Abstract
Behavioral research has led to the view that items in short-term memory can be parsed into two categories: a single item in the focus of attention that is available for immediate cognitive processing and a small set of other items that are in a heightened state of activation but require retrieval for further use. We examined this distinction by using an item-recognition task. The results show that the item in the focus of attention is represented by increased activation in inferior temporal representational cortices relative to other information in short-term memory. Functional connectivity analyses suggest that activation of these inferior temporal regions is maintained via frontal- and posterior-parietal contributions. By contrast, other items in short-term memory demand retrieval mechanisms that are represented by increased activation in the medial temporal lobe and left mid-ventrolateral prefrontal cortex. These results show that there are two distinctly different sorts of access to information in short-term memory, and that access by retrieval operations makes use of neural machinery similar to that used in long-term memory retrieval.
Keywords: focus of attention, inferior temporal cortex, working memory, medial temporal lobe, functional magnetic resonance imaging (fMRI)
Fundamental questions about the psychological and neural architecture of short-term memory (STM) have been the subject of research for over a century. This interest is fueled by demonstrations that variations in the amount of information that can be held in mind explain differences in IQ, reasoning, reading comprehension, and problem-solving (1–3). Hence, understanding the STM system that affords online maintenance of information will help us understand a great deal about cognition. In this context, two questions have received much attention: “What is the capacity of STM?” and “What is the relationship of STM to long-term memory (LTM)?”
With regard to capacity, converging behavioral and neural evidence has estimated a limit of approximately four items (4–8). A large body of behavioral research has demonstrated sharp performance discontinuities when STM is loaded with more than four items (4). This behavioral evidence is supplemented by neural data showing that areas within the intraparietal sulcus (IPS) and lateral occipital cortex track these patterns of performance, supporting the idea of a capacity limit of four (5–8).
A different line of behavioral research indicates that there are also sharp distinctions between the single most recently processed item in STM and other recently presented items (9, 10). McElree and colleagues examined retrieval times for probes in an item-recognition paradigm (9, 10). The paradigm used rapid presentation and a very short delay interval so that the most recently presented item was presumably in the focus of attention. Retrieval time for the most recently presented item was faster than retrieval times for all other items in the list. Other studies have demonstrated that updating memory representations is substantially more difficult when one must switch the focus of attention between two representations held in mind (11, 12). These results have led to proposals that within memory, a single item lies in the focus of attention and is uniquely immediately accessible for cognitive operations (9–13). Although some authors have interpreted this result to mean that STM is limited to just one item (9, 10), other authors have proposed that the focus of attention is only one of multiple states of items within STM (11, 13). Either way, there does appear to be excellent behavioral evidence to distinguish the representation of an item within the focus of attention from other items that are not within the focus.
What characteristic distinguishes an item within the focus of attention from other items in STM? One possibility is that compared with other items in STM, the item in the focus of attention is distinguishable from other items simply by greater memory strength. Neurally, this may be realized by enhanced activation in regions of memory storage for the item that is in the focus of attention relative to other items. Such regions of storage most likely include posterior aspects of cortex in the inferior temporal (IT) lobe responsible for recognizing and representing words, objects, faces, and other visual stimuli (14–18). By this account, all items in STM are represented by activation in IT cortex, but an item in the focus of attention may be distinguished by enhanced activation in this region. In fact, several studies have demonstrated maintenance-related activation in IT regions during short-term retention (16–21). However, none of these studies has compared the level of activation of the item presumed to be in the focus of attention with the level of activation of other items. Hence, it remains open whether the focus of attention is represented by enhanced IT activation or whether other neural circuitry is involved.
It is thought that information retained in STM within IT regions is mediated by selection mechanisms of the ventrolateral prefrontal cortex (VLPFC; refs. 13 and 16) and attentional mechanisms of posterior parietal cortex (PPC) (13, 22). The interactions between IT and frontal–parietal attentional regions may be enhanced for the focus of attention relative to other information in STM. Therefore, an item within the focus of attention may also be distinguished by increased synchrony between IT regions of storage on the one hand, and frontal and parietal attentional sites on the other hand. To our knowledge, no research has investigated this matter.
Beyond the focus of attention, what other distinctions can be drawn in memory? Most prominently, researchers have distinguished between information held actively and online in STM and information that is not actively maintained, but is available for retrieval in LTM (23). Much of the evidence in support of this proposal is based on the claim of unique involvement of the medial temporal lobe (MTL) in LTM, but not STM (24, 25). For example, it is often asserted that patients with damage to the MTL demonstrate deficits in LTM, but not STM (24). Moreover, these deficits become larger with increasing time between the study of memoranda and memory tests (26–31). Based on these findings, it has been suggested that the MTL is critical for maintaining associations underlying LTM, but not STM, supporting the idea that STM and LTM represent distinct memory stores (23).
