Abstract
Members of the Bcl-2 family include pro- and antiapoptotic proteins that regulate programmed cell death of developing tissues and death in response to cellular damage. In developing mice, the antiapoptotic Bcl-xL is necessary for survival of neural and hematopoietic cells, and consequently, bcl-x–deficient mice die around Day 13.5 of embryogenesis. Furthermore, adult bcl-x+/− heterozygous male mice have reduced fertility because of testicular degeneration. Bax, a multi-BH (Bcl-2 homology) domain proapoptotic member of the Bcl-2 family, is regulated by Bcl-xL and is required for the neuropathological abnormalities seen in bcl-x–deficient embryos. The BH3 domain only subgroup of the Bcl-2 family includes proapoptotic members that are essential for the initiation of apoptotic signaling. In this study, we investigated the role for Bim, a BH3 domain only protein, in the embryonic lethality and increased developmental cell death in bcl-x–deficient animals and the perturbed testicular function in bcl-x+/− adults. Our studies show that bim deficiency attenuates hematopoietic cell death in the fetal liver of bcl-x–deficient animals, indicating that Bim contributes to programmed cell death in this cell population. In addition, we found that testicular degeneration of adult bcl-x+/− males was rescued by concomitant Bim deficiency. However, concomitant Bim deficiency had no effect on the embryonic lethality and widespread nervous system abnormalities caused by bcl-x deficiency. Our work identifies Bim as an important regulator of bcl-x deficiency–induced cell death during hematopoiesis and testicular development. (J Histochem Cytochem 56:921–927, 2008)
Keywords: Bcl-2, Bim, apoptosis, neurodegeneration, hematopoiesis, testicular development
Members of the Bcl-2 protein family are critical regulators of developmentally programmed cell death and stress-induced apoptosis and play prominent roles during the development of many tissues, including the nervous system (Akhtar and Roth 2006). This family of proteins consists of both antiapoptotic members and two distinct proapoptotic subgroups that interact in a tissue-specific and death stimulus–regulated manner to control cell fate. Identification of these molecules as regulators of cell death has been greatly facilitated by the analysis of gene-targeted and transgenic mice. For example, targeted gene disruption of the antiapoptotic Bcl-2 family member bcl-x (bcl-x−/− mice) causes embryonic lethality with a marked increase in apoptosis of immature neurons throughout the developing brain, spinal cord, and dorsal root ganglia (DRG), as well as immature hematopoietic cells in the fetal liver (Motoyama et al. 1995). Mice lacking one allele of bcl-x (bcl-x+/−) survive to adulthood and appear largely normal with the exception of testicular degeneration and reduced mature sperm counts in males (Kasai et al. 2003). In healthy cells, the proapoptotic Bcl-2 family member Bax is kept in check by Bcl-xL, but when unopposed, causes permeabilization of the mitochondrial outer membrane and subsequent activation of caspase-dependent intrinsic apoptotic signaling. Although bax deficiency does not rescue bcl-x−/− mice from embryonic lethality, the unrestrained activity of Bax seems to be critical for a component of the abnormal cell death caused by bcl-x loss, because concomitant bax deficiency markedly reduces the bcl-x−/−-associated developmental neuropathology (Shindler et al. 1997) and the bcl-x+/−-associated germ cell depletion (Rucker et al. 2000), although it does not rescue bcl-x−/− mice from embryonic lethality. The neurodevelopmental abnormalities of bcl-x−/− mice, but not the embryonic lethality, can also be attenuated by concomitant loss of the initiator caspase caspase-9, its activator apaf-1, or the effector caspase caspase-3 (Roth et al. 2000; Zaidi et al. 2001; Cecconi et al. 2004), which all act downstream of Bax in the intrinsic apoptotic pathway. Comparatively little is known about the role of the proapoptotic BH3-only Bcl-2 subgroup, the critical initiators of apoptotic signaling (Huang and Strasser 2000), in the developmental defects caused by loss of Bcl-xL. Furthermore, it is unclear whether a single BH3-only protein or perhaps several are critical for the diverse pathologies seen in bcl-x−/− and bcl-x+/− mice.
