Abstract
Oxidant burden has been suggested to be a contributor to the pathogenesis of idiopathic pulmonary fibrosis (IPF). The study focused on peroxiredoxin (Prx) II, an antioxidant that has been associated with platelet-derived growth factor (PDGF) signaling and consequent cell proliferation. Localization and expression of Prx II, PDGF receptors (PDGFRα, PDGFRβ), Ki67, and nitrotyrosine were assessed in control (n=10) and IPF/usual interstitial pneumonia (UIP) (n=10) lung biopsies by immunohistochemistry and morphometry. Prx II oxidation was determined by standard and non-reducing Western blots, two-dimensional gel electrophoresis, and mass spectrometry. Prx II localized in the IPF/UIP epithelium and alveolar macrophages. Prx II–positive area in the fibroblastic foci (FF) was smaller than in other parenchymal areas (p=0.03) or in the hyperplastic epithelium (p=0.01). There was no major Prx II oxidation in IPF/UIP compared with the normal lung. The FF showed only minor immunoreactivity to the PDGFRs; Ki67, a marker of cell proliferation; and nitrotyrosine, a marker of oxidative/nitrosative stress. The results suggest that Prx II oxidation does not relate to the pathogenesis of IPF/UIP and that Prx II, PDGFRs, and proliferating cells colocalize in the IPF/UIP lung. Unexpectedly, FF represented areas of low cell proliferation. (J Histochem Cytochem 56:951–959, 2008)
Keywords: pulmonary fibrosis, lung, fibroblast foci, oxidative stress, peroxiredoxin, platelet-derived growth factor, nitrotyrosine, cell proliferation
Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease with poor prognosis and unknown etiology (American Thoracic Society/European Respiratory Society 2002). A characteristic feature in this disease is the occurrence of fibroblastic foci (FF), i.e., patchy focal areas of fibroblasts (American Thoracic Society/European Respiratory Society 2002). Progression of the disease and survival of the patients correlate with the numbers of FF lesions in the lung (Nicholson et al. 2002; Tiitto et al. 2006). However, the pathogenesis of IPF is still poorly understood.
Several studies indicate that the pathogenesis of IPF is associated with redox imbalance in the lung (Cantin et al. 1989; Kharitonov and Barnes 2001; Lakari et al. 2002; Cho et al. 2004; Hunninghake 2005; Kinnula et al. 2005). Furthermore, the antioxidant N-acetylcysteine (NAC) is the only drug thus far that has shown any positive effect on the deterioration of the lung function values in patients with IPF (Demedts et al. 2005). Reactive oxygen species (ROS) contribute to the regulation of growth factor, such as transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) activation or function (Koli et al. 2008). ROS activate TGF-β, PDGF, and their receptors, and conversely, PDGF and TGF-β promote ROS generation (Sundaresan et al. 1995; Gonzalez-Rubio et al. 1996; Bae et al. 2000; Koli et al. 2008). IPF is also characterized by the parenchymal accumulation of alveolar macrophages, which release increased amounts of PDGF, mainly of PDGF-B but also PDGF-A (Nagaoka et al. 1990). PDGF activity is regulated by the relative expression of its receptors PDGFRα and PDGFRβ on the surface of myofibroblasts. These receptors are induced during fibrogenesis in association with the expansion of the myofibroblast population and production of extracellular matrix (ECM) proteins (Bonner 2004). In addition, PDGFRα is induced by a variety of factors such as pollutants, asbestos fibers, and ROS (Gonzalez-Rubio et al. 1996; Bonner et al. 1998; Lasky et al. 1998; Bonner 2007). In this context, PDGFRα has been suggested to play a central role in the pathogenesis of lung fibrosis (Lasky et al. 1998).
Peroxiredoxins (Prxs) are involved in redox signaling, antioxidant defense, cell proliferation, and apoptosis (Rhee et al. 2005). Oxidative stress, e.g., hydrogen peroxide (H2O2), causes oxidation at the Prx cysteines and subsequent formation of cysteine sulfenic acid (Cys-SOH). Under severe oxidative stress, Prxs can be further oxidized to sulfinic acid (Cys-SOOH), which results in final Prx inactivation. All six Prxs (Prx I–Prx VI) have been detected in human lung, with a cell-specific expression (Kinnula et al. 2002a). Furthermore, Prx II functions as a negative regulator of PDGF signaling resulting in an enhanced H2O2 production and PDGFR activation on Prx II deficiency (Choi et al. 2005). Prx II plays also a role in cell proliferation because loss of Prx II caused neointimal thickening of smooth muscle cells on endothelial injury in mice (Choi et al. 2005). Similar fibrotic responses to epithelial injury can occur in human IPF.