Contradicting classic models of memory, more recent research has demonstrated that damage to the MTL can produce memory deficits with retention intervals as short as 2–10 sec (26–30). Such deficits appear to be especially prevalent when the material to be remembered is novel or involves relational information (30, 32). In these patients, memory deficits grow with increased lag between study and test (26–32), suggesting that the MTL is involved in both STM and LTM, but that the MTL is increasingly critical as retention intervals increase. Based on these findings, some recent models have called for a unitary view of memory in that STM and LTM are seen as fundamentally similar and not distinct stores (13, 32, 33). By these models, memory is best thought of as a continuum with structures that are involved in memory showing increased recruitment as memory demands increase by variables such as retention time.
Despite the claims of unitary models of memory, neuroimaging investigations have yielded mixed evidence regarding the involvement of the MTL in STM. By using within-subjects designs, two studies directly compared tasks thought to tap STM with those thought to tap LTM and found MTL recruitment for both (34, 35). However, the STM tasks used in these studies had long retention intervals, leaving open the possibility that attention could have been drawn away from the information in STM, thereby requiring maintenance and retrieval processes from LTM (13). Therefore, these studies do not unequivocally relate the MTL to STM processes.
By using a more careful procedure, a recent study presented subjects with 12-item lists, followed by a recognition probe (25). Contrasting early-list items presumed to be in LTM with late-list items presumed to be in STM, the authors demonstrated enhanced recruitment of the MTL for LTM but not STM. Although the authors accepted this as evidence for multistore models of memory, they did not separate out the contributions of the MTL for individual items within STM to determine whether MTL recruitment might also vary with retrieval demands in STM. If memory is unitary in character, there may be differential recruitment of the MTL depending on the degree to which an item is active in STM with a greater need for MTL processes when an item is less active within STM. Furthermore, in this study retrieval of late-list items presumed to be in STM did not produce enhanced activation in any region compared with control probes with minimal memory demands, suggesting that there was inadequate power to examine STM processes overall. Therefore, whether the MTL contributes to STM remains an open issue.
In addition to the MTL, studies of both STM and LTM often implicate the left VLPFC as a region involved in memory retrieval (13, 32, 35–43). In earlier work, we demonstrated that during short-term item recognition, the left VLPFC shows increased functional connectivity with the MTL when retrieval-demands are increased by proactive interference (40). We hypothesized that when retrieval-demands increase, the left VLPFC works with the MTL to select appropriate contextual details for successful memory performance. The left VLPFC has also been shown to vary with retrieval-demands during long-term episodic retrieval (42, 43) and semantic retrieval (41), and studies comparing STM and LTM within the same subjects have found common left-VLPFC recruitment across memory tasks (35–38). Thus, this region may commonly participate in the two kinds of memory as well, corroborating the functional similarity between STM and LTM.
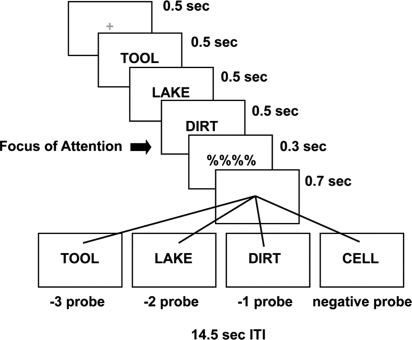
In the present study, we contrasted mechanisms of retrieval for information held in the focus of attention and other information in STM. First, we collected neural evidence to examine whether there is a special status of the focus of attention within STM. Second, we explored the roles of the VLPFC and the MTL in short-term retrieval. To do so, we used event-related functional magnetic resonance imaging (fMRI) while subjects responded to recognition probes that queried very recently presented material. The item-recognition paradigm (Fig. 1) used here has revealed unique differences in retrieval times to the most recently presented item relative to other items in a list (9, 10). Rapid presentation of materials and a very brief delay minimized the use of rehearsal and chunking strategies, so that retrieval processes related to different items within STM could be dissociated without the intrusion of rehearsal and chunking processes (4). Our presentation parameters provided reasonable assurance that the most recently presented item was in the focus of attention (9, 10). STM load was set to three items so that we could stay well within current estimates of STM capacity, and so that we could contrast an item in the focus of attention from the other two items in the list (4).
