Abstract
Many cell types wear up to 20-μm-wide hyaluronidase-sensitive surface coats, detected by exclusion of sedimenting particles like fixed erythrocytes. The structure of the coat is enigmatic, being apparently too thick to be accounted by random coils or even extended chains of just hyaluronan attached to cell surface. We have shown that hyaluronan synthesis enforced by green fluorescent protein–hyaluronan synthase transfection creates microvillous protrusions. The idea that the plasma membrane protrusions rather than hyaluronan alone is responsible for the exclusion space was studied with a fluorescent probe for hyaluronan and a dye with membrane affinity, applied to live cell cultures. Mesothelial and smooth muscle cells, fibroblasts, and chondrocytes, all known for their endogenously active hyaluronan synthesis, showed hyaluronan-coated plasma membrane protrusions, barely visible in phase contrast microscopy. Treatment with hyaluronidase and inhibition of hyaluronan synthesis caused retraction of the protrusions unless they were attached to substratum. Hyaluronan and the exclusion space were reduced, but did not disappear, by purified hyaluronan hexasaccharides that compete with hyaluronan attached to CD44. The results suggest that slender plasma membrane protrusions are an inherent feature of hyaluronan coats, form their scaffold, and largely result from ongoing hyaluronan synthesis in their plasma membrane. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 56:901–910, 2008)
Keywords: hyaluronan, protrusions, coat, CD44, glycocalyx
Hyaluronan (HA) is an extensive linear glycosaminoglycan mainly located in the pericellular and extracellular space of vertebrate cells. It provides a highly hydrated environment to protect cells, regulate their shape, and facilitate their migration and growth (Evanko et al. 2007). It is synthesized by hyaluronan synthases (HASs), a family of plasma membrane enzymes that deliver the growing polysaccharide through plasma membrane into extracellular space.
A classical particle exclusion assay, established 40 years ago, showed that many cell types with active hyaluronan production are surrounded by thick pericellular coats (Clarris and Fraser 1968). These assays are performed using fixed erythrocytes (Clarris and Fraser 1968), carbon particles, bacteria, or lymphocytes (McBride and Bard 1979), allowed to settle on sparse cell cultures. The coat disappears by hyaluronidase treatment (Goldberg and Toole 1984; Heldin and Pertoft 1993; Knudson 1993) but not by enzymes such as RNases, trypsin, and neuraminidase (Clarris and Fraser 1968), suggesting that hyaluronan is the main constituent of the coat.
The hyaluronan-rich exclusion space was first described in synovial cells and fibroblasts (Clarris and Fraser 1968), but many cultured eukaryotic cells of mesenchymal origin with active hyaluronan synthesis, such as fibrosarcoma cells (McBride and Bard 1979; Goldberg and Toole 1983), chondrocytes (Knudson 1993), mesothelial cells (Heldin and Pertoft 1993), and vascular smooth muscle cells (Evanko et al. 1999), are surrounded by this highly hydrated mucous zone. Many cell types normally without a visible exclusion zone can be induced to present one by transfection of Has (Brinck and Heldin 1999; Itano et al. 1999,2004; Li and Heldin 2001; Kakizaki et al. 2004) or by factors such as epidermal growth factor and platelet-derived growth factor that upregulate the expression of endogenous Has (Heldin and Pertoft 1993; Pienimäki et al. 2001). The diameter of the space seems to correlate with the level of hyaluronan secretion (Li and Heldin 2001), and the space completely disappears by expression of antisense Has2 RNA (Nishida et al. 1999; Itano et al. 2004) or by subjecting the cultures to 4-methylumbelliferone (4-MU), an inhibitor for hyaluronan synthesis (Kakizaki et al. 2004; Kultti et al. 2006).
It has been hypothesized that the pericellular zone consists of hyaluronan chains attached to HAS (Heldin and Pertoft 1993) or bound to receptors such as CD44 (Knudson 1993; Knudson et al. 1996). Additionally, depending on cell type, aggrecan (Knudson et al. 1996), versican (Evanko et al. 1999), inter-α-trypsin inhibitor, and tumor necrosis factor-α–stimulated gene 6 (de la Motte et al. 2003) can be constituents of the hyaluronan-rich coats and presumably expand their volume. In general, the pericellular matrix has been difficult to characterize because it collapses during fixation and routine processing for histochemistry and electron microscopy (Clarris and Fraser 1968), and in live cells it creates little if any signal for phase contrast microscopy because of its highly hydrated structure with a low content of organic material (Hunziker and Schenk 1984).
We have found that hyaluronan synthesis enhanced by green fluorescent protein (GFP)-HAS transfection induces the formation of up to 20-μm microvilli covering the apical surface in several epithelial cell types (Kultti et al. 2006). GFP-HAS3 is particularly abundant on plasma membrane extensions (Rilla et al. 2005). In this study, we show that slender membrane protrusions in different orientations are a common feature in hyaluronan-secreting cells and that the protrusions form a scaffold for the classical hyaluronan coat.
Materials and Methods
Cell Culture
The human breast adenocarcinoma cell line, MCF-7, was cultured in αMEM (Life Technologies; Paisley, Scotland) supplemented with 5% inactivated FBS (PAA Laboratories; Pasching, Austria), 2 mM glutamine (Sigma; St. Louis, MO), 50 μg/ml streptomycin sulfate, and 50 U/ml penicillin (Sigma). Cells were passaged twice a week at a 1:5 split ratio using 0.05% trypsin (w/v) 0.02% EDTA (w/v) (Biochrom; Berlin, Germany).
