Abstract
Objectives
This study examined the influence of lower extremity body composition and muscle strength on the severity of mobility-disability in community-dwelling older adults.
Methods
Fifty-seven older males and females (age 74.2 ± 7 yrs; BMI 28.9 ± 6 kg/m2) underwent an objective assessment of lower extremity functional performance, the Short Physical Performance Battery test (SPPB). Participants were subsequently classified as having moderate (SPPB score > 7: n = 38) or severe mobility impairments (SPPB score ≤7: n = 19). Body composition was assessed using dual-energy X-ray absorptiometry and provided measures of bone mineral density (BMD), total leg lean mass (TLM) and total body fat. Maximal hip extensor muscle strength was estimated using the bilateral leg press exercise. Multiple logistic regression analysis was utilized to identify the significant independent variables that predicted the level of mobility-disability.
Results
TLM was a strong independent predictor of the level of functional impairment, after accounting for chronic medical conditions, BMD, body fat, body weight and habitual physical activity. In a separate predictive model, reduced muscle strength was also a significant predictor of severe functional impairment. The severity of mobility-disability was not influenced by gender (p = 0.71). A strong association was elicited between TLM and muscle strength (r = 0.78, p < 0.01).
Conclusions
These data suggest that lower extremity muscle mass is an important determinant of physical performance among functionally-limited elders. Such findings may have important implications for the design of suitable strategies to maintain independence in older adults with compromised physical functioning. Additional studies are warranted to assess the efficacy of lifestyle, exercise or therapeutic interventions for increasing lean body mass in this population.
Keywords: Aging, Sarcopenia, Mobility, Muscle Mass, Strength
Introduction
As the life expectancy of older adults continues to increase, prevention or postponement of age-associated mobility-disability is now of major public health importance (1, 2). Elders who lose their mobility have higher rates of falls and injury, chronic disease, dependency, institutionalization, and mortality (2–6). Consequently, the need to identify specific factors that influence mobility-disability has become increasingly important for optimizing appropriate intervention strategies.
Existing evidence suggests that the age-associated decline in muscle strength and changes in body composition contribute to the onset and progression of disability with advancing age. The role of muscle strength is supported by numerous cross-sectional studies that have typically shown a strong association between low muscle strength and decreased mobility in the elderly (7). Cross-sectional (8, 9) and longitudinal (10, 11) studies also report a direct association between body weight or body mass index and functional limitations among older adults. However, several large-scale studies that have examined functional limitation in relation to other indices of body composition report conflicting results. Higher levels of body fat, rather than lower levels of lean or fat-free mass, were associated with a greater likelihood of disability among older adults with self-reported disability (12, 13). Yet, in other investigations, the age-related loss of skeletal muscle mass has been shown to be a major precursor for mobility-disability in high-functioning elders and those with self-reported disability (14, 15), and decrements in muscle mass are directly related to the decline in muscle strength with advancing age (16). The relationship between mobility-disability and other measures of body composition, such as bone mineral density (BMD), also remain unclear (17).
One possible reason for the discordant functional status - body composition relationship reported in the foregoing studies may be because many of the participants were relatively healthy, high-functioning, or were classified as being functionally-limited through use of self-report techniques. Self-reported ascertainment of disability can lead to an underestimation or overestimation of functional capabilities, while alternatively, objective performance-based measures of physical performance offer advantages in quantifying mobility-disability in terms of validity, reproducibility and applicability (18, 19). Moreover, characterizing potentially modifiable determinants of mobility-decline among a population that exhibit objective limitations in physical functioning could provide more accurate information and lend more precision in developing interventions to reduce the burden and prevalence of mobility-disability with advancing age.
Therefore, the purpose of the present study was to explore the influence of measures of body composition and muscle strength on the level of mobility-impairment in older adults using the Short Physical Performance Battery test (SPPB). The SPPB is a well-established, reliable and objective performance test characterizing lower extremity function using measures of gait speed, standing balance, and lower extremity strength. Scores obtained on the SPPB have been shown to be highly predictive of subsequent disability, institutionalization, and mortality (3, 19).
Methods
Study Design
This study employed a cross-sectional design and data was collected as part of a 12-week randomized controlled exercise intervention trial. Only baseline data are presented in the analyses below.
Study Population
Subjects were recruited from the Boston area through local advertisements and community newsletters. Potential subjects were initially screened by telephone or in person and were eligible for the study if they were aged 65 years or older, community dwelling, and demonstrated mobility impairments as defined by a score of 9 or below on the Short Physical Performance Battery (SPPB).