Fig. 1.
Experimental protocol. On each trial, subjects committed three rapidly presented words to memory and responded to a recognition probe after a very brief delay. The −1 probes matched the most recently presented item, and hence, the focus of attention. The −2 probes matched the second-most recently presented item and the −3 probes matched the least recently presented item.
On each trial, subjects received three rapidly presented words, followed by a recognition probe (Fig. 1). We contrasted activation to recognition probes matching the most recently presented item (focus of attention; minimal retrieval demands) versus the other two serial positions (greater retrieval demands). This allowed us to examine regions demonstrating heightened activation for the focus of attention. Additionally, if mechanisms of STM retrieval are similar to those of LTM, we would expect the MTL and left VLPFC to show increased activation with increased retrieval demands. In addition to traditional univariate analyses, we used functional connectivity analyses (44) to better understand how regions of interest perform retrieval operations.
Results
Behavioral Results.
Reaction times (RTs) were calculated for correct trials only. One-way ANOVAs were computed separately on RTs and error rates. There was a significant effect of probe-type on RT [F (3, 20) = 11.213, P < 0.001], but not error rate [F (3, 20) = 1.341, P > 0.2]. Corroborating previous results, planned contrasts revealed a significant recency effect with faster responses to −1 probes compared with −2 probes [t (22) = 4.436, P < 0.001] and −3 probes [t (22) = 2.901, P < 0.01]. In addition, there was a significant primacy effect with faster responses to −3 probes than −2 probes [t (22) = 3.574, P < 0.01]. Error-rate data mirrored the RT data, but did not achieve statistical significance (P > 0.3 for all tests). Accuracy was high overall (95%). Data are summarized in supporting information (SI) Table S1.
These data replicate previous findings of retrieval facilitation for the most recently presented item in short-term recognition and are consistent with the hypothesis that this item was retained in the focus of attention (9, 10).
Inferior Temporal Contributions to the Focus of Attention.
To examine the neural correlates of the focus of attention, we looked for regions demonstrating increased retrieval-related activation for −1 probes (i.e., focus of attention) versus −2 and −3 probes. Psychological models that posit a distinct single-item focus of attention in STM hypothesize that the item in the focus is unique in its availability for immediate cognitive operation (9–13). As such, an item in the focus of attention does not need to be retrieved per se. We speculated from this assumption that regions involved in representing the item in the focus of attention might show heightened activation when a recognition decision was made on that item. This speculation derives from results in the object-based attention literature demonstrating that top–down attentional modulation produces an increase in bottom–up perceptual activation (22, 45). That is, when attentional processes are focused on a representation, a stimulus that matches that representation will produce an enhanced neural response in regions supporting the representation. Similar ideas have been used to explain match enhancement effects in IT cortex, but without explicit mention of a differentiable focus of attention (46, 47). Therefore, although we examined retrieval-related activation here, we argue that this activity should reveal regions supporting the representation of the item in the focus of attention.
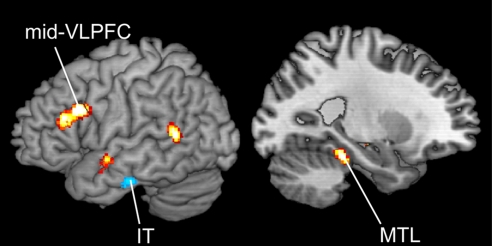
Contrasting retrieval-related activation for −1 probes with −2 and −3 probes revealed activation increases in bilateral IT cortex (BA 20/21; Fig. 2; Table S2). The IT regions were somewhat more anterior and lateral to regions previously reported to be involved in short-term maintenance (17–21), a point to which we return in Discussion. Post hoc contrasts in these regions revealed that activation for −1 probes differed from all other probe-types [−2, −3, and negative, t (18) > 2.5, P < 0.01 for all tests], but no other probe types differed from each other [F (2, 18) = 1.155 for right IT, F (2, 18) = 1.047 for left IT, P > 0.3].
Fig. 2.
Left hemisphere rendering of the contrast between the −2 and −3 probes and −1 probes. Contrast maps are thresholded at P < 0.01 for display purposes. The −2 and −3 probes > −1 probes in hot colors (increased retrieval demands); −1 probes > −2 and −3 probes in cool colors (focus of attention).