Members of the proapoptotic BH3 domain only subgroup of the Bcl-2 family are essential for the initiation of apoptotic cell death and are thought to act by activating proapoptotic molecules (e.g., Bax or Bak) either directly or indirectly by binding and inhibiting antiapoptotic Bcl-2–like proteins, thereby unleashing Bax or Bak (Huang and Strasser 2000). The interactions between the different members of the Bcl-2 family are highly cell type and death stimulus-specific and seem to link a diverse number of proapoptotic stimuli to the apoptosis effector machinery. The BH3-only protein Bim, which can interact with Bcl-xL (O'Connor et al. 1998), is required for developmentally regulated programmed death of autoreactive B and T cells (Bouillet et al. 2002; Enders et al. 2003), as well as leukocyte apoptosis induced by cytokine deprivation, ER stress, or other cytotoxic insults (Bouillet et al. 1999; Puthalakath et al. 2007). In addition, Bim expression is increased in neurons in response to a variety of apoptotic insults (Harris and Johnson 2001; Putcha et al. 2001; Biswas and Greene 2002; Linseman et al. 2002), and Bim loss partially protects sympathetic neurons from nerve growth factor deprivation in vitro (Putcha et al. 2001). Interestingly, loss of even one allele of bim prevents the fatal polycystic kidney disease and lymphopenia seen in Bcl-2–deficient mice, and loss of both alleles also prevents the premature graying of hair seen in Bcl-2–deficient mice (Bouillet et al. 2001).
We hypothesized that Bim provides a critical proapoptotic stimulus that causes the neurological, hematopoietic, and gonadal abnormalities seen in bcl-x−/− and bcl-x+/− mice, respectively, and tested this hypothesis by intercrossing bim−/− with bcl-x+/− mice. We found that concomitant Bim deficiency does not prevent embryonic lethality or neuropathology associated with bcl-x deficiency. However, we show that bim is critical for the abnormal hematopoietic cell death in bcl-x−/− embryos, and concomitant bim deficiency rescues testicular degeneration seen in bcl-x+/− adult mice. Our studies identify Bim as an important regulator of testicular and hematopoietic development and highlight the complexity of bcl-x–dependent survival pathways.
Materials and Methods
Mice
The generation of mice with gene disruptions in bcl-x and bim has been described previously (Motoyama et al. 1995; Bouillet et al. 1999). The two lines were backcrossed six and eight times, respectively, onto the C57BL/6 background. Endogenous and disrupted genes were detected by PCR analysis of DNA extracts from limb or tail samples as described previously (Shindler et al. 1997; Bouillet et al. 1999). The morning on which a vaginal plug was seen was designated as embryonic Day 0.5 (E0.5). Pregnant mice were anesthetized with pentobarbital and killed on gestational Day 12.5 by cervical dislocation. Adult male mice were similarly anesthetized and sacrificed for testis analysis. Mice were cared for in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Immunohistochemistry
Embryos and testes were fixed at 4C in 4% paraformaldehyde overnight. Tissues were dehydrated and paraffin embedded, and 5-μm sections were cut. Sections were deparaffinized and stained with hematoxylin and eosin (H&E) as described previously (Shindler et al. 1997). For terminal deoxynucleotidyltransferase-mediated dUPT nick end labeling (TUNEL) staining, sections were deparaffinized, and endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in PBS. Sections were permeabilized for 10 min in PBS containing 0.3% Triton X-100. Sections were hybridized with Dig-11-dUTP (Roche Applied Science; Indianapolis, IN) for 1 hr at 37C according to the manufacturer's instructions. Sections were blocked for 20 min at room temperature in PBS-BB (PBS with 0.1% BSA, 0.3% Triton X-100, and 0.2% non-fat powdered dry milk). Mouse antidigoxigenin monoclonal antibody (Abcam; Cambridge, MA) conjugated with horseradish peroxidase was diluted in PBS-BB and applied to sections overnight at 4C. After washes with PBS, biotin-labeled tyramide was deposited using a tyramide signal amplification system (PerkinElmer Life Sciences; Boston, MA) according to manufacturer's instructions. After three washes with PBS, sections were incubated for 45 min at room temperature with streptavidin-conjugated horseradish peroxidase (Jackson ImmunoResearch; West Grove, PA) diluted in PBS-BB. Immunostaining was detected using DAB-metal (Pierce; Rockford, IL) according to the manufacturer's instructions. TUNEL-stained sections were counterstained with hematoxylin. H&E- and TUNEL-stained sections were imaged using a Zeiss (Oberkochen, Germany) Axioscop equipped with an Axiocam MRc camera. On H&E-stained sections, apoptotic nuclei were defined as nuclei appearing condensed and hyperchromic, fragmented, and/or exhibiting a marginated chromatin staining pattern. Apoptotic nuclei were counted in multiple fields from each animal using a ×100 oil-immersion objective.