In this study, the expression levels of all six Prxs were studied to verify if they were altered in the fibrotic lung. Based on the oxidant burden in pulmonary fibrosis (Hunninghake 2005; Kinnula et al. 2005) and the role of Prx II in PDGF signaling (Choi et al. 2005), the study focused further on Prx II localization and expression. Because ROS can inactivate Prxs by oxidation and hyperoxidation (Lehtonen et al. 2005; Rhee et al. 2005), Prx II oxidation was analyzed by non-reducing Western blot, an antibody detecting hyperoxidized Prx II, two-dimensional gel electrophoresis (2-DE), and mass spectrometry (MS). Because Prx II can cause the suppression of PDGFR activation (Choi et al. 2005), the expression of PDGFRs and cellular proliferation was further studied.
Materials and Methods
Patients
Tissue samples were collected by lung surgery from patients treated at the Helsinki University Central Hospital. The control samples were obtained from normal areas of local lung tumors or hamartomas. The fibrotic tissue was obtained from lung transplantations or thoracoscopic biopsies from patients with IPF/usual interstitial pneumonia (UIP). The Ethics Committee of the Helsinki University Central Hospital approved the study, and the use of the clinical material is registered at www.hus.fi/clinicaltrials. All patients received written information and gave their permission to use the samples.
Peroxiredoxins
IHC
Four-μm-thick paraffin-embedded tissue sections (control, n=10; IPF/UIP, n=10; Table 1) were deparaffinized in xylene and rehydrated in graded alcohol. Antigens were retrieved by heating the sections in citrate buffer (pH 6.0). Endogenous peroxidase activity was neutralized with 0.3% H2O2. For immunostaining, the Histostain-Plus Kit (Zymed Laboratories; San Francisco, CA) was used according to the manufacturer's instructions. The primary antibodies used were rabbit anti-Prx I–VI (LabFrontier; Seoul, Korea). Detection was performed with 3-amino-9 ethyl-carbazole (AEC) chromogen substrate solution for horseradish peroxidase (Zymed). The sections were counterstained with Mayer's hematoxylin and mounted on glass slides. Control sections were treated with PBS or rabbit primary antibody isotype control (Zymed) to determine the specificity of the staining.
Table 1.
Demographic and physiological profiles of the control subjects and IPF/UIP patients whose lung tissue samples were used in the IHC studies
| Control | IPF/UIP | |
|---|---|---|
| Patients (n) | 10 | 10 |
| Age (years) | 57.1 ± 4.55 | 61.2 ± 3.00 |
| Sex (M/F) | 8/2 | 6/4 |
| Smoking/non-smoking | 3/7 | 4/6 |
| FVC (%) | 85.0 ± 12.8 | 56.1 ± 6.77 |
| FEV1 (%) | 86.7 ± 11.4 | 58.6 ± 6.93 |
| DLCO | 70.5 ± 23.3 | 36.0 ± 6.93 |
| DLCO/VA | 72.3 ± 17.5 | 55.8 ± 6.63 |
Data are presented as mean ± SEM. IPF, idiopathic pulmonary fibrosis; UIP, usual interstitial pneumonia; FVC, forced vital capacity; FEV1, forced expiratory flow in 1 sec; DLCO, diffusion capacity; DLCO/VA, diffusion coefficient.
Prx antibodies have shown to be specific and sensitive in detecting these proteins both by Western blotting and immunohistochemistry (IHC) in human lung (Kinnula et al. 2002a,b).
Morphometry
Digital morphometry of the stained tissue sections was conducted as previously described (Myllärniemi et al. 2008). Three representative images from the lung parenchyma of each stained section were taken with an Olympus U-CMAD3 camera (Olympus Corporation; Tokyo, Japan) and QuickPHOTO CAMERA 2.1 software (Promicra; Prague, Czech Republic). The areas of positive and negative staining were assessed with Image-Pro Plus 6.1. software (Media Cybernetics; Silver Spring, MD).
Western blot
Tissue biopsies (control, n=4; IPF/UIP, n=4; Table 2) were homogenized in PBS, and 50 μg of protein was used in standard reducing or non-reducing SDS-PAGE and blotted onto membranes. The membranes were stained with 0.2% Ponceau S (Sigma-Aldrich; St. Louis, MO) in 1% acetic acid to ensure equal loading of proteins. Membranes were probed as previously described (Lehtonen et al. 2005) with rabbit anti-Prx II antibody (LabFrontier) or anti-peroxiredoxin-SO3 (LabFrontier), followed by anti-rabbit secondary antibody treatment. The enhanced chemiluminescence system (Amersham Pharmacia Biotech Europe; Freiburg, Germany) or Odyssey Infrared Imaging System (LiCor Biotechology; Lincoln, NE) was used for detection and for quantification of the band intensity.