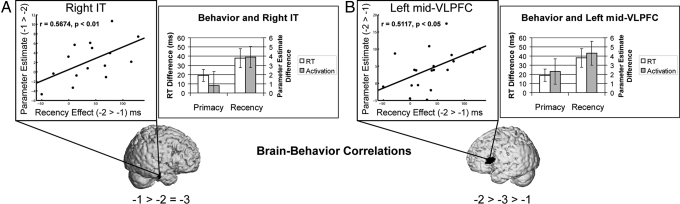
To investigate whether activation in the IT regions was related to behavioral performance, we examined correlations between the recency effect (faster responses in RT for −1 probes versus −2 probes), and activation (greater activation for −1 probes versus −2 probes), restricting ourselves to regions showing significant activation differences at P < 0.01 (see SI Methods). A region of right IT cortex (MNI center: 60, −20, −18) showed a strong correlation with the recency effect in RT (r = 0.57, P < 0.05, 15 voxels; Fig. 3A). That is, greater behavioral recency effects were related to greater retrieval-related activation to −1 probes relative to −2 probes. Note that this region did not demonstrate a corresponding primacy effect (i.e., −3 probes > −2 probes, t (14) < 1). So, it seems not to be the case that the region simply tracked ease of retrieval; rather, it appears to be associated with facilitation in retrieving the item in the focus of attention.
Fig. 3.
Brain–behavior correlations. (A) The recency effect in RT, indexed by faster responses for the −1 probes relative to −2 probes, showed a strong correlation with activation increases for the −1 probes relative to −2 probes in right IT cortex (Left). The bar graph (Left) demonstrates that although there was a significant recency effect in brain activation, there was no corresponding primacy effect (−3 probes > −2 probes). Hence, this region appears to be uniquely associated with the focus of attention. (B) The recency effect in RT showed a strong correlation with activation increases for the −2 probes relative to −1 probes in left mid-VLPFC (Right). The bar graph (Right) demonstrates that activation patterns mirrored behavioral recency and primacy effects (slower responses to the −2 probes relative to −3 probes and greater activation for the −2 probes relative to −3 probes). Hence, this region appears to vary with retrieval demands.
Functional Interactions with Inferior Temporal Cortex.
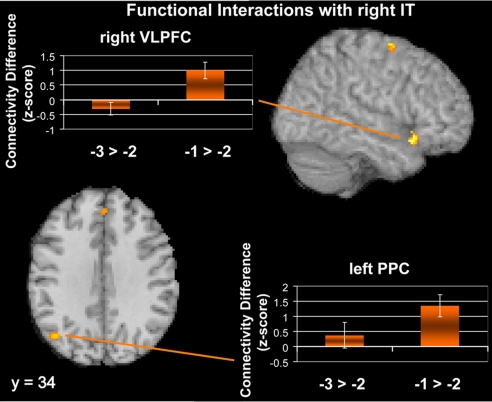
Having confirmed that right IT cortex was strongly related to behavioral performance, we were interested in investigating whether this region is truly responsible for representing the item in the focus of attention in STM. Current theories of STM posit that the item in the focus of attention is actively maintained via the support of frontal- and parietal-attentional systems (13). That is, ventral regions of the frontal lobe are thought to shield active representations from interference (13–16, 19), and PPC is thought to be involved in maintaining attention on these representations to keep them active (13, 22, 45). To investigate these claims, we performed functional connectivity analysis (44) by using the right IT cortex as a seed. We looked for regions demonstrating greater connectivity with the right IT seed region when responding to −1 probes relative to −2 and −3 probes. This analysis revealed regions that show increased connectivity related to the focus of attention relative to other information in STM.
Confirming our predictions, right VLPFC (Table S3; Fig. 4) demonstrated stronger connectivity with right IT cortex when subjects responded to −1 probes relative to −2 and −3 probes. A similar pattern was also observed in left PPC. This region was lateral to the IPS region that has been demonstrated to correlate strongly with capacity (Fig. S1; refs. 5–8).
Fig. 4.
Regions demonstrating functional connectivity increases with right IT cortex for −1 probes relative to the −2 and −3 probes. Results suggest that the focus of attention is mediated by top–down biasing from the right VLPFC and the left PPC. The bar graphs demonstrate that although these regions show connectivity increases that correspond to recency effects (−1 probes > −2 probes), there were no commensurate primacy effects (−3 probes > −2 probes). Therefore, these regions appear to be associated exclusively with enhanced connectivity related to the focus of attention.
MTL and Left Mid-VLPFC Contributions to STM Retrieval.