Statistics
All data points represent mean ± SEM. At least three animals per group were analyzed in all experiments. Statistical significance was established by one-way or two-way ANOVA, followed by Bonferroni's test for all pairwise comparisons.
Results
Generation of bcl-x−/− bim−/− Embryos
The bim and bcl-x genes are both located on mouse chromosome 2 (Eppig et al. 2005). Therefore, double-deficient mice can only be generated if mutated alleles of both genes are recombined on the same chromosome during gametogenesis. To begin, bcl-x+/− bim+/− mice were intercrossed, and a small number of bcl-x+/− bim−/− offspring were identified. These animals were crossed with wild-type (wt) mice to isolate the bcl-x− bim− chromosome, and such bcl-x+/− bim+/− progeny were intercrossed to determine whether loss of bim could prevent the embryonic lethality caused by bcl-x deficiency. In 15 litters with 80 total living offspring, 21 wt mice (26.25%; 25% expected frequency), 43 bcl-x+/− bim+/− mice (53.75%; 50% expected frequency), and no live-born bcl-x−/− bim−/− mice (25% expected frequency) were identified. In addition, germ cell recombination in a single parent led to six bcl-x+/− bim+/+ live-born mice, two bcl-x+/− bim−/− mice, and eight bcl-x+/+ bim+/− mice, but no live-born bcl-x−/− bim+/− mice were found. To confirm that bcl-x–deficient embryos were generated from these crosses, 61 mice were harvested at E12.5 from the F1 crosses described above. Fourteen wt embryos (22.95%; 25% expected frequency), 26 bcl-x+/− bim+/− embryos (42.62%; 50% expected frequency), and 7 bcl-x−/− bim−/− embryos (11.48%; 25% expected frequency) were identified. Parental recombination events generated embryos with a variety of genotypes for bcl-x and bim; one bcl-x+/− bim+/+ embryo, three bcl-x+/− bim−/− embryos, six bcl-x+/+ bim+/− embryos, three bcl-x−/− bim+/− embryos, and one bcl-x+/+ bim−/− embryo were identified. Thus, although bcl-x−/− bim−/− and bcl-x−/− bim+/− embryos were viable at E12.5, none survived to birth.