Table 2.
Demographic and physiological profiles of the control subjects and IPF/UIP patients whose lung tissue samples were used in the Western blot and 2-DE studies
| Control | IPF/UIP | |
|---|---|---|
| Patients (n) | 4 | 4 |
| Age (years) | 58.8 ± 6.63 | 51.7 ± 6.06 |
| Sex (M/F) | 3/1 | 3/1 |
| Smoking/non-smoking | 0/4 | 1/3 |
| FVC (%) | 77.0 ± 16.7 | 37.0 ± 4.93 |
| FEV1 (%) | 76.8 ± 17.3 | 54.7 ± 18.4 |
| DLCO | 55.5 ± 23.3 | 22.7 ± 11.6 |
| DLCO/VA | 66.0 ± 45.0 | 48.0 ± 7.02 |
Data are presented as mean ± SEM. 2-DE, two-dimensional gel electrophoresis.
Two-dimensional Gel Electrophoresis (2-DE) and Protein Identification
Lung tissue samples (control, n=4; IPF/UIP, n=4; Table 2) were powdered when frozen and further purified by acetone precipitation. The protein extract was resuspended in urea buffer [7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 0.15% (w/v) DTT, 0.5% (v/v) carrier ampholytes 3-10, Complete Mini protease inhibitor cocktail; Roche, Basel, Switzerland], disrupted for 10 min in an ultrasonic bath, and centrifuged. Protein aliquots (100 μg) were stored at −20C. The protein separation for each sample, done in triplicate, was performed as previously described (Lehtonen et al. 2008). In brief, the protein solution was adjusted with urea buffer to a final volume of 350 μl, and in-gel rehydration was performed overnight. Isoelectric focusing (IEF) was carried out in IPG strips (pH 4–7, 18 cm; GE Healthcare, Buckinghamshire, UK) with the Multiphor II system (GE Healthcare) under paraffin oil for 55 kWh. SDS-PAGE was done overnight in polyacrylamide gels (12.5% T, 2.6% C) with the Ettan DALT II system (GE Healthcare) at 1–2 W per gel and 12C. The gels were silver stained and analyzed with the 2-DE image analysis software Melanie 3.0 (GeneBio; Geneva, Switzerland).
For protein identification, excised spots were in-gel digested as described earlier (Lehtonen et al. 2008). Peptide masses were measured with a VOYAGER-DE STR (Applied Biosystems; Foster City, CA), and proteins were identified with ProFound database version 2005.02.14 (http://prowl.rockefeller.edu/prowl-cgi/profound.exe) and the following parameters [20 ppm; one missed cut; MH+; +C2H2O2@C (complete), +O@M (partial)]. Detailed analysis of cysteine oxidation within the detected peptides was done with PeptideMass (http://au.expasy.org/tools/peptide-mass.html).
PDGFR
Detection of PDGFR expression in the lung tissue sections from control subjects (n=10) and IPF/UIP patients (n=10) (Table 1) was performed by IHC, as described above. The primary antibodies used were a 1:100 dilution of rabbit anti-PDGFRα (Cell Signaling Technology; Danvers, MA) and a 1:100 dilution of rabbit anti-PDGFRβ (Cell Signaling Technology). Control sections were treated with PBS or rabbit primary antibody isotype control (Zymed) to determine the specificity of the staining.
Cell Proliferation and Oxidative/Nitrosative Stress
Cell proliferation in lung tissue sections (control, n=10; IPF/UIP, n=10; Table 1) was assessed by IHC, as described above. Detection of the proliferation marker Ki67 was performed with a 1:100 dilution of rabbit anti-Ki67 (Thermo Scientific; Fremont, CA). Nitrotyrosine was used as a marker for oxidative/nitrosative stress in the IPF/UIP lungs because it reflects both superoxide (through myeloperoxidase) and nitric oxide–mediated reactions in the cells (Davis et al. 2001). Nitrotyrosine expression in lung tissue sections (control, n=10; IPF/UIP, n=10) was assessed by IHC, as described above. Detection of nitrotyrosine was performed with a rabbit anti-nitrotyrosine antibody (Upstate; Lake Placid, NY). Control sections were treated with PBS or rabbit primary antibody isotype control (Zymed) to determine the specificity of the staining.
Statistical Analyses
Data were analyzed using SPSS 12.0.1 for Windows (SPSS; Chicago, IL). Intergroup differences were analyzed with the non-parametric Mann-Whitney U test; p<0.05 was considered statistically significant.
