Abstract
Inactivation of voltage-gated calcium channels is crucial for the spatiotemporal coordination of calcium signals and prevention of toxic calcium buildup. Only one member of the highly conserved family of calcium channel β-subunits—CaVβ—inhibits inactivation. This unique property has been attributed to short variable regions of the protein; however, here we report that this inhibition actually is conferred by a conserved guanylate kinase (GK) domain and, moreover, that this domain alone recapitulates CaVβ-mediated modulation of channel activation. We expressed and refolded the GK domain of CaVβ2a, the unique variant that inhibits inactivation, and of CaVβ1b, an isoform that facilitates it. The refolded domains of both CaVβ variants were found to inhibit inactivation of CaV2.3 channels expressed in Xenopus laevis oocytes. These findings suggest that the GK domain endows calcium channels with a brake restraining voltage-dependent inactivation, and thus facilitation of inactivation by full-length CaVβ requires additional structural determinants to antagonize the GK effect. We found that CaVβ can switch the inactivation phenotype conferred to CaV2.3 from slow to fast after posttranslational modifications during channel biogenesis. Our findings provide a framework within which to understand the modulation of inactivation and a new functional map of CaVβ in which the GK domain regulates channel gating and the other conserved domain (Src homology 3) may couple calcium channels to other signaling pathways.
Keywords: auxiliary subunit, modular structure, regulation
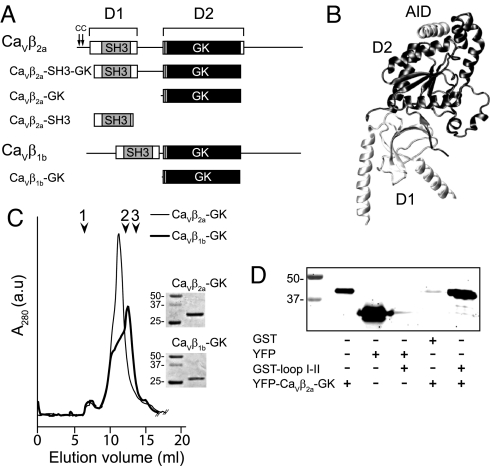
Calcium signals mediate various cellular processes, including neurotransmission, excitation-contraction coupling, hormone secretion, and gene expression (1). Voltage-gated calcium channels (VGCCs) are activated and inactivated on membrane depolarization, allowing transient increases in cytosolic Ca2+ concentration. Voltage-dependent activation and inactivation of VGCCs depend strongly on the association of the ancillary β-subunit (CaVβ) to a highly conserved sequence within the intracellular loop joining the first and second repeats (loop I-II) of the pore-forming subunit (CaVα1), known as the α-interaction domain (AID) (2). CaVβ is encoded by four nonallelic genes (β1–4), each with multiple splice variants. Except for a few short splicing forms (3), all of these genes share a common structural arrangement, consisting of two highly conserved regions separated and flanked by shorter variable sequences (Fig. 1A). Crystallographic studies have revealed that whereas the first region encompasses a Src homology 3 (SH3) domain, the second region encompasses a guanylate kinase (GK) domain. The AID sequence forms an α-helix that fits into a hydrophobic cleft of the GK module that lies on the opposite side of the SH3 domain (Fig. 1B) (4–6). This suggests that the isolated GK module may preserve at least some of the modulatory capabilities of the full CaVβ.
Fig. 1.
Domain structure, purification, and binding assay of CaVβ constructs. (A) Schematic representation of CaVβ2a, CaVβ1b, and derived protein constructs used in this study. CaVβ consists of two highly conserved regions, D1 and D2 (boxes), which are connected and flanked by variable regions (continuous lines). The gray box is the SH3 module; the black box is the GK module. The two cysteine residues at the N terminus of CaVβ2a that undergo palmitoylation are indicated by arrows. (B) A ribbon diagram of the crystal structure of CaVβ in complex with AID (PDB accession code 1T3L). (C) Size-exclusion chromatography elution profile on the Superdex 200 10/30 column (GE Healthcare) of refolded CaVβ2a-GK and CaVβ1b-GK. Here 1 indicates the void volume, 2 indicates the elution volume of albumin (67 kDa), and 3 indicates the elution volume of ovalbumin (43 kDa). The inset shows Coomassie-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) gels of the indicated proteins. Numbers indicate the molecular mass of standards, in kDa. (D) SDS/PAGE gel of the binding reaction with the indicated proteins. Yellow fluorescence protein (YFP)-CaVβ2a-GK is seen to bind specifically to GST–loop I-II (last lane). The binding assay was repeated three times.