Human mesothelial cells (LP-9) were cultured in MCDB 110 medium (Sigma) and medium 199 (Sigma) in a ratio of 1:1, supplemented with 15% FBS (PAA Laboratories), 2 mM glutamine (Sigma), 50 μg/ml streptomycin sulfate, 50 U/ml penicillin (Sigma), 10 ng/ml epidermal growth factor (Sigma), and 0.05 μg/ml hydrocortisone (Sigma).
Human cutaneous tumor stromal fibroblasts (from Dr. Reidar Grenman, Turku, Finland) were cultured in DMEM (high glucose; Gibco, Paisley, Scotland) supplemented with 10% FBS (PAA Laboratories), 2 mM glutamine, 50 μg/ml streptomycin sulfate, 50 U/ml penicillin (Sigma), and 1% non-essential amino acids (NEAA; Gibco). Bovine primary chondrocytes isolated from bovine patellar articular cartilage as described previously (Kopakkala-Tani et al. 2006) were cultured in DMEM (high glucose; Euroclone, West York, UK) supplemented with 10% FBS (PAA Laboratories), 2 mM glutamine, 50 μg/ml streptomycin sulfate, 50 U/ml penicillin (Sigma), 50 μg/ml fungizone (Gibco), and ascorbic acid (0.05 mg/ml; Sigma). Rabbit vascular smooth muscle cells (RAAs; from Dr. Marika Ruponen, Kuopio, Finland) were cultured in DMEM (high glucose; Gibco) supplemented with 10% FBS, 2 mM glutamine, 50 μg/ml streptomycin sulfate, and 50 U/ml penicillin (Sigma).
Transfections
Subconfluent cell cultures grown on chambered coverslips (Nalge Nunc; Naperwille, IL) coated with collagen type I (BD Biosciences; Bedford, MA) were transiently transfected with mouse Has3 cDNA in-frame with an N-terminal GFP fusion protein in the pCIneo vector or with a GFP fusion protein in the pCIneo vector as a control (Rilla et al. 2005), using manufacturer's instructions (Roche Molecular Biochemicals; Indianapolis, IN) and examined a day after transfection.
Fluorescent Hyaluronan Binding Complex (HABC)
For visualization of hyaluronan on live cells, a fluorescent group was directly coupled to HABC, using a modification of the method described earlier (Tammi et al. 1994). Briefly, lyophilized HABC attached to high molecular mass hyaluronan (Sigma) was dissolved in 0.5 ml of 0.1 M Na-bicarbonate and incubated for 2 hr with stirring in Alexa Fluor 594 carboxylic acid succinimidyl ester (A10239; Molecular Probes, Eugene, OR). Solid guanidinium chloride was added to the approximate final concentration of 4 M, and the reaction mixture was chromatographed on a 1 × 30-cm column of Sephacryl S-400 and eluted with 4 M guanidinium chloride in 50 mM Na-acetate, pH 5.0. The void volume, containing the hyaluronan, and the total volume, containing the free dye, were discarded, and the protein peak at Kav ∼0.6 was pooled, dialyzed against distilled water, and lyophilized. The probe was dissolved in PBS with 0.02% sodium azide and 1% BSA for storage and diluted to 1–5 μg/ml final concentration for use. The specificity of the staining for hyaluronan was controlled by removing hyaluronan with Streptomyces hyaluronidase (Seikagaku Kogyo; Tokyo, Japan). Furthermore, the specificity of the fluorescent HABC probe was verified by incubating it with hyaluronan oligosaccharides (HA6 and HA10, kindly provided by Seikagaku, or HA22, Tammi et al. 1998) 1:1 w/w for 2 hr at room temperature before diluting it for staining.
Microscopy of Live Cells
The fluorescent images were obtained with an Ultraview confocal scanner (PerkinElmer Life Sciences; Wallac-LSR, Oxford, UK), on a Nikon Eclipse TE300 microscope (Melville, NY) with ×60 NA 1.4 and ×100 NA 1.3 oil immersion objectives. For the three-dimensional imaging, a series of horizontal optical sections were captured through the whole cell at 200-nm steps. The three-dimensional images were created and further modified using ImageJ 1.32 software (http://rsb.info.nih.gov/ij/) and Adobe Photoshop, Version 8.0 (Adobe Systems Inc.; San Jose, CA). Phase contrast microscopy was done with Olympus IX71 microscope (Tokyo, Japan) equipped with a ×60 oil immersion objective and a digital CCD camera (ORCA-ER; Hamamatsu, Japan). The cells were grown on collagen-coated chambered coverslips (Nalge Nunc). The images were taken at room temperature, but during longer treatments between imaging, cells were kept in the incubator at 5% CO2 and 37C.
