, those studies were based on light microscopic approaches to studying presynaptic and/or postsynaptic elements, and presynaptic and postsynaptic structural changes may not necessarily directly reflect alterations in the number of synapses (36). As our preliminary report demonstrated (37), in line with the present results, proper electron microscopic stereology revealed considerable synaptogenic responses to estradiol across the hippocampus of female rats, including the CA3 and dentate gyrus. Further work is needed to elucidate the relationship between estradiol-induced changes in spine synapses and larger-scale remodeling of dendritic structure observed in such brain regions as the CA1.
As mentioned earlier, due to an accidental leakage from a Silastic capsule, one EB+BPA-treated monkey had a very high serum estradiol level (450 pg/ml), five times higher than that in any of the other estradiol-treated animals. However, even this high level of circulating estradiol was unable to overcome the inhibitory effect of BPA. In this estradiol-overtreated monkey, the number of spine synapses was within the range of that of other EB+BPA-treated animals in every brain region examined.
Functional Considerations and Relevance to Human Health.
Alterations in patterns of synaptogenesis appear to play critical roles in some neurologic/neuropsychiatric disorders, including mental retardation and developmental disabilities (38), Alzheimer's disease (39), schizophrenia (40, 41), and mood disorders (42–44). The neurobiology of these disorders remains unclear, although growing evidence indicates that the intricate balance of effects from growth factors and hormones, which may be required to maintain normal synaptic plasticity, becomes derailed in patients with these diseases. Estrogens derived both from circulation and from local biosynthesis within the brain itself represent an important contributory factor to these mechanisms (45–47). Estradiol has long been known to induce a strong synaptogenic response in limbic brain areas (31, 48, 49). Remodeling of dendritic spines and their synapses may play critical roles both in learning and memory (50) and in the neurobiology of mood disorders (29, 44). Thus, synapse formation on pyramidal cell dendritic spines in the hippocampus and PFC, areas critically involved in mnemonic functions and mood regulation, may contribute to the modulatory effect of gonadal steroids on cognition and mood (28–30, 51, 52). On this basis, the ability of BPA to interfere with spine synapse formation in the hippocampus and PFC has profound implications. The perturbation of play and maze learning behaviors reported in both female and male rodents after developmental BPA exposure (13–17) may be based, at least in part, on this mechanism. In addition, exposure to BPA and the resulting loss of hippocampal spine synapses may elicit depressive behavior. Although limited data are available, two studies have demonstrated that BPA indeed promotes helpless behavior in the learned helplessness paradigm (53) and increases immobility in the forced swim test (54), signs of depressive behavior in two widely accepted animal models of depression.
The mechanism by which BPA exerts its inhibitory effect on synaptogenesis is currently unknown. Because BPA has a relatively low affinity for the ERs (9), a potential explanation could be that BPA directly targets intracellular mechanisms, such as the ERK and Akt pathways, that are activated by estradiol (20, 55) and are involved in the remodeling of spine synapses and cognitive functions (56–59). For example, low concentrations of BPA interfere with estradiol action in cerebellar granule cells, possibly through activation of protein phosphatase 2A–like ERK dephosphorylation (20). A similar mechanism may contribute to the inhibition of estradiol/phospho-ERK–mediated synaptogenesis in the hippocampus and PFC. Moreover, it is not yet clear whether BPA acts directly or indirectly on limbic brain areas. Critical prefrontal and hippocampal functions, such as memory and attention, are all modulated by subcortical cholinergic, dopaminergic, and serotonergic systems (60). Recent studies have demonstrated that oral administration of BPA results in hyperactivity at 4–5 weeks of age, degeneration of mesencephalic dopaminergic neurons at 7 weeks of age, and decreased gene expression levels for dopamine transporter in adult rats (11). It also has been reported that prenatal and neonatal exposure of mice to BPA induces memory impairment as measured by a step-through avoidance test, associated with a dramatic reduction in the cholinergic innervation of the hippocampus at 7 weeks of age (61).
In summary, this study demonstrates for the first time an adverse effect of BPA on spine synapse numbers in the brain of a nonhuman primate model. The powerful inhibition of estradiol-induced hippocampal and PFC spine synapse formation by BPA was observed at the low exposure level defined by the EPA as the reference safe daily limit. Although additional studies are needed, especially in primates, these findings further amplify concerns about the widespread use of BPA in the production of materials used in food preparation and storage.
Materials and Methods
Animals.