Despite several attempts to use different CaVβ constructs and experimental approaches (7–11), the functional competence of isolated GK and its ability to mimic CaVβ function remain incompletely understood. A contributing factor may be the reduced stability of some GK-containing constructs (11). To overcome this difficulty, we expressed and refolded the GK domain of two CaVβ isoforms, CaVβ1b and CaVβ2a. These isoforms share modulatory effects on voltage-dependent activation and exhibit opposite actions on voltage-dependent inactivation. We studied the effect of the refolded GK modules on Xenopus oocytes expressing two types of α1 pore-forming subunits (CaV1.2 and CaV2.3) and compared it with the action of the recombinant full length and also the core of the CaVβ protein containing both SH3 and GK domains. Whereas CaVβ2a is unique in its ability to inhibit voltage-dependent inactivation, the other CaVβ isoforms facilitate this inactivation (12–17). CaVβ2a decelerates inactivation, increases the fraction of noninactivating current, and shifts the steady-state inactivation curve toward more positive potentials. These distinguishing modulatory properties of CaVβ2a have been broadly attributed to palmitoylation of the two contiguous cysteine residues at positions 3 and 4 in the N terminus region (15, 18–20). Here, however, we report that instead, the GK modules derived from both CaVβ2a and CaVβ1b inhibit inactivation of CaV2.3 channels. This finding indicates that the structural determinants of inhibition of inactivation by CaVβ2a are encoded not in variable regions but rather within the GK domain. GK appears to endow calcium channels with a brake to impair voltage-dependent inactivation; therefore, masking the inhibitory effect of GK facilitates inactivation. We show that CaVβ acquires this capability when co-expressed with CaVα1 but not when added later during channel biogenesis. Moreover, CaVβ2a-GK increases peak currents and shifts the activation curve toward more negative potentials of CaV1.2 channels. Thus, GK emerges as a functional unit that recapitulates the hallmarks of CaVβ modulation.
Results
Refolding and Binding Assay of Cavβ-GK Domain.
The GK domain derived from CaVβ1b (CaVβ1b-GK) and CaVβ2a (CaVβ2a-GK) (Fig. 1A) were expressed in bacteria, where they accumulated in inclusion bodies and were refolded by batch dilution. The purified GK domains were concentrated up to 0.1 mg/ml, because further concentration resulted in progressive protein aggregation, as demonstrated by high–molecular mass peaks appearing in the void volume of the size-exclusion chromato graphy column. At 0.1 mg/ml, CaVβ2a-GK eluted from the size-exclusion chromatography in a predominant peak, whereas CaVβ1b-GK exhibited a shoulder at this position and a main peak eluting near the albumin peak (Fig. 1C). Both GK constructs migrated with an apparent molecular mass larger than that predicted by their amino acid sequences. This may reflect either an aberrant migration or formation of higher oligomeric states. To determine whether the refolded GK domains form multimers, we conducted a sucrose gradient analysis [supporting information (SI) Figs. S1–S3]. The CaVβ2a-GK distribution overlapped with a protein standard of 66 kDa, whereas CaVβ1b-GK appeared over a wider range, reaching a second standard of 29 kDa. Thus, our data are consistent with CaVβ2a-GK being essentially a dimer and CaVβ1b-GK being a mixture of dimers and monomers.
To assess the binding ability of CaVβ2a-GK to loop I-II, we fused the former to YFP (YFP-CaVβ2a-GK) and the latter to GST (GST–loop I-II). CaVβ2a-GK was seen to bind specifically to loop I-II; no association between GST and YFP-CaVβ2a-GK or between YFP and GST–loop I-II was detected (Fig. 1D).
Cavβ2a-GK Increases Peak Current Amplitude and Shifts the Current–Voltage Relationship of Cav1.2 Channels.
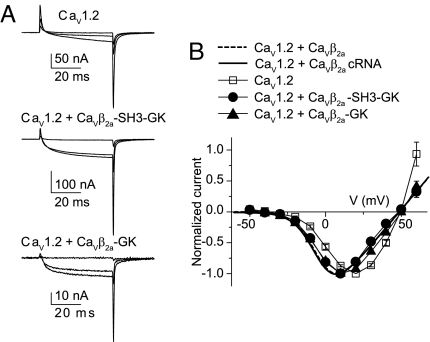
To investigate the modulation of voltage-dependent activation by CaVβ2a-GK, we used the CaV1.2α1 subunit, because it exhibits little inactivation, and the coupling between voltage sensor and channel opening is extremely sensitive to the presence of CaVβ. This CaVα1 isoform also is more suitable for monitoring channel expression through gating current measurements (21). Injection of recombinant full-length CaVβ2a into oocytes expressing CaV1.2 channels results in an increase in the ionic current to charge movement ratio (I/Q) and a leftward shift in the current–voltage relationship (22). CaVβ2a-SH3-GK proved to be equally robust in modulating the activation of CaV1.2 channels (Fig. 2A, Fig. S4, and Table S1). Here, as in a previous report (22), we measured charge movement by integrating outward transient currents at the onset of depolarizing pulses to the ionic current reversal potential. Injection of CaVβ2a-SH3-GK into CaV1.2-expressing oocytes increased the I/Q from 0.3 ± 0.06 nA/pC (n = 15) to 4.6 ± 0.7 nA/pC (n = 17), comparable to the values obtained after injection of CaVβ2a (4.5 ± 0.9 nA/pC; n = 18). The effect of CaVβ2a-GK was seen more clearly at reduced channel expression levels, where gating currents were barely visible and thus I/Q plots were not readily measurable. Nevertheless, the normalized current–voltage relationship was shifted to more-negative potentials and to similar extents with CaVβ2a (as protein or cRNA), CaVβ2a-SH3-GK, and CaVβ2a-GK (Fig. 2B).