The differences in retrieval times for −1, −2, and −3 probes provided a parametric assay of retrieval demands that mirrored previous studies (9, 10). Hence, based on the RT data, we hypothesized that retrieval demands were greatest for −2 probes, next greatest for −3 probes, and least for −1 probes. We further hypothesized that −1 probes required minimal use of retrieval processes because this item was presumed to be in the focus of attention and hence available for immediate cognitive operation. Therefore, we used activation to −1 probes as a baseline to index retrieval demands in STM.
Based on the rationale above, we hypothesized that regions involved in STM retrieval would demonstrate heightened activation to −2 and −3 probes relative to −1 probes. If retrieval dynamics in STM mimic those of LTM, we would expect increases in the MTL and left VLPFC as a reflection of retrieval demands. Confirming this hypothesis, compared with −1 probes, −2 and −3 probes produced enhanced retrieval-related activation in left parahippocampal and entorhinal cortex of the MTL [Brodmann's area (BA) 36 and 35]. Activation increases were also observed in left lateral prefrontal cortex, both in mid-VLPFC (BA 45) and more dorsal regions (BA 9 and 46) (Fig. 2; Table S4). The left PFC region was in the opposite hemisphere and 3 cm superior to the region that showed connectivity with the IT cortex, indicating that it is not part of the network involved in representing the item in the focus of attention. Post-hoc contrasts within these regions demonstrated that both MTL and left PFC showed strong recency effects, with greater activation for −2 probes relative −1 probes [t (18) = 3.65, P < 0.001 for MTL; t (18) = 4.1, P < 0.001 for left PFC], and marginal primacy effects, with greater activation for −2 probes relative to −3 probes [t (18) = 1.7, P = 0.05 for MTL; t (18) = 1.62, P = 0.06 for left PFC]. These activation results reflected the behavioral effects and demonstrated that these regions vary parametrically with retrieval demands.
To bolster the claim that the MTL and left PFC are retrieval-related, we examined whether activation differences in these regions correlated with behavioral performance. Mirroring the analyses above, we searched for regions where the behavioral recency effect measured by the RT difference between −1 probes and −2 probes predicted brain activation differences between −1 and −2 probes. We restricted this analysis to regions that demonstrated greater activation for −2 probes relative to −1 probes at P < 0.01.† Left mid-VLPFC (MNI center −58 24 20, BA 45) demonstrated a strong positive correlation with the behavioral recency effect (r = 0.51, P < 0.05, 33 voxels; Fig. 3B). As demonstrated in Fig. 3B, this region also showed a marginal primacy effect with greater activation for −2 probes relative to −3 probes [t (18) = 1.69, P = 0.054]. These results closely mirrored the behavioral data and suggest that the left mid-VLPFC responds to increased retrieval demands. Similar results were found in the MTL (see SI Results).
Functional Interactions with Left Mid-VLPFC.
To understand the mechanisms by which left mid-VLPFC participates in short-term retrieval, we performed functional connectivity analysis, searching for regions demonstrating increased correlation with the left mid-VLPFC for −2 and −3 probes relative to −1 probes (44). That is, we wished to explore what regions covary with the left mid-VLPFC with increased retrieval demands. This analysis penetrates the mechanisms by which retrieval is achieved.
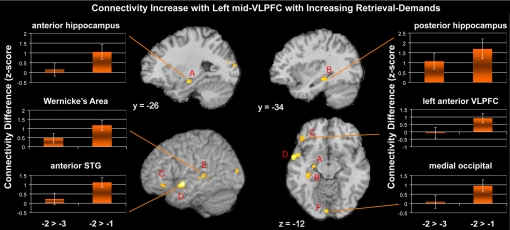
The results revealed that the left mid-VLPFC demonstrated increased connectivity with the hippocampus with increasing retrieval demands (Table S5). Increased functional connectivity was also observed in posterior superior temporal regions near Wernicke's area, occipital cortex, anterior superior temporal gyrus, and ventromedial prefrontal cortex (Fig. 5). These results suggest that as retrieval demands increase, the left VLPFC accrues information from several memory sources to arrive at a correct decision. These sources include phonological information (Wernicke's area; ref. 48), semantic information (anterior superior temporal gyrus; ref. 49), contextual information (hippocampus; refs. 50 and 51), and visual information (occipital cortex).
Fig. 5.