The treatments of live cells were performed as follows: the hyaluronan coat around cells was digested by Streptomyces hyaluronidase (10 turbidity reducing units/ml; Seikagaku). To control the possible effect of protease contaminations in the hyaluronidase preparation, digestions were also done in the presence of a protease inhibitor cocktail including aprotinin, bestatin hydrochloride, E-64, leupeptin hemisulfate salt, and pepstatin A (Sigma). The secretion of hyaluronan was inhibited by 4-MU (1 mM; Sigma). To remove hyaluronan bound to receptors on plasma membrane, cells were treated with 0.2 mg/ml of hyaluronan hexasaccharides, kindly provided by Seikagaku. The plasma membrane of live cells was labeled with 5 μg/ml of lipophilic dye FM1-43 (Molecular Probes).
Red Blood Cell Exclusion Assay
Fixed sheep red blood cells (Sigma) were suspended in PBS containing 0.1% BSA, washed three times and suspended in PBS, added to the culture, allowed to settle on cells for 15 min at 37C, and examined by confocal microscopy. Autofluorescence or phase contrast microcopy was used to visualize the red blood cells.
Assay of Hyaluronan Secretion
The culture media (containing serum) were collected for hyaluronan enzyme-linked sorbent assay, performed as described previously (Rilla et al. 2005). Maxisorp 96-well plates (Nalge Nunc) were precoated with HABC (1 μg/ml in 50 mM carbonate buffer, pH 9.5), washed with 0.5% Tween-PBS, and blocked with 1% BSA-PBS. The medium samples, diluted 1:40 or 1:50, and the hyaluronan standards (0-50 ng/ml; Provisc, Alcon, Fort Worth, TX) were added into the wells and incubated for 1 hr at 37C. The plates were sequentially incubated with biotinylated HABC (1.0 μg/ml) and horseradish peroxidase streptavidin (1:20,000 in PBS; Vector Laboratories, Burlingame, CA), each for 1 hr at 37C and for 10 min at room temperature in the substrate-chromogen solution containing 0.01% of 3,3′,5,5′-tetramethybenzidine and 0.005% H2O2 in 0.1 M sodium acetate and 1.5 mM citric acid buffer. The absorbances were measured at 450 nm after the addition of 50 μl of 2 M H2SO4.
Results
Hyaluronan Coat on HAS-transfected MCF-7 Cells
GFP-HAS3–transfected MCF-7 cells displayed a wide pericellular space that excluded fixed erythrocytes, and the space was occupied by GFP-HAS3–positive microvilli (Figure 1A), as reported previously (Kultti et al. 2006). The contour of the exclusion space around GFP-HAS–transfected cells correlated with the length of the GFP-HAS–positive extensions (Figure 1A). At 1 μg/ml in the growth medium, the fluorescent HABC concentrated on cell surface hyaluronan in a density high enough for direct visualization of the coat (Figure 1B). The hyaluronan shown by the probe faithfully followed the GFP-HAS3–positive protrusions, with the HABC signal extending along the protrusions as a tight cover up to ∼2 μm thick (Figure 1C).
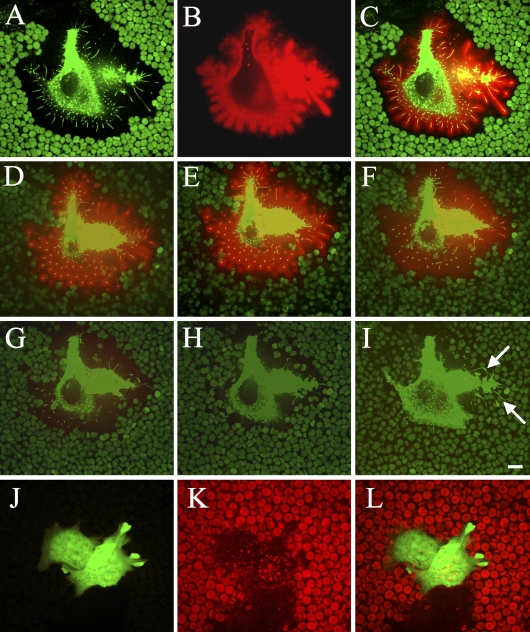
Figure 1.
Confocal images of hyaluronan-coated microvilli on live MCF-7 cells transfected with green fluorescent protein (GFP)-hyaluronan synthase (HAS)3. In single optical sections, the exclusion space is indicated by erythrocytes, and GFP-HAS3 in cell protrusions is shown (A), hyaluronan around the same cell visualized with a hyaluronan binding probe (B), and a composite image (C). (D–I) Three-dimensional images composed of horizontal stacks of the same cell before (D) and 1 (E), 2 (F), 3 (G), 4 (H), and 5 min (I) after the addition of Streptomyces hyaluronidase (10 TRU/ml). Arrows in I point out hyaluronidase-resistant extensions adhered to the substratum. Red, hyaluronan; green particles, red blood cell autofluorescence; green color in the cell, GFP-HAS3. Control cells transfected with a GFP-expressing vector are presented in J–L; green color, GFP; red particles, red blood cells; red color in the cell, hyaluronan. Bar = 10 μm.
Time-lapse confocal microscopy indicated that when hyaluronidase was applied to gradually remove the hyaluronan layer on the plasma membrane, most of the extensions were disrupted, and erythrocytes approached the cell body, leading to the disappearance of the exclusion space (Figures 1D–1I, SM1). This suggests that the protrusions are supported by the hyaluronan layer around them, and they form a “skeleton” for the hyaluronan-rich coat. However, protrusions attached to the substratum did not retract after hyaluronidase digestion (arrows in Figure 1I). The control cells transfected with only a GFP-expressing vector did not show any growth of microvilli, exclusion space, or HABC signal using the same culturing conditions (Figures 1J–1L).