Fig. 2.
Refolded CaVβ2a-GK and CaVβ2a-SH3-GK shift the current–voltage relationship of CaV1.2-mediated currents. (A) Representative gating and ionic currents traces from oocytes expressing CaV1.2 cRNA alone and after injection of either CaVβ2a-SH3-GK or CaVβ2a-GK. Currents were evoked by 50-ms pulses to −30, 0, and +30 mV from a holding potential of −80 mV. (B) Normalized current–voltage plot from oocytes expressing the subunit combinations CaV1.2 cRNA (n = 11), CaV1.2 + CaVβ2a-SH3-GK (n = 14), and CaV1.2 + CaVβ2a-GK (n = 16). For comparison, normalized current–voltage curves for CaV1.2 + CaVβ2a, either injected as a protein (dashed line) or co-injected as cRNA (continuous line) are shown (see Table S1 for details).
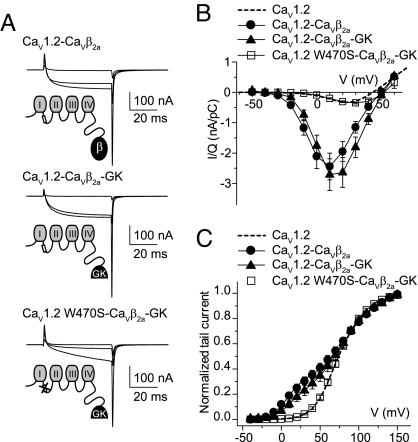
We attribute the limited activity of refolded CaVβ2a-GK to the low concentration of this protein, calculated as 0.3 μM for a 525-nl oocyte. In addition, at high expression levels, a large amount of CaV1.2 subunit remained in the cytoplasm and may have acted as a sink. Another contributing factor could be the protein's decreased stability (see below). To overcome this potential problem, and inspired by a previous experiment demonstrating that CaVβ2a recapitulates channel modulation when attached to the C-termini of CaVα1 (23), we covalently linked CaVβ-GK to CaV1.2 (CaV1.2-CaVβ2a-GK) and compared this with CaV1.2 linked to CaVβ2a (CaV1.2-CaVβ2a). Fig. 3 shows gating and ionic currents from oocytes expressing CaV1.2-CaVβ2a or CaV1.2-CaVβ2a-GK. When covalently linked to CaV1.2, CaVβ2a-GK appears to be as efficient as full-length CaVβ2a in increasing I/Q and shifting the voltage dependence of activation. This effect was abolished by a mutation in the conserved tryptophan within the AID (W470S) shown to prevent binding to CaVβ (22), indicating that the changes in gating properties occurred through specific association of the fused GK moiety to the AID site and not from changes in channel activity generated by the GK linkage.
Fig. 3.
CaVβ2a-GK covalently linked to CaV1.2 WT, but not to CaV1.2 W470S, increases peak current amplitudes and shifts the current–voltage relationship. (A) Representative gating and ionic current traces from oocytes expressing CaV1.2 WT covalently linked to either CaVβ2a (CaV1.2-CaVβ2a) or CaVβ2a-GK (CaV1.2-CaVβ2a-GK), and CaV1.2 W470S covalently linked to CaVβ2a-GK (CaV1.2 W470S-CaVβ2a-GK). Currents were evoked by 50-ms pulses to −30, 0, and +30 mV from a holding potential of −80 mV. (B) Ionic current from oocytes expressing the different constructs were normalized by charge movement (I/Q) and plotted versus voltage. For CaV1.2-CaVβ2a, the peak I/Q was 2.44 ± 0.44 nA/pC (n = 17); for CaV1.2-CaVβ2a-GK, it was 2.71 ± 0.52 nA/pC (n = 17); and for CaV1.2 W470S-CaVβ2a-GK, it was 0.35 ± 0.03 nA/pC (n = 17). For comparison, the average I/Q from 15 oocytes expressing CaV1.2 alone are shown as dashed lines (0.30 ± 0.06 nA/pC). (C) Normalized tail currents from oocytes expressing the different constructs. The continuous lines correspond to the fit of the sum of two Boltzmann distributions, and the dashed line corresponds to the fit obtained from CaV1.2-expressing oocytes (see Fig. S4 and Table S1 for details). The fit to CaV1.2 W470S-CaVβ2a-GK was excluded from the plot for clarity.
Cavβ2a-GK Inhibits Inactivation of Cav2.3 WT Channels.