Young adult female African green monkeys (Chlorocebus aethiops sabaeus) of reproductive age were used (n = 12; body weight, 4–5 kg). Animals and sterile surgical facilities were provided by the Saint Kitts Biomedical Research Foundation (SKBRF). The SKBRF traps or breeds its animals and has a complete 24-h veterinary service. The facility operates in full compliance with all applicable U.S. regulations and has provided an assurance of compliance (#A3005) to the Office of Laboratory Animal Welfare. All animal protocols used in this study were in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by SKBRF's Institutional Animal Care and Use Committee.
Surgery and Hormone Treatment.
All monkeys were anesthetized (20 mg/kg ketamine, i.m, followed by 20 mg/kg pentobarbital i.v.), intubated, and ovariectomized through a median laparatomy under sterile conditions. In the same surgical session, animals received the following treatments:
Vehicle-treated controls (three animals): a cholesterol-filled 4-cm long Silastic capsule (Dow Corning; 3.5 mm i.d., 4.65 mm o.d.) and a vehicle-filled minipump (Alzet 2ML4 osmotic pump, delivering fluid at a rate of 2.5 μl/h for 4 weeks).
EB-treated group (three animals): a Silastic capsule containing crystalline EB and an Alzet minipump loaded with vehicle.
BPA-treated group (three animals): a cholesterol-filled Silastic capsule and a BPA-filled Alzet minipump.
EB+BPA-treated group (three animals): a Silastic capsule containing crystalline EB and an Alzet minipump loaded with BPA.
We demonstrated that this type of estradiol treatment induces marked changes in CA1 spine synapse density in female monkeys (31). The Alzet minipumps delivering BPA were filled with 4.17 μg/μl of BPA dissolved in propylene glycol (Sigma) to supply BPA at a rate of 50 μg/kg/day. The Silastic capsules and minipumps were implanted below the skin of the back. Minipump performance was verified by measuring the volume of fluid residue extracted from the pumps after the animals were euthanized. After surgery and recovery, the animals were housed in individual cages. They were given buprenorphine analgesia (Buprenex, 0.01 mg/kg i.m.) at the conclusion of surgery, followed by carprofen (Remidil, 2 mg/kg by mouth) every 6 h for the first 2 postoperative days. Water and food intake (soybean-free diet: TD.06476; Harlan Teklad) and incision sites were monitored until complete wound healing occurred.
Euthanasia and Tissue Processing.
Twenty-eight days later, the animals were deeply anesthetized (ketamine 20 mg/kg i.m, followed by an overdose of pentobarbital, 100 mg/kg i.v.). After blood samples were collected, the animals were euthanized by transcardial perfusion of heparinized saline (1.0 liter), followed by a fixative [1.5 liters, containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4)] with the descending aorta clamped. Brains were dissected out and postfixed overnight in the same, but glutaraldehyde-free, fixative. The tissues were stored and transported to Yale University in phosphate buffer containing 0.1% sodium azide.
Unbiased Quantitative Synaptology.
The number of spine synapses in the CA1sr, CA3sl/sr, DGsm, and layer II/III of Walker's area 46 (PFC) was calculated as described in ref. 62. Serial sections (200 μm) were cut in the coronal plane throughout the entire PFC and the hippocampus on a vibratome, and then systematically sorted into 10 groups. One randomly selected group of sections was postfixed in 1% osmium tetroxide (40 min), dehydrated in 70% ethanol containing 1% uranyl acetate for 40 min, and flat-embedded in Durcupan (Electron Microscopy Sciences) between slides and coverslips. The volume of the sampling areas was estimated using the Cavalieri estimator module of the Stereo Investigator system (MicroBrightField). Hippocampal sampling areas could be readily identified in each section due to the highly organized cytoarchitecture of the hippocampus. The boundaries of Walker's area 46 were determined according to the method described by Tang et al. (49).