Bim Loss Reduces Hematopoietic Cell Death in bcl-x−/− Embryos but Has No Effect on Neuronal Degeneration
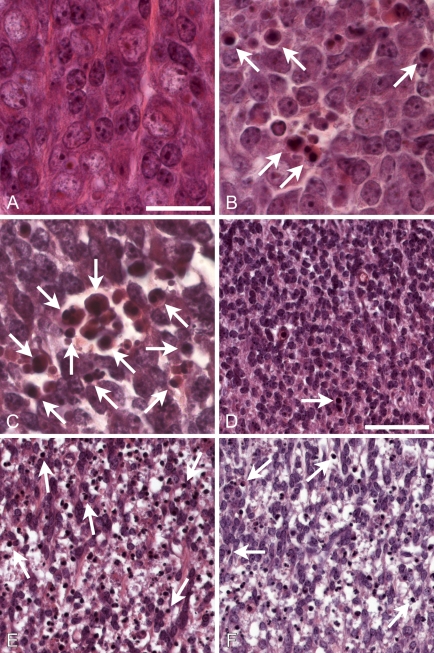
Embryos (12.5) from the crosses described above were prepared for histological and immunohistochemical analysis and assessed for the abundance of apoptotic nuclei. As expected, few apoptotic cells were found in DRG (Figure 1A) or ventral thoracic spinal cord (Figure 1D, example indicated by arrow) of bcl-x+/o bim+/o mice (the +/o designation includes both +/+ and +/− genotypes). Furthermore, there was no significant increase in the number of apoptotic cells in bcl-x+/o bim−/− mice (data not shown). In contrast, numerous apoptotic cells were detected in these regions in bcl-x−/− bim+/o mice (Figures 1B and 1E), consistent with previous analysis of bcl-x−/− embryos (Motoyama et al. 1995). Loss of both alleles of bim did not reduce the number of apoptotic cells in the DRG (Figure 1C) or spinal cord (Figure 1F) in bcl-x−/− mice. TUNEL staining and immunohistochemical staining for activated caspase-3 confirmed that bcl-x+/o bim+/o (Figure 2A) and bcl-x+/o / bim−/− (data not shown) embryos had only few apoptotic cells in their spinal cords. In contrast, large numbers of TUNEL-positive cells were detected in spinal cords of bcl-x−/− bim+/o (data not shown) and bcl-x−/− bim−/− (Figure 2B) embryos. Quantification of TUNEL-positive cells showed no significant difference between bcl-x−/− bim+/o and bcl-x−/− bim−/− animals (Figure 3). These findings indicate that bim and bax do not possess equivalent proapoptotic function in this context, because Bax loss attenuates bcl-x deficiency–induced embryonic neuropathology (Shindler et al. 1997), whereas Bim loss does not.
Figure 1.
Bim loss has no effect on neurodegeneration caused by bcl-x deficiency. (A) Dorsal root ganglia (DRG) in bcl-x+/o bim+/o E12.5 embryos contained few apoptotic cells as determined by hematoxylin and eosin staining. (B) In bcl-x−/− bim+/o embryos, many cells with fragmented, condensed nuclei were visible in the DRG (arrows). (C) Numerous apoptotic cells were also visible in bcl-x−/− bim−/− embryos (arrows). (D) Ventral spinal cord (SC) of bcl-x+/o bim+/o E12.5 embryos showed occasional apoptotic cells (arrow). (E) In contrast, large numbers of apoptotic cells and degenerative changes were noted in bcl-x−/− bim+/o embryos (arrows). (F) Loss of Bim in bcl-x−/− bim−/− embryos did not alleviate these defects (arrows). Bars: A–C = 20 μm; D–F = 50 μm.
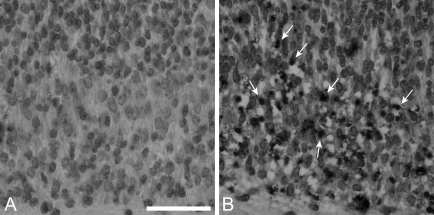
Figure 2.
Bim loss does not alter terminal deoxynucleotidyltransferase-mediated dUPT nick end labeling (TUNEL) reactivity in bcl-x–deficient spinal cord. (A) Spinal cord in bcl-x+/o bim+/o E12.5 embryos contained few apoptotic cells as determined by TUNEL staining. Previous reports have described significant apoptosis characterized by TUNEL positivity in spinal cord of bcl-x−/− bim+/o mice (Motoyama et al. 1995 and data not shown). (B) Concomitant Bim deficiency (bcl-x−/− bim−/−) did not rescue this phenotype and resulted in significant numbers of TUNEL-positive neurons (indicated by arrows). Bar = 50 μm.
Figure 3.
Bim loss significantly reduces the abnormal apoptosis of hematopoietic cells in the fetal liver but not spinal cord caused by bcl-x deficiency. TUNEL staining was performed on sections from E12.5 embryos, and TUNEL-positive cells within multiple ×100 fields were quantitated. Fields were assessed in spinal cord (left) and liver (right). ns, not significant. *p<0.005.