Eligible subjects completed a medical history questionnaire and underwent a physical examination and medical screening by the study physician. In addition, all subjects underwent a supervised graded exercise test on a treadmill prior to enrollment. Subjects were excluded from participation if they had acute or terminal illness, myocardial infarction in the past 6 months, unstable cardiovascular disease or other medical condition, upper or lower extremity fracture in the past 6 months, upper or lower extremity amputation, cognitive impairment according to the Folstein Mini-Mental State Examination (MMSE) (score <23), current participation in regular exercise sessions (>1×/week), or unwillingness to complete the study requirements. Other exclusion criteria included uncontrolled hypertension (>150/90 mmHg), the presence of neuromuscular disease or drugs affecting neuromuscular function, and estrogen therapy in females. Subjects meeting the study entry criteria and given medical clearance by the study physician and written approval from their primary care physician were deemed eligible for participation. All volunteers signed an informed consent form and, prior to enrollment, were made aware of all potential risks and benefits associated with procedures of the study. The Boston University Institutional Review Board approved this study.
Outcome Measures
Mobility-Disability Classification using the SPPB
The SPPB has been previously described and validated in large-scale epidemiologic studies, and scores obtained on the 12-point summary scale indicate a gradient of functional decline that can be used to identify subgroups at low (score ≥ 10), moderate (score 7–9) and severe (score 4–6) risk of disability (3, 4, 19, 20). In this study, we stratified eligible participants into two distinct categories based on their SPPB score: elders who exhibited moderate limitations in physical functioning (score > 7), or elders that exhibited severe functional limitations (score ≤7).
Muscle Strength
Lower extremity muscle strength was quantitatively assessed by using 1 repetition maximum (1RM) measures of bilateral leg extension exercise, using Keiser pneumatic resistance training equipment (Keiser Sports Health Equipment Inc., Fresno, CA) (21). The 1RM was defined as the maximum load that could be moved only once throughout the full range of motion while maintaining proper form. Subjects performed the concentric phase, maintained full extension, and performed the eccentric phase of each repetition over 2, 1, and 2 seconds, respectively.
Bone Mineral Density and Body Composition
Dual-energy X-ray absorptiometry (DXA) was used to determine bone mineral density (BMD) of the total hip, total percentage body fat, and total lean leg muscle mass (TLM, left + right leg) (fat free mass of leg minus bone tissue) utilizing the Hologic QDR 4500 fan beam scanner (Hologic Inc., Waltham, MA). Body mass was recorded on a standard platform scale to the nearest 0.1 kg. Height was measured to the nearest 0.5 cm with a scale stadiometer.
Health Status and Physical Activity Levels
Health and cognitive status, and the number of medical diagnoses and daily medications were assessed via medical history questionnaires and the physical examination. Habitual occupational, household, and leisure physical activity was estimated using the Physical Activity Scale for the Elderly (PASE) (22). Because both cross-sectional and longitudinal studies have demonstrated that regular physical activity is associated with maintenance of physical performance and functional status (23, 24), the potential influence of this variable was also controlled for in the statistical analysis.
Statistical Analysis
Data were analyzed using SPSS software (Version 15.0 for Windows, Chicago, IL). All data were first examined visually and statistically for normality of distribution. Values are presented as means ± standard deviation (SD) unless otherwise stated. Significance level was set at p ≤ 0.05. Descriptive statistics were calculated and an independent samples t-test was used to determine baseline differences between males and females. Multiple logistic regression analysis was used to assess whether the level of mobility-disability was associated with measures of lower extremity strength or body composition. Dummy variables for the level of mobility-disability variable were created and coded as follows: moderate = 0, severe = 1, and Odds Ratios (OR) for these factors were subsequently computed. In order to determine the most appropriate logistic regression model, exploratory data analysis (Pearson correlation coefficients) was used to assess the degree of association and potential for multicollinearity between independent variables. Initially, a chi-squared test of cross tabulation between sex and mobility status was performed in order to assess the influence of sex on the level of mobility-disability. Goodness-of-fit tests such as the Log Likelihood ratio, the Cox & Snell R Square statistic and the Hosmer and Lemeshow Test were also used are as indicators of model appropriateness, and the Wald statistic was used to test the significance level of individual independent predictor variables. Model assumptions were examined both graphically and analytically, and were adequately met. A general additive model (GAM) was also applied to aid in describing the potential underlying relationships between mobility status and the independent variables (25).