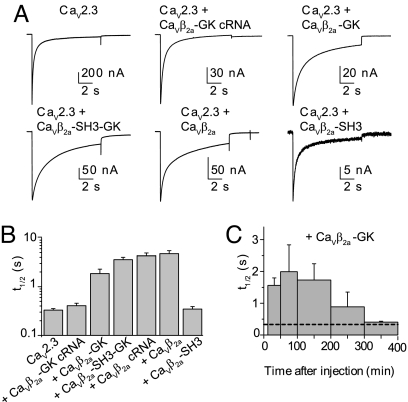
Next, we investigated the ability of CaVβ2a-GK to regulate inactivation kinetics, using the fast-inactivating CaV2.3 channels (Fig. 4). Injection of refolded CaVβ2a-GK into CaV2.3-expressing oocytes resulted in a sixfold increase in the decay time to half-peak current amplitude (t½; Fig. 4 A and B); however, CaVβ2a-GK co-injected as cRNA failed to modulate the CaV2.3-mediated currents. A greater increase in t½ was observed after injection of full-length CaVβ2a, either as protein or co-injected as cRNA, and CaVβ2a-SH3-GK. Consistent with our earlier findings, CaVβ2a-SH3 decreased ionic currents without changing the time course (24). The effect of refolded CaVβ2a-GK vanished 5 h after injection, indicating some degree of protein instability (Fig. 4C); this finding also may explain the lack of effect of CaVβ2a-GK cRNA.
Fig. 4.
CaVβ-GK slows inactivation of CaV2.3-mediated currents. (A) Representative current traces from oocytes expressing CaV2.3 cRNA alone or after injection of the specified protein during a 10-s pulse to 0 mV from a holding potential of −90 mV. (B) Average decay times to half-peak current amplitude (t½) for the different subunit combinations: CaV2.3 cRNA, t½ = 0.33 ± 0.03 s (n = 26); CaV2.3 + CaVβ2a-GK cRNA, t½ = 0.41 ± 0.05 s (n = 13); CaV2.3 + CaVβ2a-GK, t½ = 1.85 ± 0.44 s (n = 13); CaV2.3 + CaVβ2a-SH3-GK, t½ = 3.57 ± 0.42 s (n = 21); CaV2.3 + CaVβ2a cRNA, t½ = 4.11 ± 0.65 s (n = 12); CaV2.3 + CaVβ2a, t½ = 4.76 ± 0.70 s (n = 16); CaV2.3 + CaVβ2a-SH3, t½ = 0.35 ± 0.04 s (n = 13). The t½ values for CaV2.3 + CaVβ2a-GK, CaV2.3 + CaVβ2a-SH3-GK, and CaV2.3 + CaVβ2a were significantly different from those measured in oocytes expressing CaV2.3 alone (t test; P < .01). (C) Time course of inhibition of inactivation by CaVβ2a-GK. Each bar corresponds to the average t½ measured at different time intervals after protein injection. The first bar includes recordings from 12–50 min (n = 4), and the second bar includes recordings from 51–100 min (n = 6) and every 100 min thereafter (n = 7, 2, and 4, respectively). The dashed line corresponds to t½ for CaV2.3 alone.
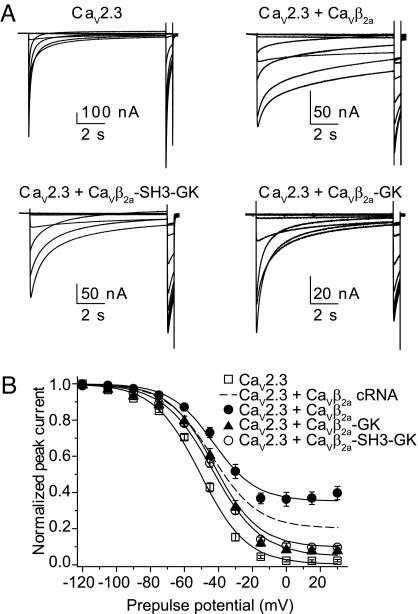
Both CaVβ2a-GK and CaVβ2a-SH3-GK shifted the steady-state inactivation curve of CaV2.3-mediated currents to more positive potentials. A residual current at the end of the pulse also emerged, but it was only a fraction of that seen with full-length CaVβ2a (Fig. 5). Half-inactivation voltages (V½), derived from the fit to a Boltzmann distribution plus the residual component, were similar with CaVβ2a-GK, CaVβ2a-SH3-GK, and full-length CaVβ2a but were significantly more positive than those with CaV2.3 alone (Fig. 5B and Table S2). Overall, CaVβ2a derivatives appear to be less effective than full-length CaVβ2a in inhibiting inactivation and may reflect a contribution of unoccupied CaV2.3 subunits. Nevertheless, inactivation of CaV2.3 channels bearing the W386S mutation that disrupt binding to CaVβ (25) were not modulated by CaVβ2a or CaVβ2a-GK (Fig. S5), indicating that the action of CaVβ2a-GK is AID-dependent and does not involve nonspecific binding. As in previous work (25), we also found that this substitution results in CaV2.3 channels that inactivate more slowly than wild-type channels. Taken together, these findings indicate that the N terminus of CaVβ2a is not mandatory for inhibiting inactivation and predict (owing to the highly conserved nature of the GK domain) that inhibition of inactivation is a property shared by all GKs in the various CaVβ isoforms.