Next, the effect of Bim loss on hematopoietic cell death in bcl-x−/− embryos was examined. bcl-x+/o bim+/o and bcl-x+/o bim−/− embryos had only low numbers of TUNEL-positive cells in the liver, but bcl-x–deficient mice had abnormally increased numbers of apoptotic cells (Figure 3), as previously reported (Motoyama et al. 1995). Bim deficiency led to a reduction of TUNEL-positive nuclei in the liver of bcl-x–deficient embryos (Figure 3), showing that Bim is an important initiator of the abnormal death of hematopoietic cells that lack antiapoptotic Bcl-xL.
Bim Loss Rescues Testicular Degeneration Seen in Adult bcl-x+/− Animals
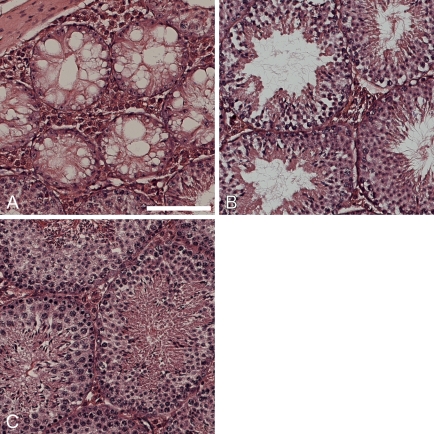
Bim is expressed during spermatogenesis (O'Reilly et al. 2000) and combined loss of the two BH3-only proteins Bim and Bik inhibited apoptosis of immature germ cell progenitors as did loss of Bax (Coultas et al. 2005). Although embryos were generated for the studies described above, adult male bcl-x+/− bim+/− and bcl-x+/− bim−/− mice displayed improved fertility compared with bcl-x+/− bim+/+ animals (data not shown), and we hypothesized that Bim may be essential for the testicular atrophy observed in bcl-x+/− adult males. The average adult (90 days old) testicular weight did not differ significantly between bcl-x+/+ bim−/−, bcl-x+/+ bim+/−, and wt males (Figure 4), consistent with previous observations that bim disruption alone does not affect testicular size (Coultas et al. 2005). In contrast, in bcl-x+/− bim+/+ males, average testicular weight was <40% of that seen in wt animals. Concomitant bim deficiency restored normal testes weight in bcl-x+/− mice (bcl-x+/− bim−/− mice) and even loss of a single allele of bim (bcl-x+/− bim+/− mice) provided a partial rescue (Figure 4). In accordance with a previous report (Kasai et al. 2003), histological analysis showed degenerative changes in the testes of bcl-x+/− bim+/+ males (Figure 5A). Consistent with the data on testes weights, loss of one allele of bim led to a partial rescue of the testicular atrophy seen in bcl-x+/− mice (Figure 5B), and complete deficiency of bim restored normal testicular morphology (Figure 5C). These findings showed that Bim is essential for the testicular degeneration caused by loss of Bcl-xL.
Figure 4.
Bim loss reduces testicular hypoplasia in bcl-x happloinsufficient adult mice. Testis weights from multiple adult male mice of the indicated genotypes were assessed, and average testis weight (g)/total body weight (kg) was calculated. Testes of adult bcl-x+/− mice were 57% smaller than those of bcl-x+/+ mice. This size difference was reduced to 27% in bcl-x+/− bim+/− adults and eliminated in bcl-x+/− bim−/− adults. ns, not significant. *p<0.005.
Figure 5.
Bim loss rescues testicular degeneration caused by loss of one allele of bcl-x. (A) Hematoxylin and eosin–stained testis from bcl-x+/− bim+/+ showed significant degenerative changes with vacuole formation and disrupted testicular morphology. (B) Vacuolar degenerative changes were not observed in bcl-x+/o bim+/o adult testis, although some parenchymal loss was evident. (C) Adult bcl-x+/− males lacking both alleles of bim (bcl-x+/− bim−/−) had normal testicular morphology. Bar = 50 μm.