Results
Subject Characteristics
A total of 71 subjects met the acceptable SPPB score criteria of ≤ 9, however, 14 subjects were no longer eligible after the screening visit because of medical exclusions. Therefore, 57 subjects (31 females) were eligible and participated in the study. Baseline characteristics are presented in Table 1. Age and height were significantly greater in males compared to females (p < 0.05). Similarly, leg strength, total hip BMD, and TLM were significantly greater among males (p < 0.05). Total body fat percentage was significantly higher among females (p < 0.05).
Table 1.
Baseline characteristics (n = 57)
| Variables | Males (n = 26) | Females (n = 31) | Total |
|---|---|---|---|
| Age, yrs | 76.8 ± 7 | 72.0 ± 6* | 74.2 ± 7 |
| Body Weight, kg | 80.9 ± 15 | 77.5 ± 18 | 78.8 ± 16 |
| BMI, kg/m2 | 27.7 ± 4 | 30.1 ± 7 | 28.9 ± 6 |
| Medical Diagnoses, n | 1.3 ± 1 | 1.4 ± 1 | 1.4 ± 1 |
| Medications per day, n | 2.4 ± 3 | 2.2 ± 2 | 2.3 ± 2 |
| SPPB | 7.6 ± 1 | 7.8 ± 1 | 7.7 ± 1 |
| BMD Total Hip, g/cm2 | 0.94 ± 0.2 | 0.83 ± 0.1* | 0.88 ± 0.2 |
| Total Body Fat, % | 28.0 ± 7 | 37.8 ± 9* | 33.1 ± 10 |
| TLM, kg | 17.1 ± 3 | 14.6 ± 3* | 15.7 ± 3.2 |
| Leg Strength, N | 658 ± 202 | 504 ± 144* | 574 ± 188 |
| Physical Activity, (PASE) | 99.4 ± 52 | 99.6 ± 48 | 99.5 ± 49 |
Values are mean ± SD. BMI: body mass index; SPPB: short physical performance battery score; BMD: bone mineral density; TLM: Total Lean Leg Muscle Mass; N: newtons; PASE: physical activity scale for the elderly;
= p ≤ 0.05
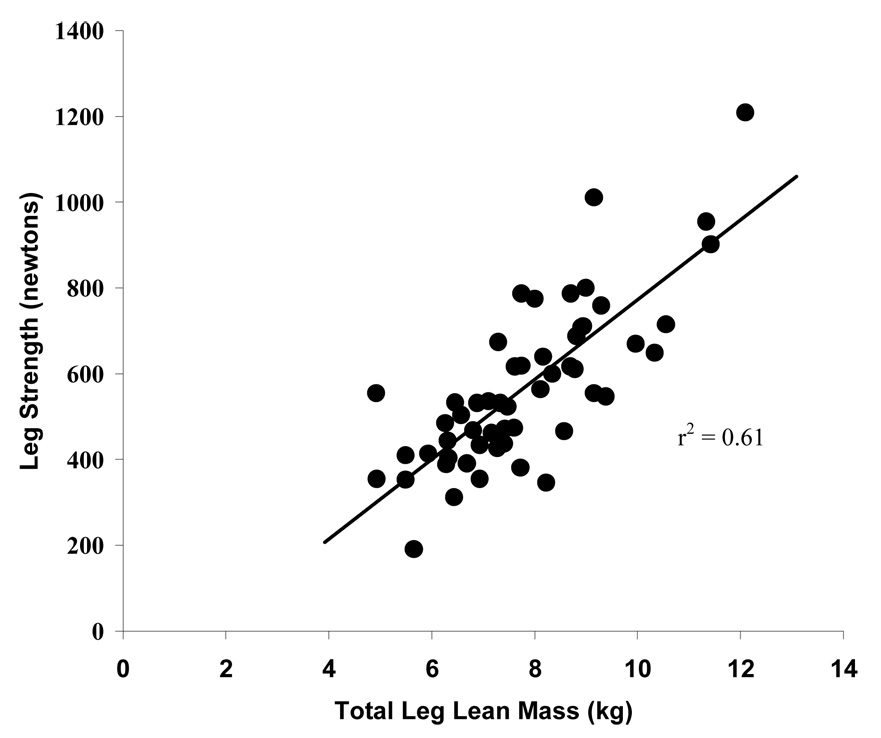
Table 2 displays the correlation coefficients and interrelationships between the independent variables. A strong relationship was elicited between TLM and muscle strength (r = 0.78, p ≤ 0.01, Figure 1).
Table 2.