Results
Prx Expression in Normal and IPF/UIP Lung
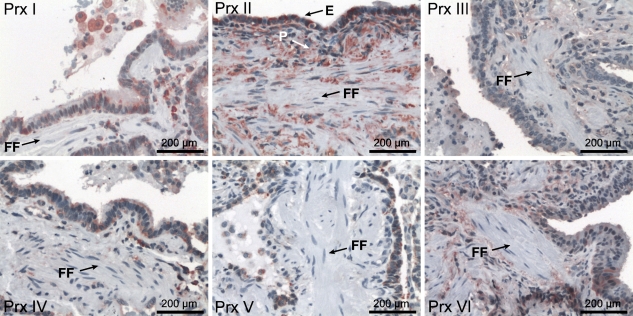
Prxs are expressed in the bronchial and alveolar epithelium as well as in alveolar macrophages (Kinnula et al. 2002a). To verify if their localization in lung tissue—especially in the FF lesions—is affected in IPF, all six Prxs were analyzed by IHC. Generally, a similar localization of Prxs was observed in IPF/UIP and healthy control lungs. However, very low, if any, expression of Prxs could be detected in the FF (Figure 1). Control slides were treated with PBS or appropriate rabbit primary antibody isotype control instead of the primary antibody to determine the specificity of the staining. Both IPF/UIP and healthy lung control slides showed only blue hematoxylin staining, indicating that the red staining achieved with the primary antibodies was specific and restrained to the antigens in question (Prx I–VI, PDGFRα, PDGFRβ, Ki67, or nitrotyrosine).
Figure 1.
Peroxiredoxin (Prx) I–VI expression and localization in idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP) lungs. Positive Prx immunoreactivity is seen mainly in the alveolar epithelium (E) and alveolar macrophages and lung parenchyma (P), but not at fibroblastic foci areas (FF).
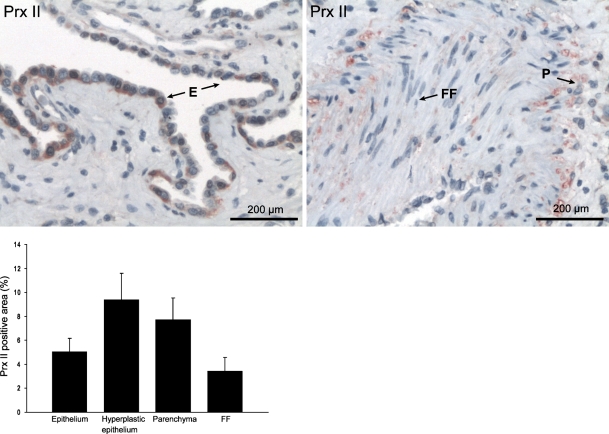
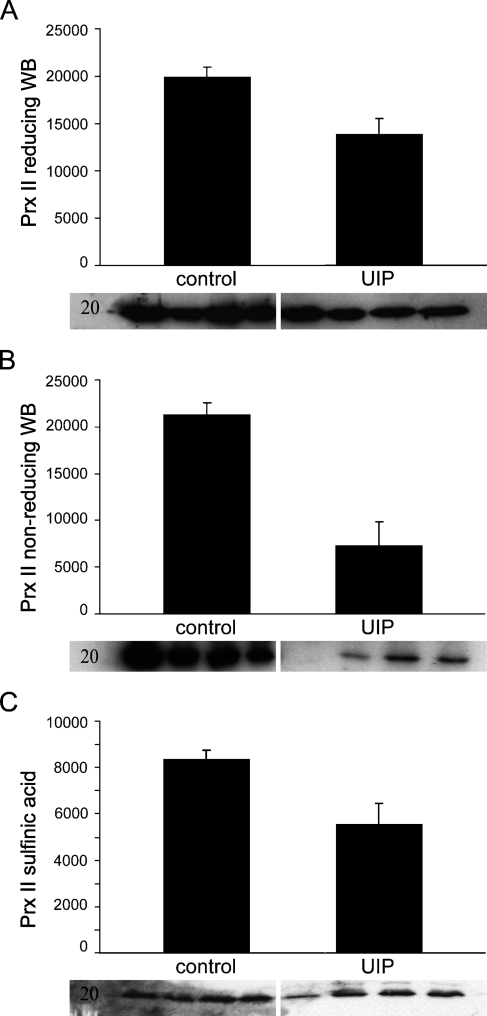
Because Prx II plays a special role in the redox balance and in PDGF signaling (Choi et al. 2005), this study focused on Prx II. Prx II expression was negative or very weak in the FF of the IPF/UIP lungs (Figures 1 and 2). By morphometry, the Prx II–positive area in FF was lower than in the other parenchymal areas in IPF/UIP (p = 0.03) and lower than in the hyperplastic epithelium of IPF/UIP (p=0.01; Figure 2). The comparison of the Prx II immunoreactivity in normal and IPF/UIP lung tissue by Western blot showed decreased Prx II protein levels in the diseased lungs (p=0.04; Figure 3A).