Discussion
In this report, we assessed the role of the proapoptotic BH3-only Bcl-2 family member Bim in the abnormal cell death caused by deficiency of the antiapoptotic bcl-x. This aim was accomplished by generating mice that lacked both bim and bcl-x and analyzing the consequences in neural, hematopoietic, and germinal tissues. Because both bim and bcl-x reside on the same chromosome, we identified and bred mice that underwent gametal recombination. These breeding experiments produced embryos that lacked both bim and bcl-x at E12.5, but none of these animals survived to birth. Analysis of these embryos showed that bim deficiency reduced the abnormal death of hematopoietic cells but had no effect on neurodegeneration or embryonic lethality caused by loss of Bcl-xL. We also found that adult bcl-x+/− animals displayed reduced fertility and significant testicular degeneration and showed that this defect was rescued by concomitant bim deficiency. Overall, our results showed that the Bim/Bcl-xL interaction regulates cell fate in a cell type–specific manner.
Deficiency of bcl-x results in embryonic lethality and abnormally increased apoptosis of neuronal cells in the brain stem, DRG, ventral spinal cord, and erythroid progenitors in the fetal liver. It remains unclear whether neurodegeneration, fetal anemia, or both abnormalities cause embryonic lethality. A number of double knockout mice lacking Bcl-xL plus any one of the proapoptotic factors Bax, caspase-3, or caspase-9 have been generated, but none of these mice survive to birth (Shindler et al. 1997; Roth et al. 2000; Zaidi et al. 2001; Klocke et al. 2002; Cecconi et al. 2004). However, Bax deficiency prevents neurodegeneration seen in bcl-x–deficient animals, and as seen in this report, Bim deficiency protects hematopoietic cells. It therefore seems that abnormal death of either neuronal cells or erythroid progenitors alone is sufficient to cause embryonic lethality in bcl-x−/− mice. Notably, embryonic lethality is seen in mice lacking erythropoietin (Wu et al. 1995), which have defective erythropoiesis but no neuronal abnormalities, and also in mice lacking XRCC4 (a component of the non-homologous DNA end-joining complex), which have abnormal neurogenesis but normal erythropoiesis (Gao et al. 1998). One may therefore predict that combined loss of Bax and Bim might prevent embryonic lethality of bcl-x−/− mice. However, we cannot exclude the possibility that still other cell types, such as hepatocytes, may be affected by Bcl-xL deficiency at later developmental stages and contribute to embryonic lethality in bcl-x−/− animals.
Although Bim has been shown to play a critical role in nerve growth factor deprivation–induced apoptosis of certain neuronal populations (Harris and Johnson 2001; Putcha et al. 2001; Biswas and Greene 2002; Linseman et al. 2002), Bim deficiency, unlike loss of Bax, did not rescue the degeneration of neuronal cells in the DRG and ventral spinal cord caused by loss of Bcl-xL. This indicates that another BH3-only protein may be critical for this death. Puma is a potential candidate, because, like Bim, it binds all prosurvival Bcl-2 family members (Chen et al. 2005) and its loss protects neural cells against certain apoptotic stimuli (Akhtar et al. 2006; Wyttenbach and Tolkovsky 2006). Because BH3-only proteins exhibit significant functional overlap (Coultas et al. 2005; Erlacher et al. 2006), we speculate that Bim and Puma may together cause the neurodegeneration seen in bcl-x−/− mice.
Primordial gonocytes populate the genital ridge before E11.5 and some undergo programmed cell death around E13.5 (Coucouvanis et al. 1993). A variety of hypomorphs for bcl-x have been generated (Rucker et al. 2000; Kasai et al. 2003) that demonstrate the requirement of bcl-x in determining the number of spermatogenic cells that survive during this period. The abnormal death of these cells caused by loss of Bcl-xL seems to require proapoptotic bax (Rucker et al. 2000), and our studies showed that bim is also required for mediating the apoptosis of these cells. Collectively, our studies showed a heretofore undescribed cell type–specific interaction between Bim and Bcl-xL. Additional studies are needed to identify yet other BH3-only proteins that contribute to the increased neuronal apoptosis in bcl-x–deficient embryos.
Acknowledgments
This work is supported by grants from the National Institutes of Health (NS35107 and NS41962). R.S.A. is supported by the University of Alabama–Birmingham (UAB) Medical Scientist Training Program (GM008361). A.S. is supported by the National Health and Medical Research Council (Canberra, Australia), the Leukemia and Lymphoma Society of America, and the Virtual Research Institute of Ageing.