Coat on LP-9 Mesothelial Cells
Do hyaluronan-dependent protrusions exist in untransfected cells with naturally thick hyaluronan coats? This question was first studied in cultured mesothelial cells (LP-9), known for their high rate of hyaluronan synthesis (Table 1). As previously reported (Heldin and Pertoft 1993), most of the mesothelial cells displayed a prominent exclusion space (Figure 2A), which was occupied by a large amount of hyaluronan (Figure 2B). The hyaluronan around the mesothelial cells was usually not homogenous but seemed to be concentrated around streaks pointing out of the cell body (Figure 2B). Fine filamentous structures, barely visible in high-resolution phase contrast microscopy, appeared on the surface of the mesothelial cells (Figure 2C, arrows), but it was technically difficult to colocalize them with the streaks of hyaluronan. Instead, simultaneous application of fluorescent HABC and the lipophilic FM1-43 dye clearly showed that there were plasma membrane protrusions within the hyaluronan coat of mesothelial cells (Figure 2D) and their exclusion space (Figure 2E). Some microvilli pointed upward (Figure 2F), like those in the GFP-HAS3–transfected cells.
Table 1.
Hyaluronan concentration in the culture media of different cell types
| Cell type | Hyaluronan production (ng/10,000 cells/24 hr) |
|---|---|
| Breast cancer cells (MCF-7, GFP transfected) | 0.8 ± 0.5 |
| Breast cancer cells (MCF-7, GFP-Has3 transfected) | 136 ± 7 |
| Arterial smooth muscle cells (RAA, rabbit) | 21 ± 4 |
| Chondrocytes (bovine patella) | 44 ± 3 |
| Mesothelial cells (LP-9, human) | 115 ± 19 |
| Tumor stromal fibroblasts (human) | 305 ± 46 |
Means ± SD of three wells of MCF-7 cells; means ± SD of six wells in other cells. GFP, green fluorescent protein; RAA, rabbit vascular smooth muscle cells.
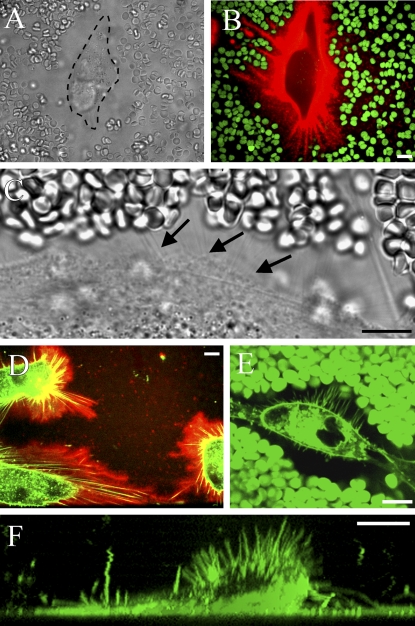
Figure 2.
Hyaluronan-coated plasma membrane extensions in live LP-9 cells. In regular phase contrast microscopy, a live human mesothelial cell is surrounded by a wide space that excludes erythrocytes (A). Hyaluronan-rich streaks (red) around a mesothelial cell correspond to areas free of erythrocytes (green), suggesting underlying cell protrusions (B). High-resolution phase contrast microscopy shows thin lines probably representing the protrusions (C, arrows), confirmed by double staining for hyaluronan and the FM1-43 membrane marker (D). Protrusions illustrated by the membrane marker FM1-43 and the exclusion space by erythrocytes (both green) are shown together in E. A vertical view of a living LP-9 cell labeled with FM1-43 also shows extensions protruding upward (F). Bar = 10 μm.
Coat Properties of Cells With Naturally High Hyaluronan Production
Despite some similarities, there were obvious differences between the hyaluronan-dependent microvilli in GFP-HAS3–transfected MCF-7cells and the extensions in LP-9 cells. The length and distribution of the protrusions in LP-9 cells were more variable than in HAS-overexpressing cells, and the hyaluronan coat was less compact. Furthermore, most of the LP-9 extensions were attached, lying flat on the substratum, and often originated as dense arrays (Figures 2D, 3A, and 3B), whereas those in MCF-7 cells were mostly unattached, slightly mobile, and evenly and less densely spaced (Figure 1A, SM1). Hyaluronan staining pattern itself in LP-9 cells appeared more fuzzy on its edges (Figure 2D) compared with GFP-HAS3–overexpressing cells.