Figure 2.
Prx II expression and localization in IPF/UIP epithelium (E), lung parenchyma (P), and fibroblastic foci (FF). Morphometric analysis shows increased Prx II–positive area (%) in the hyperplastic epithelium (p=0.01) and lung parenchyma (p=0.03) compared with fibroblastic foci.
Figure 3.
Prx II protein level by immunoblotting in control (Lanes 1–4) and IPF/UIP lung (Lanes 5–8). (A) Standard Western blot (WB) shows decreased Prx II intensity levels in IPF/UIP lungs compared with healthy control lung (p=0.04). (B) Non-reducing Western blot shows lower amount of monomeric, oxidized Prx II in IPF/UIP lungs than in normal control lungs as measured by intensity of the bands. (C) Prx II hyperoxidation was detected by standard reducing Western blot using an antibody against the sulfinic acid form of Prx. The amount of hyperoxidized Prx II was slightly but not significantly decreased in IPF/UIP lungs compared with controls.
Evaluation of Prx II Oxidation in Normal and Fibrotic Lung
Oxidation (i.e., shift from the dimeric form to monomers) of Prx II was studied by non-reducing Western blot (Figure 3B). Analysis of the non-reducing Western blot bands showed some Prx II oxidation and monomer formation both in control and IPF/UIP lungs with clear variability, as has already been shown in vitro and in control and chronic obstructive pulmonary disease (COPD) lungs (Lehtonen et al. 2005,2008). When the protein levels of the monomeric oxidized Prx II in control and IPF/UIP lungs were compared, the band intensities were lower in the IPF/UIP than in the control lung (p=0.02 control vs IPF/UIP, Mann-Whitney test; Figure 3B). Furthermore, the sum intensity of the dimeric and monomeric Prx II forms was significantly lower in IPF/UIP than in the control lung (p=0.02). Because the hyperoxidized forms are not necessarily monomers, possible Prx II hyperoxidation was further studied by Western blot with sulfinic acid–specific antibody (Figure 3C). It showed a trend toward a decrease in the level of the hyperoxidized Prx II in the IPF lungs. This result is in concordance with the overall decrease of Prx II levels in the IPF/UIP lungs.
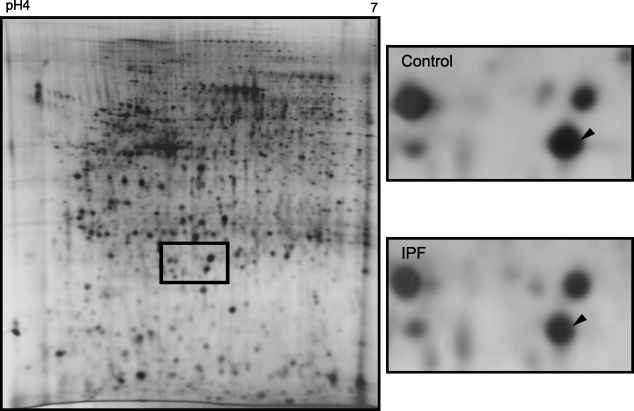
Because cysteine oxidation of Prxs causes a change in protein charge reflected by a spot shift within the 2-DE gel (Rabilloud et al. 2002; Wagner et al. 2002; Chevallet et al. 2003), the oxidation stage of Prx II in vivo was further evaluated by 2-DE of control (n=4) and IPF/UIP lungs (n=4; Figure 4). In agreement with the Western blot analyses, a slight decrease in the Prx II level was observed in IPF/UIP lungs. However, no shift of the Prx II spot was observed in 2-DE gels of the control and IPF/UIP specimens, indicating no detectable difference in the oxidation status. Because the spot matched to the non-oxidized form of an earlier study (Wagner et al. 2002), this indicates the presence of non-oxidized Prx II. MS analyses confirmed with a spot-specific peptide (3058.5422) that cysteine at position 51 was not oxidized. These data indicate that the majority of Prx II in the control and IPF/UIP lungs is not irreversibly oxidized.
Figure 4.