Regions demonstrating functional connectivity increases with the left mid-VLPFC for the −2 and −3 probes relative to −1 probes. Increased connectivity with the left mid-VLPFC was found in anterior hippocampus (A), posterior hippocampus (B), anterior VLPFC (BA 47) (C), anterior superior temporal gyrus (STG) (D), posterior STG (Wernicke's area) (E), and medial occipital cortex (F). This pattern suggests that with increased retrieval demands, the left mid-VLPFC accrues information from several sources, including episodic (hippocampus), phonological (Wernicke's area), semantic (anterior STG), and visual (occipital) information.
Discussion
Recent psychological models include the assumption that of the information in STM, a single item resides in the focus of attention that is available for immediate processing without explicit retrieval needing to operate on that item (9–13). The results presented here suggest that the item in the focus of attention in STM is represented by enhanced activation in IT cortex. This region demonstrated a strong correlation with behavioral measures of retrieval facilitation associated with the focus of attention, solidifying the idea that it performed a central role in the task.
Corroborating unitary models of memory (13, 32, 33), we found regions of the MTL and left mid-VLPFC involved during short-term retrieval. These regions varied with retrieval demands and demonstrated strong correlations with behavioral measures of retrieval processes. Furthermore, we demonstrated that the MTL varied with retrieval demands in STM by using both traditional univariate analyses and functional connectivity analyses with an independent seed. The combination of these methods provides robust support for MTL involvement in STM. These results demonstrate that processes of STM retrieval mimic those of LTM, suggesting common mechanisms between STM and LTM.
Role of Inferior Temporal Cortex in the Focus of Attention.
Examinations of STM have demonstrated maintenance-related activation in IT regions involved in perceptual representations of objects (17–20) and words (21). Much of this work was inspired by non-human primate studies that demonstrated stimulus-specific delay activity in IT cortex during short-term retention tasks (52, 53). The regions we demonstrated here were somewhat anterior and lateral to regions found in previously published fMRI investigations of STM (17–21). It is thought that as information flows through IT cortex in a posterior to anterior direction, there is a greater degree of convergence and integration of information (49). Therefore, the regions we found here may be representing somewhat more integrated information, such as semantic or conceptual information, about the item in the focus of attention. Corroborating this idea, these IT regions are highly activated during semantic retrieval (54–56). This raises the interesting possibility that what distinguishes the item in the focus of attention from other information in STM is the elaboration of additional semantic/conceptual content. It is this elaboration that may be at the heart of why the item in the focus of attention is unique in its immediate availability for cognitive operation. That is, operations can be performed on this item because additional semantic/conceptual content needed to perform these operations has already been retrieved. Notably, although other STM imaging work on humans has not targeted more anterior regions of IT cortex, much of the nonhuman primate work that inspired this tradition has been drawn from anterior areas near the temporal pole (52, 53, 57).
Frontal and Parietal Interactions Supporting the Focus of Attention.
We found that activation representing the focus of attention was associated with enhanced connectivity with the right VLPFC regions and PPC. These results are consistent with the idea that top–down biasing from frontal-parietal attentional regions produces enhanced posterior match responses to goal-relevant stimuli (46, 47, 49, 58). The right VLPFC has been hypothesized to play a role in selection processes that enhance relevant information and inhibit irrelevant information in STM by modulating posterior regions involved in representing information (14–16). The region found here was by no means the right hemisphere homologue of the left mid-VLPFC region that varied with retrieval demands, because the right VLPFC region was a full 3 cm inferior to the region on the left. The right lateralization may suggest that subjects used visual information for their recognition decisions, which may have been preferable to verbal information given the very short retention interval.
The posterior parietal region demonstrating enhanced focus-related connectivity with IT cortex was somewhat lateral to the IPS (Fig. S1). Several studies have demonstrated that the IPS tracks capacity limits within STM (5–8). The IPS is thought to be involved in maintaining attention to relevant information, so increases in STM load are reflected by increases in attentional demand in this region (22, 45). However, parietal regions directly adjacent to, but excluding the IPS, demonstrated connectivity differences with right IT cortex while subjects retrieved different information in STM. In monkeys, homologous posterior parietal regions in area 7a have direct connections to IT cortex and the lateral intraparietal area, considered to be the homologue of the IPS (59). As a result, this region may provide an interface between attentional mechanisms of the IPS and representational regions of IT cortex (see also SI Discussion).
Multiple Routes to Retrieval.