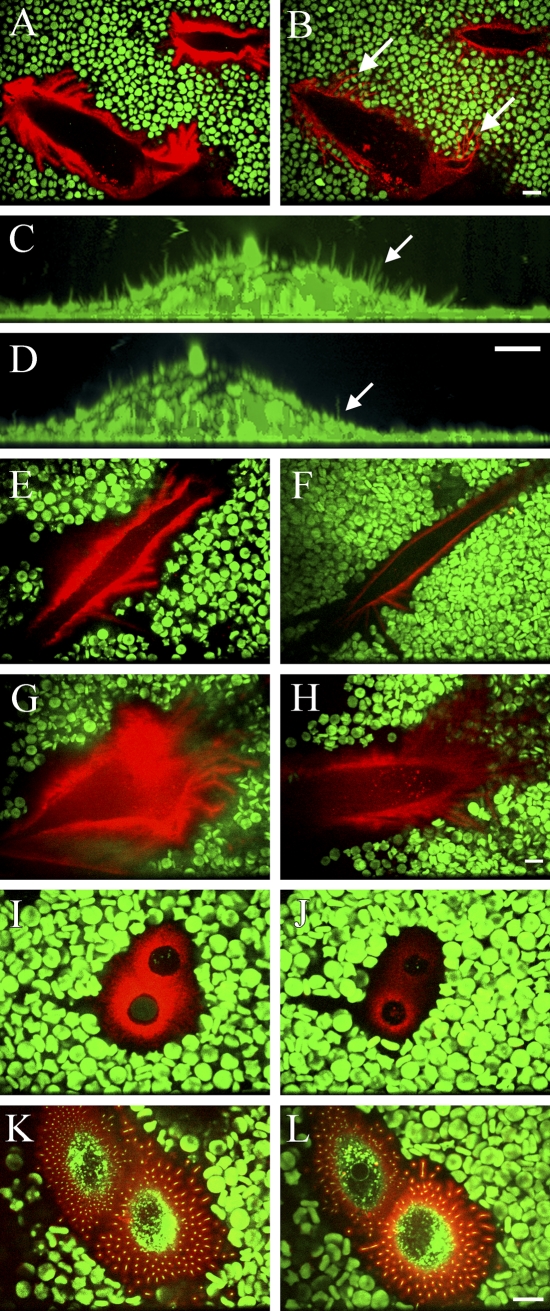
Figure 3.
Role of hyaluronan in the maintenance of the exclusion space and membrane protrusions. LP-9 mesothelial cells (A,B) and fibroblasts (C,D) before (A,C) and after (B,D) treatment with the Streptomyces hyaluronidase (10 turbidity reducing units/ml, 20 min). Note that the extensions adhered to the substratum do not retract concomitantly with the removal of hyaluronan (B, arrows), whereas most of the upward oriented, shorter extensions do retract (C,D, arrows). The exclusion space of a mesothelial cell and hyaluronan before (E) and after (F) treatment with the hyaluronan synthesis inhibitor, 4-methylumbelliferone (4-MU; 1 mM, 4 hr). A mesothelial cell (G,H), chondrocyte (I,J), and GFP-Has overexpressing MCF-7 cell (K,L) before (G,I,K) and after (H,J,L) treatment with hyaluronan hexasaccharides (0.2 mg/ml, 2 hr). Single optical sections are shown in A, B, and E–L, and vertical sections from compressed image stacks are shown in C and D. The same cell is represented in each pair of figures. Hyaluronan, red; FM1-43 membrane marker, green (C,D); GFP-HAS3, green (K–L); erythrocytes, green. Bar = 10 μm.
Streptomyces hyaluronidase treatment (10 TRU/ml) gradually removed the coat of LP-9 cells, obviously by truncating the growing hyaluronan chains until an equilibrium was reached in ∼20 min, when stubs barely able to bind the HABC probe were left (Figures 3A and 3B). The removal of hyaluronan was associated with erythrocyte rolling on top of the extensions that remained attached to the substratum (Figures 3A and 3B). In contrast, the microvillous membrane protrusions on the upper surface of LP-9 cells, and fibroblasts almost disappeared on hyaluronidase digestion (Figures 3C and 3D, arrows), thus resembling the situation in transfected MCF-7 cells. Treatment of LP-9 cells with 4-MU, an inhibitor of hyaluronan synthesis (Rilla et al. 2004), diminished the exclusion space and hyaluronan around the cells in 4 hr (Figures 3E and 3F), indicating that the coat is dependent on ongoing hyaluronan synthesis. Furthermore, even the size and number of the protrusions attached to substratum were diminished by 4-hr treatment with 4-MU, suggesting that, in a longer time frame, they were also dependent on hyaluronan synthesis (Figures 3E and 3F).
The human tumor fibroblasts, which had the highest hyaluronan secreting capacity of the cell lines studied (Table 1), expressed the longest protrusions, pointing to all directions (Figures 4A and 4D). RAAs (Figures 4C and 4F) expressed long, irregular, mainly horizontal extensions, which were present only in cells with an elongated or rounded morphology. The presence, length, and localization of the extensions of fibroblasts and smooth muscle cells correlated with those of the exclusion space. In contrast, the exclusion space of chondrocytes was usually more extensive than the average length of the protrusions (Figures 4B and 4E), suggesting that aggrecan–hyaluronan interactions increase the diameter of the exclusion space beyond the length of the protrusions in chondrocytes.
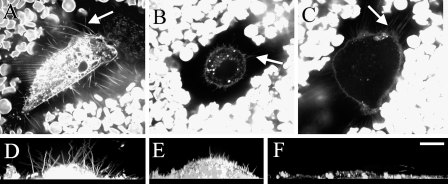
Figure 4.