Two-dimensional gel electrophoresis (2-DE) shows no difference in Prx II oxidation in IPF/UIP lungs. Lung homogenates were separated by 2-DE (pH 4–7) and Prx II was identified by mass spectrometry. A 2-DE gel representative for a lung with IPF is shown on the left. The representative Prx II expression in normal lungs (control, n=4) and IPF (n=4) is shown on the right. The non-oxidized spot is marked with an arrow.
Associations Between Prx II, PRGFR, Proliferation, and Oxidative/Nitrosative Stress
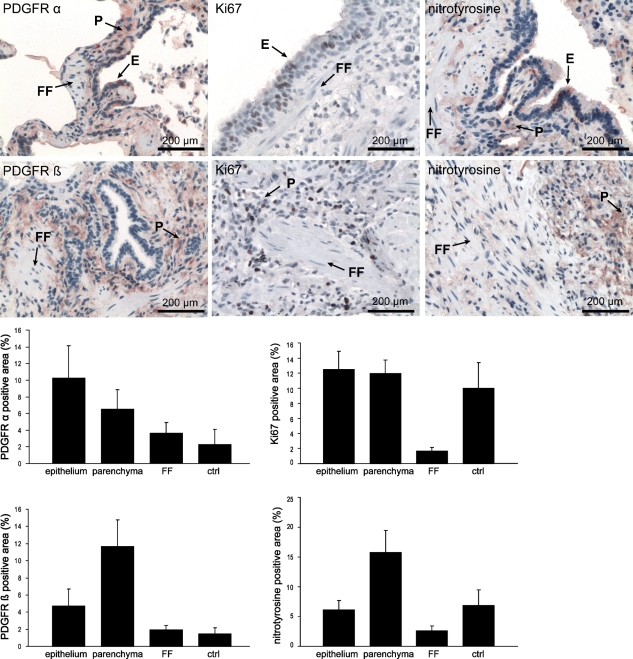
The expression of the PDGF receptor and the proliferation marker Ki67 was studied by IHC in the different compartments of the IPF/UIP lungs (the FF, remaining parenchymal tissue, and the epithelium overlying the FF, which are easily identifiable morphologically). By morphometry, PDGFRα- and PDGFRβ-positive areas were found in the IPF/UIP epithelium and the lung parenchyma outside the FF, whereas low PDGFR-positive areas were seen in the FF (Figure 5). Similarly, Ki67-positive cells were localized predominantly in the parenchyma and epithelium, outside the FF (Figure 5).
Figure 5.
Expression and localization of platelet-derived growth factor receptors PDGFRα and PDGFRβ, Ki67, and nitrotyrosine in IPF/UIP lungs. Morphometrical analysis shows an increase in the PDGFRα-, PDGFRβ-, nitrotyrosine-, and Ki67-positive area (%) in the epithelium (E) and parenchyma (P) in IPF/UIP compared with the fibroblastic foci (FF) or healthy control lung (ctrl).
Prx II deficiency has been associated with oxidative stress, at least in vitro (Choi et al. 2005), but whether this phenomenon occurs in vivo is unclear. Local oxidative/nitrosative stress was evaluated by positive immunoreactivity to nitrotyrosine in the control and IPF/UIP lung. These results showed nitrotyrosine-positive cells in the epithelium and inflammatory cells but again the FF in the IPF/UIP lung showed only weak positivity or remained negative. The nitrotyrosine-positive area in the control lung localized to the epithelium (Figure 5).
Discussion
The regulation of intracellular H2O2 levels has been shown to be associated with Prx II. Prx II–deficient fibroblasts exhibit twice as much H2O2, enhanced activation of PDGFR, and enhanced proliferation. This study showed that the levels of Prx II are very low in the FF of the fibrotic lung, whereas Prx II expression can be seen at the lung parenchyma outside the FF. Prx II expression colocalized with the other Prx proteins, the PDGFRs, nitrotyrosine, and the proliferation marker Ki67.
Peroxidase reactions of Prxs can cause the thiol group (–SH) of cysteine residues to be oxidized to sulfenic acid (–SOH). This is highly unstable and quickly forms either a disulfide bridge (–S–S–) with another thiol or is converted to sulfinic acid (–SOOH) or even sulfonic acid (–SOOOH). However, even the overoxidized forms of Prxs can reverse back to the reduced forms (Rhee et al. 2005). There are very few studies on Prx oxidation in human material. In our previous study on COPD specimens, no major Prx oxidation could be detected by non-reducing Western blot or 2-DE/MS (Lehtonen et al. 2008). The results of this study are very similar (i.e., no major differences could be detected between the control and diseased lung by non-reducing Western blot, by an anti-Prx II antibody directed toward the sulfinic acid form of the molecule, or by 2-DE/MS). Individual Cys molecules of Prx II did not differ between the control and diseased lung. Moreover, unpublished data showed no thioredoxin oxidation by 2-DE/MS in the IPF/UIP samples. In contrast, oxidation of Prxs has been documented in lung cells that have been exposed to H2O2 in vitro (Lehtonen et al. 2005). These studies have shown that the oxidation of Prxs is generally transient, which may explain the unchanged findings in the lung specimens and also the high resistance of the Prx system against oxidative stress in vivo.