Subsequently, 20 sampling sites for electron microscopic analysis were localized in each sampling area using a systematic-random approach, as modified from MacLusky et al. (62). In brief, we used a two-dimensional coordinate system with length (L) and height (H) axes. Using the same group of sections that previously underwent volume estimation, the length of the CA1 stratum pyramidale was measured in each section and combined to create the L-axis. The L-axis was then divided by the number of desired sampling sites (L/20), and a random number (N) between 0 and L/20 was selected. The coordinate of the first sampling site on the L axis was localized at N μm and the subsequent coordinates at L/20 μm apart, going along the L axis (the CA1 stratum pyramidale) in the direction of the CA3 > subiculum and from rostral to caudal. Subsequently, the height of CA1sr was measured at each L-axis sampling coordinate, along lines drawn perpendicular to the stratum pyramidale. These 20 height measurements were then combined to create the H-axis. Sampling sites along the H axis were localized using the same method as described for the L-axis, going along the height measurement lines in the direction of the stratum pyramidale > stratum lacunosum moleculare and from rostral to caudal. This technique was repeated to localize sampling sites in the remaining sampling areas. The CA3 stratum pyramidale, dentate gyrus stratum granulosum, and the brain surface represented L-axes for the CA3sl/sr, DGsm, and PFC, respectively. Finally, blocks were assembled for ultracutting and trimmed, and then approximately four 75-nm-thick consecutive ultrasections were cut at each identified sampling site using a Reichert Ultracut E ultrotome.
At each sampling site, digitized electron micrographs (Fig. 2) were obtained for the physical disector using a Tecnai-12 transmission electron microscope (FEI Company) furnished with a Hamamatsu HR/HR-B CCD camera system, at a final magnification of 11,000X. The disector technique requires picture pairs depicting identical regions in adjacent ultrasections, with these identical regions identified by landmarks, such as myelinated fibers, that do not change significantly between adjacent ultrasections due to their size. Before synapse counting, the pictures were coded for blind analysis. This sampling technique provided 20 disectors for each of the CA1sr, CA3sl/sr, DGsm, and PFC (i.e., 80 disectors total per brain).
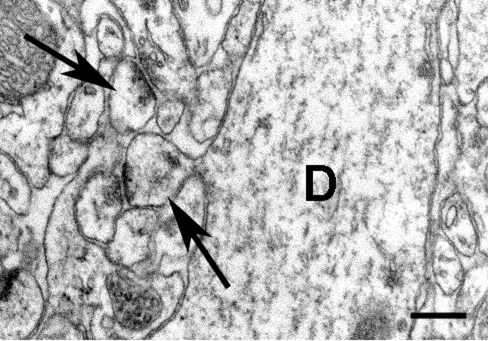
Fig. 2.
Representative high-power electron micrograph from an estradiol-treated monkey demonstrating examples of spines as they form asymmetric synapses with boutons (arrows). One of these spines is emerging from a large dendritic shaft (D). (Scale bar: 250 nm.)
Asymmetric spine synapses were counted according to the rules of the disector technique (63) within an unbiased counting frame superimposed onto each electron micrograph. Synapsing spines were identified by the presence of postsynaptic densities, and by the absence of mitochondria, microtubules, and synaptic vesicles. The average volumetric density (synapse/μm3) of spine synapses within each sampling area was then determined by dividing the sum of spine synapses counted in all samples taken from that particular sampling area by the disector volume. The disector volume was calculated by multiplying the area of the unbiased counting frame (79 μm2) by ultrasection thickness (average 75 nm) and by the number of disectors (i.e., 20). Thus, the average disector volume, uniformly for each sampling area, was 237.6 μm3. Finally, the volumetric density of spine synapses was multiplied by the volume of the sampling area, determined earlier, to arrive at the total number of spine synapses. The number of spine synapses was calculated independently by two different investigators (C.L. and T.H.), and the results were cross-checked to preclude systematic analytical errors.
Statistical Analysis.
Spine synapse numbers obtained from individual sampling areas were used to calculate means ± standard deviations for each treatment group. Results were analyzed using Bartlett's test for homogeneity of variance and two-way (treatment × sampling area) ANOVA, to test for significant interaction effects that might indicate that responses to treatment were region-dependent. The data for each brain region were then analyzed individually by one-way ANOVA, followed by the conservative Tukey-Kramer post hoc test for comparison of individual group means. A criterion for statistical confidence of P < 0.05 was adopted. Typically with these methods, standard deviations for spine synapse numbers are ≈5% of the mean. With a standard deviation of 5% and sample sizes of n = 3 per group, a 15% change in mean spine synapse numbers can be detected with α = 0.05 and 80% power.
Hormone Assay.
Blood samples were allowed to clot, then centrifuged to separate serum. Serum samples were frozen at −80°C until being assayed using an IMMULITE LKE-21 estradiol chemiluminescent enzyme immunoassay kit (Siemens Healthcare Diagnostics). According to the manufacturer's specifications, the analytical sensitivity of the kit is 15 pg/ml, with coefficients of variance of 9.5% for intraassay precision and 9.3% for interassay precision in the concentration range of our samples.