Plasma membrane protrusions (arrows) and the exclusion space of different cells (A–C). Membranes were labeled with FM1-43, and sedimenting erythrocytes show the exclusion space in single optical sections (A–C). Plasma membrane morphology as a side view obtained from compressed series of optical sections (D–F). Human tumor stromal fibroblast (A,D), bovine primary chondrocyte (B,E), and rabbit aortic smooth muscle cell (C,F) secreting ∼305, 44, and 21 ng hyaluronan/10,000 cells/24 hr, respectively (Table 1). Bar = 10 μm.
Role of Receptor-bound Hyaluronan in Coat Formation
The role of the hyaluronan–CD44 interaction in coat formation was studied by treatment with hyaluronan hexasaccharides, which have been reported to displace high molecular mass hyaluronan from this receptor in many cell types (Knudson 1993; Knudson et al. 1993). The size of the exclusion space was diminished, and the intensity of the hyaluronan layer was decreased both in mesothelial cells (Figures 3G and 3H) and chondrocytes (Figures 3I and 3J). However, hexasaccharide treatment did not influence the dimensions of the hyaluronan layer or exclusion space of GFP-HAS–overexpressing breast cancer cells (Figures 3K and 3L). This suggests that a part of hyaluronan in LP-9 cells and chondrocytes is attached to receptors, but the hyaluronan attached to HAS is enough to maintain the exclusion space, particularly in HAS-overexpressing cells. The same concentration of hyaluronan tetrasaccharides was used to control the possible nonspecific effect of oligosaccharides on the hyaluronan coat detected by fluorescent HABC. Tetrasaccharides had no influence on cell surface hyaluronan (data not shown).
Decasaccharides or longer hyaluronan oligosaccharides effectively compete for the binding of fluorescent HABC to hyaluronan, as shown by experiments where HAS3-overexpressing cells treated with decasaccharides did not bind fluorescent HABC, although the exclusion assay indicated that they still had an intact hyaluronan coat around them. Moreover, HA14 did not affect the erythrocyte exclusion space more than hexasaccharides in chondrocytes and in fibroblasts (data not shown).
Discussion
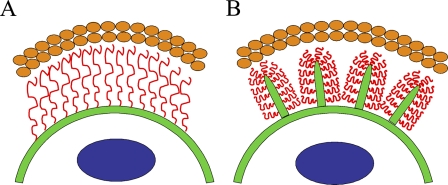
Although the extended length of a high-molecular-mass hyaluronan chain can exceed 20 μm, a similar size hyaluronan in free solution forms a random coil <2 μm in diameter (Laurent 1989). However, the thickness of the exclusion space often exceeds 20 μm (Evanko et al. 2007). Therefore, in the absence of factors such as high concentrations of aggrecan, a single layer of hyaluronan alone is unlikely to create an exclusion space on the cell surface wider than a few micrometers. In this work, we showed that hyaluronan-covered plasma membrane protrusions account for, or contribute to, the exclusion space (coat) around many cell types with active hyaluronan synthesis. Accordingly, disruption of the actin cytoskeleton of the protrusions by cytochalasin or latrunculin eliminates the exclusion space (Knudson et al. 1999). There is a reciprocal dependence: hyaluronan attached to HAS on plasma membrane is a prerequisite for certain membrane protrusions, and membrane protrusions are required for a thick hyaluronan coat. Most of the thickness of the hyaluronan coat (exclusion space) is thus based on the protrusions rather than just extended hyaluronan chains arising from a flat cell surface, as schematically depicted in Figure 5.
Figure 5.
Schematic models for the hyaluronan coat. (A) A model in which extended hyaluronan chains (red), attached to plasma membrane (green) by hyaluronan receptors or hyaluronan synthases, account for the whole thickness of the exclusion space. (B) These data suggest a model where plasma membrane protrusions give a scaffold to the hyaluronan coat that excludes erythrocytes (orange ovals). The protrusions are supported by hyaluronan on their surface. Blue circles in the cell represent nucleus.
The hyaluronan-covered protrusions described in this work can explain the wide pericellular exclusion space previously shown around cancer cells that secrete large amounts of hyaluronan (McBride and Bard 1979; Toole 2004). In fact, plasma membrane protrusions within a thick, hyaluronan-containing glycocalyx are found on many normal cell types such as oocytes (Makabe et al. 2006), synovial cells (Lukoschek and Addicks 1991), mesothelial cells (Mutsaers 2004), and chondrocytes (Hale and Wuthier 1987).
Using samples freeze-dried for scanning electron microscopy, it was shown already in 1983 that cells with a hyaluronan coat display numerous microvilli (Bard et al. 1983; Cohen et al. 2003), but the microvilli were ignored as an important structural component of the hyaluronan coat. Because the microvilli are highly sensitive to routine fixation and poorly visible in phase contrast microscopy, their importance for hyaluronan coats were ignored for decades, and only application of fluorescent probes on live cells allowed their role to be recognized.
Receptor-bound Hyaluronan on Non-transfected Cells
High concentrations of purified hyaluronan hexasaccharides or higher oligomers had no influence on the layer of hyaluronan or the microvilli of GFP-HAS3–transfected MCF-7 cells, indicating that interaction with CD44 was not necessary for the coat formation (Kultti et al. 2006). Instead, inhibition of hyaluronan synthesis and treatment with hyaluronidase reduced the size of the coat and the length of the protrusions, indicating that hyaluronan chains under synthesis, still attached to HAS, accounted for the hyaluronan layer on the MCF-7 microvilli. The role of HAS activity and HAS-associated hyaluronan in the coat formation was supported by the positive correlation between the rate of hyaluronan synthesis with the size of the coat. The protrusions pointing upward were especially well developed in cells with the highest hyaluronan synthesis.