An important question still remaining to be answered is the association between low antioxidant levels and oxidative stress in the FF lesions, and whether there is any major oxidant burden in the FF. Several studies have shown that, besides low Prx II expression, many other antioxidant enzymes are very low or absent in the FF, whereas they are elevated, especially in the hyperplastic epithelium of the IPF/UIP lungs (Lakari et al. 1998; Tiitto et al. 2003; Peltoniemi et al. 2004; Tiitto et al. 2004; Kinnula et al. 2006). The same is true with the expression of inducible nitric oxide synthase (iNOS), which is intensively expressed in the inflammatory areas and alveolar epithelium but weak or absent in the FF of IPF/UIP lungs (Lakari et al. 2002; Kinnula and Myllarniemi 2008). Antioxidant enzymes are activated because of oxidative stress and activation of oxidant-producing enzymes, such as iNOS. The results suggest that, in the IPF/UIP lungs, this activation does not occur in the FF but rather in the inflammatory and alveolar epithelial cells, which is the main site of the injury. In contrast, oxidant/antioxidant imbalance in the FF lesions may contribute to the activation of growth factors and their receptors and thus increase cell proliferation. This study could not confirm this phenomenon to occur with Prx II. Despite the very low Prx II levels in FF, no marked cell proliferation could be seen in these lesions. This finding is in full agreement with the fact that oxidant burden was very low in the FF. High antioxidant defense system and oxidative metabolism are generally associated with actively proliferating cells that have high metabolic activity and uncontrolled expansion (Kinnula and Crapo 2004). This study showed that the high amount of oxidant-related metabolism is located predominantly outside the FF, colocalizing with proliferating cells in vivo. Nitrotyrosine immunoreactivity in the epithelium of control lung may be caused by changes in the oxidative conditions during anesthesia and surgery as the lung biopsies were obtained.
In conclusion, we showed a colocalization of Prx II immunoreactivity, PDGFR immunoreactivity, oxidant burden, as shown by nitrotyrosine immunoreactivity, and proliferating cells in the IPF/UIP lung. A prominent feature was the low level of Prx II, nitrotyrosine, and Ki67, as a marker of cell proliferation, in the fibroblastic foci in IPF/UIP. The absence of evidence for Prx II oxidation suggests the high resistance of this enzyme against oxidative modifications in vivo.
Acknowledgments
This work was financially supported by the Finnish Antituberculosis Association Foundation, Finnish Cultural Foundation, Finnish Medical Foundation, Pulmonary Association Heli, Sigrid Juselius Foundation, Yrjö Jahnsson Foundation, and a special governmental grant for health sciences research (HUCH-EVO).
The authors thank Anitra Ahonen and Tiina Marjomaa for excellent technical assistance.
References
- American Thoracic Society/European Respiratory Society (2002) International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 165:277–304 [DOI] [PubMed]
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG (2000) Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem 275:10527–10531 [DOI] [PubMed] [Google Scholar]
- Bonner JC (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255–273 [DOI] [PubMed] [Google Scholar]
- Bonner JC (2007) Lung fibrotic responses to particle exposure. Toxicol Pathol 35:148–153 [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Lindroos PM, O'Brien PO, Dreher KL, Rosas I, Faro-Moreno E, et al. (1998) Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. Am J Respir Cell Mol Biol 19:672–680 [DOI] [PubMed] [Google Scholar]
- Cantin AM, Hubbard RC, Crystal RG (1989) Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139:370–372 [DOI] [PubMed] [Google Scholar]
- Chevallet M, Wagner E, Luche S, Van DA, Leize-Wagner E, Rabilloud T (2003) Regeneration of peroxiredoxins during recovery after oxidative stress: only some overoxidized peroxiredoxins can be reduced during recovery after oxidative stress. J Biol Chem 278:37146–37153 [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR (2004) The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 18:1258–1260 [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Parks HS, et al. (2005) Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435:347–353 [DOI] [PubMed] [Google Scholar]
- Davis KL, Martin E, Turko IV, Murad F (2001) Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 41:203–236 [DOI] [PubMed] [Google Scholar]
- Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, et al. (2005) High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353:2229–2242 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rubio M, Voit S, Rodriguez-Puyol D, Weber M, Marx M (1996) Oxidative stress induces tyrosine phosphorylation of PDGF alpha-and beta-receptors and pp60c-src in mesangial cells. Kidney Int 50:164–173 [DOI] [PubMed] [Google Scholar]