Fig. 5.
CaVβ-GK shifts midpoint voltage for the steady-state inactivation of CaV2.3-mediated currents. (A) Representative traces of CaV2.3-mediated currents in the presence of the specified protein during a steady-state inactivation pulse protocol. This consisted of a 10-s conditioning period to voltages of increasing amplitude, from −120 mV to +30 mV in 15-mV increments, followed by a 0.4-s test pulse to 0 mV. Pulses were delivered once every 50 s from a holding potential of −90 mV. (B) Average steady-state inactivation curves from oocytes expressing CaV2.3 alone (n = 22) or after injection of full-length CaVβ2a (n = 13), CaVβ2a-SH3-GK (n = 23), or CaVβ2a-GK (n = 15). The continuous lines correspond to Boltzmann distributions plus a noninactivating current component that best described each set of data. For comparison, the Boltzmann distributions that best described the CaV2.3 + CaVβ2a cRNA data (dashed line) also are shown. The V½ values for CaV2.3 + CaVβ2a-GK and CaV2.3 + CaVβ2a-SH3-GK are significantly different than the V½ measured in oocytes expressing CaV2.3 alone (t test; P < .01) (see Table S2 for details).
Cavβ1b-GK Resembles Cavβ2a in Inhibiting Inactivation of Cav2.3 WT Channels.
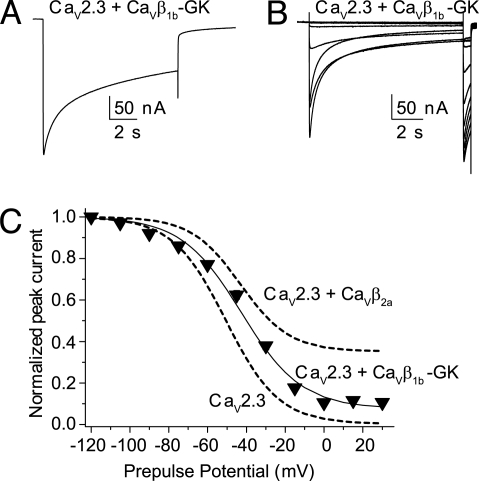
We studied the effect of a GK module derived from CaVβ1b, an isoform that accelerates inactivation and shifts the steady-state inactivation curve to more negative potentials when co-expressed as cRNA (12). Injection of refolded CaVβ1b-GK into oocytes expressing CaV2.3 channels yielded currents that inactivated slowly (t½ = 4.2 ± 0.9 s, n = 16; Fig. 6A). CaVβ1b-GK also shifted the steady-state inactivation curves toward more depolarizing potentials and, like CaVβ2a-GK, induced a residual current (Fig. 6 B and C). Based on these findings, we conclude that the GK module encompasses the minimal structural requirements to inhibit voltage-dependent inactivation. A question that naturally arises is what determines the facilitation of inactivation by full-length CaVβ proteins.
Fig. 6.
CaVβ1b-GK slows inactivation of CaV2.3-mediated currents and shifts the steady-state inactivation toward more depolarized potentials. (A) Representative current traces from a CaV2.3-expressing oocytes injected with CaVβ1b-GK during a 10-s pulse to 0 mV from a holding potential of −90 mV. (B) Current traces evoked with the steady-state inactivation pulse protocol from the same oocytes shown in (A). (C) Average steady-state inactivation curve from oocytes expressing CaV2.3 and injected with CaVβ1b-GK protein (n = 14). For comparison, the Boltzmann distributions that best describe CaV2.3 and CaV2.3 + CaVβ2a data from Fig. 5 also are shown (dashed lines). The V½ value for CaV2.3 + CaVβ1b-GK was significantly different from that measured in oocytes expressing CaV2.3 alone (t test; P < .01) (see Table S2 for details).
Full-Length CaVβ Proteins Switch CaV2.3-Inactivation Phenotype Depending on the Time of Injection.