Chondrocytes also presented microvilli, but they did not get as close to the edge of the exclusion space, as in other cells types, presumably because of the fact that aggrecans produced by chondrocytes contribute to the coat by their own exclusion volume and especially by keeping hyaluronan in an extended conformation (Knudson 1993; Lee et al. 1993; Knudson et al. 1996,1999). Unlike the GFP-HAS3–overexpressing MCF-7 cells, chondrocytes responded to hexasaccharide treatment with slightly reduced hyaluronan staining intensity and smaller erythrocyte exclusion area. This indicates that CD44 or a similar receptor activity on cell surface contributed to the coat, as reported previously (Knudson et al. 1996).
The exclusion space of mesothelial cells was partly displaced by hexasaccharides. An earlier report also found that the exclusion space of mesothelial cells is influenced by oligosaccharides and that dodecasaccharide size fragments cause the maximum decrease (Heldin and Pertoft 1993). However, we found that a part of the coat depends on HAS activity, because hyaluronan synthesis inhibition reduced the exclusion space.
Importance of the Coat
Mesothelial cells express numerous microvilli on the apical surface in vivo (Mutsaers and Wilkosz 2007) and secrete hyaluronan (Yung and Chan 2007), suggesting that these features represent important functions of the cells that line the peritoneal, pleural, and cardiac cavities. The glycocalyx of mesothelial cells has been suggested to prevent abrasion, form a protective barrier around the cells (Mutsaers and Wilkosz 2007), and regulate inflammation (Yung and Chan 2007). In cancer cells, the hyaluronan-rich glycocalyx may protect from lymphocyte-mediated cytolysis (McBride and Bard 1979), and microvilli may mediate multidrug resistance (Lange and Gartzke 2001).
The exclusion space is especially thick in mitotic and migrating smooth muscle cells (Evanko et al. 1999) and fibrosarcoma cells (McBride and Bard 1979), as well as mesothelial cells (Heldin and Pertoft 1993) and prostate cancer cells (Ricciardelli et al. 2007) and has been shown to regulate migration and morphology of these cells (Li and Heldin 2001). Faster migration is also associated with the assembly on activated keratinocytes of a hyaluronan glycocalyx, which is undetectable in the normal state (Pienimäki et al. 2001). The glycocalyx has an important role in many cell functions, such as hydration and osmotic balance, flow of nutrients and growth factors, and cell adhesion to its environment (Evanko et al. 2007).
We conclude that membrane protrusions are a typical feature of cells with active hyaluronan synthesis and that the protrusions can scaffold the hyaluronan coat observed in cell cultures where small particles are allowed to settle. Another conclusion is that studies on hyaluronan coat structure, dynamics, and composition are best done in live cells. A challenging future task is to establish the biological functions of these hyaluronan-associated membrane protrusions in vivo.
Supplementary Material
Acknowledgments
This work was supported by the Academy of Finland, Grants 40807 and 54062 (to MT) and by grants from The Finnish Cultural Foundation (to KR), Kuopio University Foundation (to KR), The North Savo Cancer Foundation (to KR), The Finnish Cancer Foundation (to RT), The Sigrid Juselius Foundation, The Mizutami Foundation, The Finnish Technology Centre TEKES (to MT), and the EVO funds of Kuopio University Hospital (to MT).
The authors thank Sari Maljanen for expert technical assistance; Kari Törrönen for help in image processing; Sanna Miettinen and Chengjuan Qu for providing the primary chondrocytes; Dr. Reidar Grenman for tumor stromal fibroblasts; and Dr. Andrew P. Spicer for a GFP-Has3 construct.