Our functional connectivity results demonstrated that with increasing retrieval demands, the left mid-VLPFC showed increased connectivity with the hippocampus, posterior superior temporal regions near Wernicke's area, occipital cortex, and anterior superior temporal gyrus. This result suggests that as retrieval demands increase, the left mid-VLPFC accrues information from several sources. These sources involve not only associational information (i.e., relating an item to a context; refs. 40, 50, and 51) presumed to be a process supported by the hippocampus and surrounding structures, but also phonological information (Wernicke's area; ref. 48), semantic information (anterior superior temporal gyrus; ref. 49), and visual information (occipital cortex). Previous work from our laboratory has also demonstrated increased functional connectivity between the left mid-VLPFC and the MTL when retrieval demands are increased by proactive interference (40). However, proactive interference is caused by irrelevant long-term information intruding on relevant short-term information, making it unclear whether the MTL was working with the left mid-VLPFC to resolve interference or whether it was the source of the interference itself. That the left mid-VLPFC and the hippocampus demonstrated increased functional connectivity in this study despite minimal interference suggests that these regions work together to arrive at correct memory decisions.
The results here seem to contradict early accounts that demonstrated that lesions to the MTL produced profound deficits in LTM, but spared STM (24). Our connectivity data suggest that multiple sources of information are used in short-term retrieval, including phonological, semantic, and sensory information. These multiple routes to retrieval may overcome the loss of contextual information that accompanies MTL damage. That is, although patients with MTL damage may not be able to place a particular memory item in time, they may still be able to determine that an item is familiar because of a match with a recently activated phonological, semantic, or sensory code. These routes may not be available (i.e., no longer active) for long-term information that was processed in the distant past, producing the previously observed dissociations. Consistent with these ideas, MTL damage produces deficits in short-term retention when the retained information is novel, but not when information is well learned (32). Novel information cannot rely on many of the various representational sources that we found here by using well learned words. Therefore, when alternative routes are not available, the brain may rely exclusively on the MTL for short-term retention (13, 32, 33).
Materials and Methods
Materials and Procedure.
Each trial began with a 0.5-s tone followed by a 0.5-s warning fixation cross. Thereafter, the target-set of three words was presented sequentially, with each word shown for 0.5 s each, followed by a mask for 0.3 s. Finally, a recognition probe was presented for 0.7 s, followed by a 14-s intertrial interval (Fig. 1). Subjects either affirmed the probe as a member of the target-set with a left index press (positive probe) or rejected it as an unpresented word with a right-index press (negative probe). Half of the probes were not members of the target set and half were members. One-third of the positive probes matched the most recently presented item (−1 probe), one-third matched the item presented before that (−2 probe), and one-third matched the least recently presented item (−3 probe). Words were drawn randomly without replacement from a set of 171 four-letter nouns, and the list was rerandomized after it was exhausted. Subjects performed 6 runs of the task, and each run consisted of 30 trials.
Whole-brain analyses were conducted by using the General Linear Model implemented in SPM2. Probe-locked predictors were convolved with a canonical hemodynamic response function and contrast images for each participant were subjected to a random-effects group analysis.
See SI Methods for additional details.
Supplementary Material
Acknowledgments.
We thank Cindy Lustig for insightful comments on an earlier version of this manuscript and Valur Olafsson for help with image reconstruction. This work was supported by National Science Foundation Grant 0520992, National Institute of Mental Health Grant MH60655, and a National Science Foundation Graduate Research Fellowship (to D.E.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802081105/DCSupplemental.
We used only the recency effect as a covariate in this analysis to mirror the analyses done on IT cortex and to provide an unbiased way to check to see whether these regions also demonstrated a corresponding primacy effect. However, this analysis could also be done by contrasting −3 and −2 probes with −1 probes by using a P < 0.005 activation threshold as in the whole-brain analyses and correlating activation with the same difference in RT. This analysis produced similar results in the left mid-VLPFC (r = 0.595, P < 0.01) and the MTL (r = 0.59, P < 0.01).