Acknowledgments.
This work was supported by National Institutes of Health Grants ES014893 (to C.L.) and MH074021 (to T.H.), and by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13705.
References
- 1.Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F. Content and release of bisphenol A from polycarbonate dental products. Dent Mater J. 2000;19:389–395. doi: 10.4012/dmj.19.389. [DOI] [PubMed] [Google Scholar]
- 2.Kang JH, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans. J Food Prot. 2003;66:1444–1447. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- 3.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 4.Howdeshell KL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safe SH. Endocrine disruptors and human health: Is there a problem? An update. Environ Health Perspect. 2000;108:487–493. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ema M, et al. Rat two-generation reproductive toxicity study of bisphenol A. Reprod Toxicol. 2001;15:505–523. doi: 10.1016/s0890-6238(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 9.Degen GH, Janning P, Wittsiepe J, Upmeier A, Bolt HM. Integration of mechanistic data in the toxicological evaluation of endocrine modulators. Toxicol Lett. 2002;127:225–237. doi: 10.1016/s0378-4274(01)00504-5. [DOI] [PubMed] [Google Scholar]
- 10.Nagel SC, et al. Relative binding affinity–serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishido M, Yonemoto J, Morita M. Mesencephalic neurodegeneration in the orally administered bisphenol A–caused hyperactive rats. Toxicol Lett. 2007;173:66–72. doi: 10.1016/j.toxlet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Miyagawa K, et al. Changes in central dopaminergic systems with the expression of Shh or GDNF in mice perinatally exposed to bisphenol-A. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007;27:69–75. [PubMed] [Google Scholar]
- 13.Farabollini F, Porrini S, Dessi-Fulgheri F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol Biochem Behav. 1999;64:687–694. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 14.Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(Suppl 3):409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr R, et al. Effect of neonatal rat bisphenol A exposure on performance in the Morris water maze. J Toxicol Environ Health A. 2003;66:2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- 17.Della Seta D, et al. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm Behav. 2006;50:301–307. doi: 10.1016/j.yhbeh.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 19.Schonfelder G, et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal–regulated kinase signaling in the rat cerebellar cortex: Potent nongenomic agonist and endocrine-disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 21.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 22.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 23.Satoh K, Ohyama K, Aoki N, Iida M, Nagai F. Study on anti-androgenic effects of bisphenol a diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem Toxicol. 2004;42:983–993. doi: 10.1016/j.fct.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Xu LC, et al. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Xu LC, Chen JF, Song L, Wang XR. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor–mediated reporter gene. Food Chem Toxicol. 2006;44:1916–1921. doi: 10.1016/j.fct.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008;149:988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 29.Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: Is hippocampal neuroplasticity the key? Neurology. 2006;66:S13–22. doi: 10.1212/wnl.66.66_suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- 30.Li C, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leranth C, Shanabrough M, Redmond DEJ. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 32.Hess DL, Hendrickx AG, Stabenfeldt GH. Reproductive and hormonal patterns in the African green monkey (Cercopithecus aethiops) J Med Primatol. 1979;8:273–281. doi: 10.1159/000460211. [DOI] [PubMed] [Google Scholar]
- 33.Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okado N, Narita M, Narita N. A biogenic amine-synapse mechanism for mental retardation and developmental disabilities. Brain Dev. 2001;23(Suppl 1):S11–S15. doi: 10.1016/s0387-7604(01)00371-0. [DOI] [PubMed] [Google Scholar]
- 39.Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: A review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 41.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 43.Castren E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 45.Kretz O, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouriec K, et al. Synthesis of estrogens in progenitor cells of adult fish brain: Evolutive novelty or exaggeration of a more general mechanism implicating estrogens in neurogenesis? Brain Res Bull. 2008;75:274–280. doi: 10.1016/j.brainresbull.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia–neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y, et al. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 50.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 51.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 52.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 53.Negishi T, et al. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112:1159–1164. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 55.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- 58.Thiels E, Klann E. Extracellular signal–regulated kinase, synaptic plasticity, and memory. Rev Neurosci. 2001;12:327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- 59.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(Suppl 1):i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 61.Miyagawa K, Narita M, Akama H, Suzuki T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci Lett. 2007;418:236–241. doi: 10.1016/j.neulet.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 62.MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- 63.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]