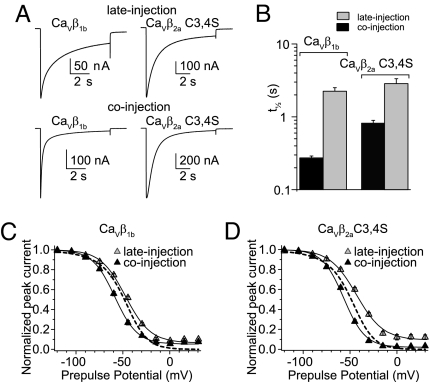
Our finding that the GK module inhibits inactivation implies that full-length proteins that facilitate inactivation must be able to counteract the GK effect. This property has been documented for CaV2.3 channels co-expressed with cRNA encoding full-length CaVβ1b or a palmitoylation-deficient mutant of CaVβ2a bearing two cysteine-to-serine substitutions at positions 3 and 4 (CaVβ2a C3,4S) (12, 18). Here we report that injection of these CaVβ constructs as proteins into oocytes already expressing CaV2.3 channels (late injection) slowed the time to inactivation compared with CaV2.3 alone (Fig. 7 A and B). Furthermore, CaVβ C3,4S shifted the steady-state inactivation curve toward more positive potentials (Fig. 7 C and D and Table S2). Assuming that these full-length proteins were folded correctly, we conjectured that posttranslational modifications requiring longer times than allowed by the experiment were needed to confer their native phenotype. Consequently, we co-injected these proteins together with the cRNA-encoding CaV2.3 subunit (co-injection) and indeed found that inactivation was accelerated (Fig. 7 A and B) and the steady-state inactivation curve shifted toward more negative potentials (Fig. 7 C and D and Table S2). These findings indicate that the recombinant proteins are properly folded, and that after posttranslational modifications during biogenesis of the channel complex, CaVβ can switch the phenotype conferred to CaV2.3 from slow-inactivating to fast-inactivating.
Fig. 7.
The CaV2.3-inactivation phenotype induced by full-length CaVβ1b and CaVβ2aC3,4S depends on the time of injection. (A) Representative current traces from oocytes expressing CaV2.3 channels with CaVβ1b or CaVβ2aC3,4S injected either 2–7 h before recording (late injection) or co-injected with CaV2.3-encoding cRNA (co-injection). Currents were evoked by a 10-s pulse to 0 mV from a holding potential of −90 mV. (B) Average t½ for both of the subunit combinations shown in (A). Using CaVβ1b, the t½ values for co-injection (0.29 ± 0.02 s; n = 11) and late injection (2.19 ± 0.25 s; n = 14) differed significantly. This was true for experiments with CaVβ2aC3,4S as well (co-injection: 0.82 ± 0.08 s, n = 13; late injection: 2.86 ± 0.48 s, n = 16; t test; P < .01). (C) Steady-state inactivation curves from oocytes either co-injected or late-injected with CaVβ1b. The continuous lines correspond to the Boltzmann distributions that best describe each set of data. For comparison, the Boltzmann distributions that best describe the CaV2.3 data from Fig. 5 are shown (dashed lines). (D) As in C, but for CaVβ2aC3,4S. With both proteins, the V½ values from the co-injection experiments were significantly more negative than those from the late-injection experiments (t test; P < .01). For CaVβ2a C3,4S, the V½ values from both the late-injection and co-injection experiments were significantly different from those values for CaV2.3 alone (t test; P < .01). The V½ value for CaVβ1b differed from that of CaV2.3 alone only in the co-injection experiments. With both proteins, t½ values in late-injection and co-injection experiments differed significantly from each other and from those values for CaV2.3 alone (t test; P < .01) (see Table S2 for details).
Discussion
We have demonstrated that the GK module of the VGCC β subunit recapitulates key modulatory properties of the full-length protein, and thus CaVβ-GK emerges as a functional competent domain. This implies that CaVα1–GK interaction suffices for channel gating regulation. Moreover, we have shown that regulation by GK is abolished by a mutation of the AID sequence, consistent with the idea that the AID–GK binding surface is critical for channel modulation (26). Although our size-exclusion chromatography and sucrose gradient analysis revealed that purified GK domains formed dimers in solution, the finding that CaVβ2a-GK covalently linked to a CaV1.2 pore-forming subunit fully recapitulated the activation properties of the channel (Fig. 3) proves that the functional unit is a single GK molecule. Our experiments also demonstrate that CaVβ-GK can sustain the inhibition of voltage-dependent inactivation of CaV2.3 channels. The prevailing view is that this phenotype is conferred uniquely by CaVβ2a, because it is anchored to the membrane and constrains the movement of the inactivating particle encoded by loop I-II (15, 27). Our findings show that instead, inhibition of inactivation is not a unique feature of CaVβ2a, but rather is an inherent property of the GK module that appears to act as a brake to impair voltage-dependent inactivation. A corollary of this conclusion is that facilitation of inactivation by full-length CaVβ requires additional structural determinants to antagonize GK's brake-like effect. Full-length proteins, CaVβ1b and CaVβ2a C3,4S, were able to counteract the action of GK only when they were co-injected with the CaV2.3-encoding cRNA, as if these determinants were acquired within a restricted time window during channel biogenesis.
We envision that in cRNA or protein co-injection experiments, formation of the CaVα1-CaVβ complex in early compartments, such as the endoplasmic reticulum, allows the necessary chemical or structural modifications to counteract GK's brake-like effect. Within this framework, palmitoylation may sequester CaVβ2a to other membranous compartments early in the course of biogenesis, hindering the formation of CaVα1-CaVβ complexes in the compartment, which is permissible for these structural modifications. Alternatively, CaVβ protein may gradually switch to a fast-inactivation–conferring phenotype over a period of several days independent of its location or association state. In any case, we conclude that fast inactivation relies on further posttranslational modifications of CaVβ, although the precise molecular mechanism remains to be identified.