References
- 1.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 1980;19:450–466. [Google Scholar]
- 2.Daneman M, Merikle PM. Working memory and language comprehension: A meta-analysis. Psychon Bull Rev. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter PA, Just MA, Shell P. What one intelligence test measures: A theoretical account of the processing in the Raven Progressive Matrices Test. Psychol Rev. 1990;97:404–431. [PubMed] [Google Scholar]
- 4.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 5.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;426:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 6.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- 7.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 9.McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. J Exp Psychol Gen. 1989;118:346–373. [Google Scholar]
- 10.McElree B. Accessing recent events. Psychol Learn Motiv. 2006;46:155–200. [Google Scholar]
- 11.Oberauer K. Access to information in working memory: Exploring the focus of attention. J Exp Psychol Learn Mem Cogn. 2002;28:411–421. [PubMed] [Google Scholar]
- 12.Garavan H. Serial attention within working memory. Mem Cogn. 1998;26:263–276. doi: 10.3758/bf03201138. [DOI] [PubMed] [Google Scholar]
- 13.Jonides J, et al. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonides J, Lacey SC, Nee DE. Processes of working memory in mind and brain. Curr Dir Psychol Sci. 2005;14:2–5. [Google Scholar]
- 16.Ranganath C. Working memory for visual objects. Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 17.Postle BR, Druzgal TJ, D'Esposito M. Seeking the neural substrate of visual working memory storage. Cortex. 2003;39:927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- 18.Druzgal TJ, D'Esposito M. Activity in fusiform face area modulated as a function of working memory load. Cogn Brain Res. 2001;10:355–364. doi: 10.1016/s0926-6410(00)00056-2. [DOI] [PubMed] [Google Scholar]
- 19.Ranganath C, Cohen MX, Dam C, D'Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 2004;24:3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganath C, DeGutis J, D'Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Fiebach CJ, Rissman J, D'Esposito M. Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron. 2006;51:251–261. doi: 10.1016/j.neuron.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobio. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 23.Baddeley AD, Hitch G. In: Recent Advances in Learning and Motivation. Bower GA, editor. New York: Academic; 1974. pp. 47–89. [Google Scholar]
- 24.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talmi D, Grady CL, Goshen-Gottstein Y, Moscovitch M. Neuroimaging the serial position curve. Psychol Sci. 2005;16:716–723. doi: 10.1111/j.1467-9280.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- 26.Holdstock JS, Shaw C, Aggleton JP. The performance of amnesic subjects on tests of delayed matching-to-sample and delayed matching-to-position. Neuropsychologia. 1995;33:1583–1596. doi: 10.1016/0028-3932(95)00145-x. [DOI] [PubMed] [Google Scholar]
- 27.Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- 28.Holdstock JS, Gutnikov SA, Gaffan D, Mayes AR. Perceptual and mnemonic matching-to-sample in humans: Contributions of the hippocampus, perirhinal and other medial temporal cortices. Cortex. 2000;36:301–322. doi: 10.1016/s0010-9452(08)70843-8. [DOI] [PubMed] [Google Scholar]
- 29.Aggleton JP, Shaw C, Gaffan EA. The performance of postencephalitic amnesic subjects on two behavioural tests of memory: Concurrent discrimination learning and delayed matching-to-sample. Cortex. 1992;28:359–372. doi: 10.1016/s0010-9452(13)80146-3. [DOI] [PubMed] [Google Scholar]
- 30.Hannula DE, Tranel D, Cohen NJ. The long and short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Nee DE, Berman MG, Moore KS, Jonides J. Neuroscientific evidence about the distinction between short- and long-term memory. Curr Dir Psychol Sci. 2008;17:102–106. doi: 10.1111/j.1467-8721.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- 35.Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 36.Braver TS, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. NeuroImage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- 37.Ranganath C, Johnson MK, D'Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 38.Marklund P, et al. Sustained and transient neural modulations in prefrontal cortex related to declarative long-term memory, working memory, and attention. Cortex. 2007;43:22–37. doi: 10.1016/s0010-9452(08)70443-x. [DOI] [PubMed] [Google Scholar]
- 39.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cognit Neurosci. 2000;9:254–265. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 40.Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. NeuroImage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 42.Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- 44.Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. Control of object-based attention in human cortex. Cereb Cortex. 2004;14:1346–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- 46.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Druzgal TJ, D'Esposito M. A neural network reflecting decisions about human faces. Neuron. 2001;32:947–955. doi: 10.1016/s0896-6273(01)00519-0. [DOI] [PubMed] [Google Scholar]
- 48.Vallar G, Baddeley AD. Fractionation of working memory. Neuropsychological evidence for a phonological short-term store. J Verbal Learn Verbal Behav. 1984;23:151–161. [Google Scholar]
- 49.Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Curr Opin Neurobio. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 50.Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobio. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. J Neurophys. 1995;74:162–178. doi: 10.1152/jn.1995.74.1.162. [DOI] [PubMed] [Google Scholar]
- 53.Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1988;331:68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- 54.Binder JR, et al. Conceptual processing during the conscious resting state: A functional MRI study. J Cognit Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 55.Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 56.Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cognit Neurosci. 2007;19:1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- 57.Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. J Neurosci. 2000;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- 59.Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.