- Hunninghake GW (2005) Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med 353:2285–2287 [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Barnes PJ (2001) Exhaled markers of pulmonary disease. Am J Respir Crit Care Med 163:1693–1722 [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD (2004) Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med 36:718–744 [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Fattman CL, Tan RJ, Oury TD (2005) Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 172:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Hodgson UA, Lakari EK, Tan RJ, Sormunen RT, Soini YM, Kakko SJ, et al. (2006) Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology 49:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Lehtonen S, Kaarteenaho-Wiik R, Lakari E, Paakko P, Kang SW, Rhee SG, et al. (2002a) Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax 57:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, Soini Y (2002b) Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol 196:316–323 [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Myllarniemi M (2008) Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid Redox Signal 10:727–738 [DOI] [PubMed] [Google Scholar]
- Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL (2008) Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal 10:333–342 [DOI] [PubMed] [Google Scholar]
- Lakari E, Paakko P, Kinnula VL (1998) Manganese superoxide dismutase, but not CuZn superoxide dismutase, is highly expressed in the granulomas of pulmonary sarcoidosis and extrinsic allergic alveolitis. Am J Respir Crit Care Med 158:589–596 [DOI] [PubMed] [Google Scholar]
- Lakari E, Soini Y, Saily M, Koistinen P, Paakko P, Kinnula VL (2002) Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol 117:132–142 [DOI] [PubMed] [Google Scholar]
- Lasky JA, Tonthat B, Liu JY, Friedman M, Brody AR (1998) Upregulation of the PDGF-alpha receptor precedes asbestos-induced lung fibrosis in rats. Am J Respir Crit Care Med 157:1652–1657 [DOI] [PubMed] [Google Scholar]
- Lehtonen ST, Markkanen PM, Peltoniemi M, Kang SW, Kinnula VL (2005) Variable overoxidation of peroxiredoxins in human lung cells in severe oxidative stress. Am J Physiol Lung Cell Mol Physiol 288:L997–1001 [DOI] [PubMed] [Google Scholar]
- Lehtonen ST, Ohlmeier S, Kaarteenaho-Wiik R, Harju T, Paakko P, Soini Y, Kinnula VL (2008) Does the oxidative stress in chronic obstructive pulmonary disease cause thioredoxin/peroxiredoxin oxidation? Antioxid Redox Signal 10:813–820 [DOI] [PubMed] [Google Scholar]
- Myllärniemi M, Vuorinen K, Pulkkinen V, Kankaanranta H, Aine T, Salmenkivi K, Keski-Oja J, et al. (2008) Gremlin localization and expression levels partially differentiate idiopathic interstitial pneumonia severity and subtype. J Pathol 214:456–463 [DOI] [PubMed] [Google Scholar]
- Nagaoka I, Trapnell BC, Crystal RG (1990) Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest 85:2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU (2002) The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166:173–177 [DOI] [PubMed] [Google Scholar]
- Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, et al. (2004) Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 35:1000–1007 [DOI] [PubMed] [Google Scholar]
- Rabilloud T, Heller M, Gasnier F, Luche S, Rey C, Aebersold R, Benahmed M, et al. (2002) Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem 277:19396–19401 [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 8:1543–1552 [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299 [DOI] [PubMed] [Google Scholar]
- Tiitto L, Bloigu R, Heiskanen U, Paakko P, Kinnula VL, Kaarteenaho-Wiik R (2006) Relationship between histopathological features and the course of idiopathic pulmonary fibrosis/usual interstitial pneumonia. Thorax 61:1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitto L, Kaarteenaho-Wiik R, Sormunen R, Holmgren A, Paakko P, Soini Y, Kinnula VL (2003) Expression of the thioredoxin system in interstitial lung disease. J Pathol 201:363–370 [DOI] [PubMed] [Google Scholar]
- Tiitto LH, Peltoniemi MJ, Kaarteenaho-Wiik RL, Soini YM, Paakko PK, Sormunen RT, Kinnula VL (2004) Cell-specific regulation of gamma-glutamylcysteine synthetase in human interstitial lung diseases. Hum Pathol 35:832–839 [DOI] [PubMed] [Google Scholar]
- Wagner E, Luche S, Penna L, Chevallet M, Van DA, Leize-Wagner E, Rabilloud T (2002) A method for detection of overoxidation of cysteines: peroxiredoxins are oxidized in vivo at the active-site cysteine during oxidative stress. Biochem J 366:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]