In clear contrast to the protein injection experiments, we observed no modulation when CaVβ-SH3-GK or CaVβ-GK were co-injected as cRNA. Most likely, different parts of CaVβ are important to either efficient translation or stability of the protein in the oocyte cytoplasm. Indeed, modulation of inactivation by CaVβ2a-GK, injected as protein, is rather transient compared with that of the full-length protein, indicating a reduced lifetime. This may explain why previous attempts by either co-expressing or co-injecting the protein more than 24 h before the recordings yielded seemingly contradictory results (7–11). Although the time course of CaVβ2a-GK effect on CaV1.2 activation could not be determined, linking GK to CaV1.2 proved to be an effective strategy for stabilizing this module and increasing its potency. So far, we have been unable to express a concatamer of CaV2.3 with either the full-length CaVβ or the GK domain.
As new protein partners are discovered, the functional role of CaVβ is expanding rapidly (28, 29). We recently found that the SH3 module of CaVβ binds to the endocytotic protein dynamin (24), and now we report that the GK module regulates calcium channel function. Together, these findings introduce a new perspective on CaVβ. Calcium entry through VGCCs on membrane depolarization ensures a transient change in intracellular calcium concentration that regulates diverse cellular functions. Integration of these different cellular processes must be tightly coordinated in living cells, and the domain architecture of CaVβ, with its two functionally independent modules, appears particularly well suited for orchestrating calcium signaling. We suggest that whereas GK regulates calcium entrance, the SH3 domain links channel activity to other cellular processes by binding to additional proteins.
Materials and Methods
Construction of cDNA and Protein Expression.
cDNA encoding the GK domain (residues 201–422), the SH3-GK core (residues 24–422) of rat β2a (Swiss-Prot Q8VGC3–2), and the GK domain (residues 209–413) of the rat β1b (Swiss-Prot P54283) were subcloned by polymerase chain reaction methods into a pRSET vector (Invitrogen). The predicted molecular masses of the CaVβ1b-GK, CaVβ2a-GK, and CaVβ2a-SH3-GK constructs, including the N-terminal His Tag, a transcript-stabilizing sequence, and the enterokinase cleavage recognition site, were 26.9 kDa, 28.6 kDa, and 48.2 kDa, respectively. CaVβ2a-SH3 was prepared as described previously (24). Full-length CaVβ2a, the mutant bearing two substitutions at positions 3 and 4 (CaVβ2a C3,4S) and CaVβ2a-SH3-GK also were prepared as described previously (22). CaVβ1b, CaVβ1b-GK, and CaVβ2a-GK were expressed in bacteria and recovered from inclusion bodies as reported previously (30). The GK domains were refolded by batch dilution (11-fold dilution) in refolding buffer (400 mM l-arginine, 2 mM NaEDTA, 0.5 mM glutathione oxidized, and 100 mM Tris base [pH 7.0]) and subsequently purified by size-exclusion chromatography onto a Superdex S-200 column (GE Healthcare) preequilibrated with nondenaturing buffer (20 mM Tris, 300 mM NaCl, and 1 mM EDTA [pH 8.0]). Proteins were concentrated up to 0.1 mg/ml by ultrafiltration (Amicon Ultra-4 10 kDa MWCO), rapidly frozen, and stored at −80°C until use. The identity of the purified proteins was confirmed by mass spectrometry analysis performed in the mass spectrometry laboratory of the Department of Pharmacology and Toxicology, Medizinische Hochschule Hannover. The protein was digested by trypsin, and the peptides were analyzed with an Ultraflex MALDI-TOF/TOF mass spectrometer (Bruker Daltonics).
The loop I-II of CaV1.2 (Swiss-Prot P15381) fused to GST (GST–loop I-II) was prepared as described previously (30). YFP was fused to CaVβ2a-GK at its N terminus (YFP-CaVβ2a-GK) and subcloned into the pcDNA 3.1 vector. The cDNA encoding CaV1.2 was fused to the GK domain at the carboxyl-terminal end (CaV1.2- CaVβ2a-GK) by overlapping extension polymerase chain reaction, which incorporated the sequence “MGRDLYDDDDKD” at residue 2164 of CaV1.2. All constructs were verified by DNA sequencing.
Binding Assay.
First, tsA201 cells were transfected with a YFP-CaVβ2a-GK or YFP-alone encoding vector and lysed after 24–36 h. Precleared cell extracts were incubated for 1 h with glutathione beads coupled to either GST–loop I-II or GST alone. The beads were pelleted and washed, and bound proteins were eluted with SDS/PAGE loading buffer. Proteins were then resolved on SDS/PAGE and visualized by fluorescence scanning (Typhoon imager; GE Healthcare).