References
- Bard JB, McBride WH, Ross AR (1983) Morphology of hyaluronidase-sensitive cell coats as seen in the SEM after freeze-drying. J Cell Sci 62:371–383 [DOI] [PubMed] [Google Scholar]
- Brinck J, Heldin P (1999) Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Exp Cell Res 252:342–351 [DOI] [PubMed] [Google Scholar]
- Clarris BJ, Fraser JR (1968) On the pericellular zone of some mammalian cells in vitro. Exp Cell Res 49:181–193 [DOI] [PubMed] [Google Scholar]
- Cohen M, Klein E, Geiger B, Addadi L (2003) Organization and adhesive properties of the hyaluronan pericellular coat of chondrocytes and epithelial cells. Biophys J 85:1996–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:Polycytidylic acid: Inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol 163:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN (1999) Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19:1004–1013 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Tammi MI, Tammi RH, Wight TN (2007) Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev 59:1351–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RL, Toole BP (1983) Monensin inhibition of hyaluronate synthesis in rat fibrosarcoma cells. J Biol Chem 258:7041–7046 [PubMed] [Google Scholar]
- Goldberg RL, Toole BP (1984) Pericellular coat of chick embryo chondrocytes: structural role of hyaluronate. J Cell Biol 99:2114–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JE, Wuthier RE (1987) The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem 262:1916–1925 [PubMed] [Google Scholar]
- Heldin P, Pertoft H (1993) Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Exp Cell Res 208:422–429 [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK (1984) Cartilage ultrastructure after high pressure freezing, freeze substitution, and low temperature embedding. II. intercellular matrix ultrastructure - preservation of proteoglycans in their native state. J Cell Biol 98:277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Sawai T, Atsumi F, Miyaishi O, Taniguchi S, Kannagi R, Hamaguchi M, et al. (2004) Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem 279:18679–18687 [DOI] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, et al. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274:25085–25092 [DOI] [PubMed] [Google Scholar]
- Kakizaki I, Kojima K, Takagaki K, Endo M, Kannagi R, Ito M, Maruo Y, et al. (2004) A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem 279:33281–33289 [DOI] [PubMed] [Google Scholar]
- Knudson CB (1993) Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol 120:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CB, Nofal GA, Pamintuan L, Aguiar DJ (1999) The chondrocyte pericellular matrix: A model for hyaluronan-mediated cell-matrix interactions. Biochem Soc Trans 27:142–147 [DOI] [PubMed] [Google Scholar]
- Knudson W, Aguiar DJ, Hua Q, Knudson CB (1996) CD44-anchored hyaluronan-rich pericellular matrices: An ultrastructural and biochemical analysis. Exp Cell Res 228:216–228 [DOI] [PubMed] [Google Scholar]
- Knudson W, Bartnik E, Knudson CB (1993) Assembly of pericellular matrices by COS-7 cells transfected with CD44 lymphocyte-homing receptor genes. Proc Natl Acad Sci USA 90:4003–4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopakkala-Tani M, Leskinen JJ, Karjalainen HM, Karjalainen T, Hynynen K, Töyräs J, Jurvalin JS, et al. (2006) Ultrasound stimulates proteoglycan synthesis in bovine primary chondrocytes. Biorheology 43:271–282 [PubMed] [Google Scholar]
- Kultti A, Rilla K, Tiihonen R, Spicer AP, Tammi RH, Tammi MI (2006) Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem 281:15821–15828 [DOI] [PubMed] [Google Scholar]
- Lange K, Gartzke J (2001) Microvillar cell surface as a natural defense system against xenobiotics: A new interpretation of multidrug resistance. Am J Physiol Cell Physiol 281:C369–C385 [DOI] [PubMed] [Google Scholar]
- Laurent T (1989) The biology of hyaluronan. introduction. Ciba Found Symp 143:1–20 [PubMed] [Google Scholar]
- Lee GM, Johnstone B, Jacobson K, Caterson B (1993) The dynamic structure of the pericellular matrix on living cells. J Cell Biol 123:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Heldin P (2001) Hyaluronan production increases the malignant properties of mesothelioma cells. Br J Cancer 85:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoschek M, Addicks K (1991) Histologic, morphometric study of the human synovial membrane. Z Orthop Ihre Grenzgeb 129:136–140 [DOI] [PubMed] [Google Scholar]
- Makabe S, Naguro T, Stallone T (2006) Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc Res Tech 69:436–449 [DOI] [PubMed] [Google Scholar]
- McBride WH, Bard JB (1979) Hyaluronidase-sensitive halos around adherent cells. their role in blocking lymphocyte-mediated cytolysis. J Exp Med 149:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers SE (2004) The mesothelial cell. Int J Biochem Cell Biol 36:9–16 [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Wilkosz S (2007) Structure and function of mesothelial cells. Cancer Treat Res 134:1–19 [DOI] [PubMed] [Google Scholar]
- Nishida Y, Knudson CB, Nietfeld JJ, Margulis A, Knudson W (1999) Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J Biol Chem 274:21893–21899 [DOI] [PubMed] [Google Scholar]
- Pienimäki JP, Rilla K, Fülop C, Sironen RK, Karvinen S, Pasonen S, Lammi MJ, et al. (2001) Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem 276:20428–20435 [DOI] [PubMed] [Google Scholar]
- Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, Marshall VR, et al. (2007) Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem 282:10814–10825 [DOI] [PubMed] [Google Scholar]
- Rilla K, Pasonen-Seppänen S, Rieppo J, Tammi M, Tammi R (2004) The hyaluronan synthesis inhibitor 4-methylumbelliferone prevents keratinocyte activation and epidermal hyperproliferation induced by epidermal growth factor. J Invest Dermatol 123:708–714 [DOI] [PubMed] [Google Scholar]
- Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH (2005) Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem 280:31890–31897 [DOI] [PubMed] [Google Scholar]
- Tammi R, Ågren UM, Tuhkanen AL, Tammi M (1994) Hyaluronan metabolism in skin. Prog Histochem Cytochem 29:1–81 [DOI] [PubMed] [Google Scholar]
- Tammi R, MacCallum D, Hascall VC, Pienimäki JP, Hyttinen M, Tammi M (1998) Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J Biol Chem 273:28878–28888 [DOI] [PubMed] [Google Scholar]
- Toole BP (2004) Hyaluronan: From extracellular glue to pericellular cue. Nat Rev Cancer 4:528–539 [DOI] [PubMed] [Google Scholar]
- Yung S, Chan TM (2007) Hyaluronan–regulator and initiator of peritoneal inflammation and remodeling. Int J Artif Organs 30:477–483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.