We thank the UAB Neuroscience Core Facilities (NS47466 and NS57098) and Cecelia B. Latham for technical assistance.
References
- Akhtar RS, Geng Y, Klocke BJ, Latham CB, Villunger A, Michalak EM, Strasser A, et al. (2006) BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci 26:7257–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RS, Roth KA (2006) Regulation of neural stem cell death. In Rao MS, ed. Neural Development and Stem Cells. Totowa, NJ, Humana Press, 97–122
- Biswas SC, Greene LA (2002) Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J Biol Chem 277:49511–49516 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM (2001) Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist bim. Dev Cell 1:645–653 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Kontgen F, Adams JM, et al. (1999) Proapoptotic Bcl-2 relative bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735–1738 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, et al. (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415:922–926 [DOI] [PubMed] [Google Scholar]
- Cecconi F, Roth KA, Dolgov O, Munarriz E, Anokhin K, Gruss P, Salminen M (2004) Apaf1-dependent programmed cell death is required for inner ear morphogenesis and growth. Development 131:2125–2135 [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, et al. (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17:393–403 [DOI] [PubMed] [Google Scholar]
- Coucouvanis EC, Sherwood SW, Carswell-Crumpton C, Spack EG, Jones PP (1993) Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp Cell Res 209:238–247 [DOI] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, Strasser A (2005) Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. EMBO J 24:3963–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A (2003) Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med 198:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JT, Bult CJ, Kadin JA, Richardson JE, Blake JA, and the members of the Mouse Genome Database (2005) The Mouse Genome Database (MGD): from genes to mice-a community resource for mouse biology. Nucleic Acids Res 33:D471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, et al. (2006) Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 203:2939–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, et al. (1998) A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell 95:891–902 [DOI] [PubMed] [Google Scholar]
- Harris CA, Johnson EM (2001) BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J Biol Chem 276:37754–37760 [DOI] [PubMed] [Google Scholar]
- Huang DCS, Strasser A (2000) BH3-only proteins: essential initiators of apoptotic cell death. Cell 103:839–842 [DOI] [PubMed] [Google Scholar]
- Kasai S, Chuma S, Motoyama N, Nakatsuji N (2003) Haploinsufficiency of Bcl-x leads to male-specific defects in fetal germ cells: differential regulation of germ cell apoptosis between the sexes. Dev Biol 264:202–216 [DOI] [PubMed] [Google Scholar]
- Klocke BJ, Latham CB, D'Sa C, Roth KA (2002) p53 deficiency fails to prevent increased programmed cell death in the Bcl-X(L)-deficient nervous system. Cell Death Differ 9:1063–1068 [DOI] [PubMed] [Google Scholar]
- Linseman DA, Phelps RA, Bouchard RJ, Le SS, Laessig TA, McClure ML, Heidenreich KA (2002) Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci 22:9287–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, et al. (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506–1510 [DOI] [PubMed] [Google Scholar]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DCS (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J 17:384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, Huang DC, et al. (2000) The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol 157:449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM (2001) Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29:615–628 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, et al. (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349 [DOI] [PubMed] [Google Scholar]
- Roth KA, Kuan C, Haydar TF, D'Sa-Eipper C, Shindler KS, Zheng TS, Kuida K, et al. (2000) Epistatic and independent functions of caspase-3 and Bcl-X(L) in developmental programmed cell death. Proc Natl Acad Sci USA 97:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L (2000) Bcl-x and bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 14:1038–1052 [DOI] [PubMed] [Google Scholar]
- Shindler KS, Latham CB, Roth KA (1997) Bax deficiency prevents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci 17:3112–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish HF (1995) Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 83:59–67 [DOI] [PubMed] [Google Scholar]
- Wyttenbach A, Tolkovsky AM (2006) The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J Neurochem 96:1213–1226 [DOI] [PubMed] [Google Scholar]
- Zaidi AU, D'Sa-Eipper C, Brenner J, Kuida K, Zheng TS, Flavell RA, Rakic P, et al. (2001) Bcl-X(L)-caspase-9 interactions in the developing nervous system: evidence for multiple death pathways. J Neurosci 21:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]