Oocyte Injection and Electrophysiologic Recordings.
cRNA was synthesized and Xenopus laevis oocytes were prepared, injected, and maintained as reported previously (30). The CaV2.3 encoding cDNA was sequenced; compared with the Swiss-Prot entry Q15878, the following changes were noted: I649M, W837L, P838A, and insertion of a glycine residue at position 839. The CaV1.2-subunit used in this study bears 60 aa deletion at the amino terminal (31). Electrophysiologic recordings were performed with the cut-open oocyte technique 4–6 days after cRNA injection and 1–7 hours after protein injection, as described previously (22). For details see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Christoph Fahlke and David Naranjo for insightful discussions and Andreas Pich for the mass spectrometry data. This work was supported by grants from the Deutsche Forschung Gemeinschaft Grant FOR 450 (to P.H.), Anillo de Ciencia y Tecnologia Grant ACT-46 (to A.N.), and German-Chilean Scientific Cooperation Program Grant DFG-CONICYT 2008 to P.H. and A.N.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806558105/DCSupplemental.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Pragnell M, et al. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 3.Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac Cavbeta2 subunit: Redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem. 2004;279:46367–46372. doi: 10.1074/jbc.M409523200. [DOI] [PubMed] [Google Scholar]
- 4.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen YH, et al. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 6.Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)beta modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12:372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase–like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci USA. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He LL, Zhang Y, Chen YH, Yamada Y, Yang J. Functional modularity of the beta-subunit of voltage-gated Ca2+ channels. Biophys J. 2007;93:834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGee AW, et al. Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron. 2004;42:89–99. doi: 10.1016/s0896-6273(04)00149-7. [DOI] [PubMed] [Google Scholar]
- 11.Richards MW, Leroy J, Pratt WS, Dolphin AC. The HOOK-domain between the SH3- and the GK-domains of CaVβ subunits contains key determinants controlling calcium channel inactivation. Channels. 2007;1:92–101. doi: 10.4161/chan.4145. [DOI] [PubMed] [Google Scholar]
- 12.Olcese R, et al. The amino termini of calcium channel β subunits set rates of inactivation independently of their effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 13.Qin N, et al. Identification of a second region of the beta-subunit involved in regulation of calcium channel inactivation. Am J Physiol. 1996;271:C1539–C1545. doi: 10.1152/ajpcell.1996.271.5.C1539. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov S, Weiss RG, Timin EN, Hering S. Modulation of slow inactivation in class A Ca2+ channels by beta subunits. J Physiol (Lond) 2000;527(Pt 3):445–454. doi: 10.1111/j.1469-7793.2000.t01-1-00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restituito S, et al. The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hering S, et al. Molecular determinants of inactivation in voltage-gated Ca2+ channels. J Physiol. 2000;528(Pt 2):237–249. doi: 10.1111/j.1469-7793.2000.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal alpha1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin N, et al. Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc Natl Acad Sci USA. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 20.Hurley JH, Cahill AL, Currie KP, Fox AP. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc Natl Acad Sci USA. 2000;97:9293–9298. doi: 10.1073/pnas.160589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo P, Gonzalez-Gutierrez G, Garcia-Olivares J, Neely A. The alpha 1-beta subunit interaction that modulates calcium channel activity is reversible and requires a competent alpha-interaction domain. J Biol Chem. 2006;281:24104–24110. doi: 10.1074/jbc.M605930200. [DOI] [PubMed] [Google Scholar]
- 23.Dalton S, Takahashi SX, Miriyala J, Colecraft HM. A single CaVβ can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol. 2005;567:757–769. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The Src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- 25.Berrou L, Klein H, Bernatchez G, Parent L. A specific tryptophan in the I-II linker is a key determinant of beta-subunit binding and modulation in Ca(V)2.3 calcium channels. Biophys J. 2002;83:1429–1442. doi: 10.1016/S0006-3495(02)73914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaVα1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berrou L, Bernatchez G, Parent L. Molecular determinants of inactivation within the I-II linker of alpha1E (CaV2.3) calcium channels. Biophys J. 2001;80:215–228. doi: 10.1016/S0006-3495(01)76008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel beta-subunit. Cell Calcium. 2007;42:389–396. doi: 10.1016/j.ceca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Zou S, Jha S, Kim EY, Dryer SE. The beta1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2008;73:369–378. doi: 10.1124/mol.107.040733. [DOI] [PubMed] [Google Scholar]
- 30.Neely A, Garcia-Olivares J, Voswinkel S, Horstkott H, Hidalgo P. Folding of active calcium channel beta(1b) subunit by size-exclusion chromatography and its role on channel function. J Biol Chem. 2004;279:21689–21694. doi: 10.1074/jbc.M312675200. [DOI] [PubMed] [Google Scholar]
- 31.Wei X, et al. Increase in Ca2+ channel expression by deletions at the amino terminus of the cardiac alpha 1C subunit. Receptors Channels. 1996;4:205–215. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.