Pearson correlation coefficients among independent variables (n = 57)
| BMD Total Hip | Body weight | Leg Strength | Total Body Fat | TLM | |
|---|---|---|---|---|---|
| Body Weight | 0.55** | ||||
| Leg Strength | 0.51** | 0.55** | |||
| Total Body Fat | 0.07 | 0.43** | −0.21 | ||
| TLM | 0.48* | 0.65** | 0.78** | −0.24 | |
| Physical Activity, (PASE) | −0.07 | 0.12 | 0.34* | −0.10 | 0.34* |
BMD: bone mineral density; TLM: Total Lean Leg Muscle Mass; N: newtons; PASE: physical activity scale for the elderly;
p ≤ 0.05
p ≤ 0.001
Figure 1.
Correlation between total leg lean mass and leg strength (n = 57)
Based on their SPPB score, 38 of the participants were classified as having moderate mobility limitations (SPPB score > 7), while 19 participants exhibited severe functional limitations (SPPB score ≤ 7). No association was elicited between gender and the level of mobility-disability (χ2 = 0.14, p = 0.71).
Table 2 outlines the variable selection procedures used to assess the relationship between the level of mobility-disability and the potential independent predictor variables. Model specification was tightly controlled in generating the logistic regression model and caution was taken not to include any variables in the same model that were a strong linear combination of each other (i.e. TLM vs. leg muscle strength). Similarly, goodness-of-fit and appropriateness of the selected models were maximized using diagnostic tests, as previously described.
Only TLM was a significant independent predictor of the level of mobility-disability, after accounting for chronic medical diagnoses, BMD, body weight, total body fat and habitual physical activity (Table 4, Model 1). When all other variables in the model are held constant, for every one unit increase in leg muscle mass (1 kg), the log odds of having severe mobility impairments decreased multiplicatively by a factor of e −0.75 resulting in an odds ratio of 0.47 (95% CI: 0.25, 0.91).
Table 4.
Individual multiple logistic regression model to assess the association between level of mobility-disability and independent predictor variables (n = 57)
| Variable | B | S.E | Wald | P Value |
|---|---|---|---|---|
| Model 1 | ||||
| Medical Diagnoses | 0.31 | 0.31 | 1.02 | 0.31 |
| BMD Total Hip | 0.45 | 2.6 | 0.03 | 0.86 |
| Body Weight | 0.12 | 0.06 | 3.8 | 0.06 |
| TLM | −0.75 | 0.34 | 5.4 | 0.02* |
| Total Body Fat | −0.14 | 0.08 | 3.2 | 0.08 |
| Physical Activity | 0.007 | 0.008 | 0.8 | 0.38 |
| Constant | 4.95 | 3.3 | 2.3 | 0.13 |
| Model 2 | ||||
| Medical Diagnoses | 0.07 | 0.3 | 0.05 | 0.82 |
| BMD Total Hip | 1.74 | 2.8 | 0.4 | 0.53 |
| Body weight | 0.04 | 0.04 | 1.5 | 0.23 |
| Leg Strength | −0.008 | 0.004 | 5.4 | 0.02* |
| Total Body Fat | −0.043 | 0.05 | 0.86 | 0.36 |
| Physical Activity | 0.002 | 0.007 | 0.07 | 0.80 |
| Constant | −0.08 | 2.3 | 0.001 | 0.97 |
BMD: bone mineral density; TLM: Total Lean Leg Muscle Mass
Similarly, in model 2 (table 4), leg strength was a significant independent predictor of mobility-disability severity, after accounting for medical diagnoses, BMD, body weight, total body fat and habitual physical activity. When all other variables in the model are held constant, for every one hundred unit increase in leg muscle strength (newtons), the log odds of being at severe risk for disability decreased multiplicatively by a factor of e −0.80 resulting in an odds ratio of 0.45 (95% CI: 0.22, 0.89).
Because baseline differences existed between males and females for leg strength and TLM, the logistic regressions were repeated to investigate whether gender was a potential effect modifier. The analysis was repeated with adjustment for gender and interaction term in the model. The observed relations were similar to results presented in table 3 for both TLM and leg strength (data not presented).
Table 3.
Individual multiple logistic regression models to assess the mediators of mobility-disability
| Variables | Model 1 | Model 2 |
|---|---|---|
| Medical Diagnoses | x | x |
| BMD Total Hip | x | x |
| Body Weight | x | x |
| Leg Strength | x | |
| TLM | x | |
| Total Body Fat | x | x |
| Physical Activity | x | x |
BMD: bone mineral density; TLM: Total Lean Leg Muscle Mass
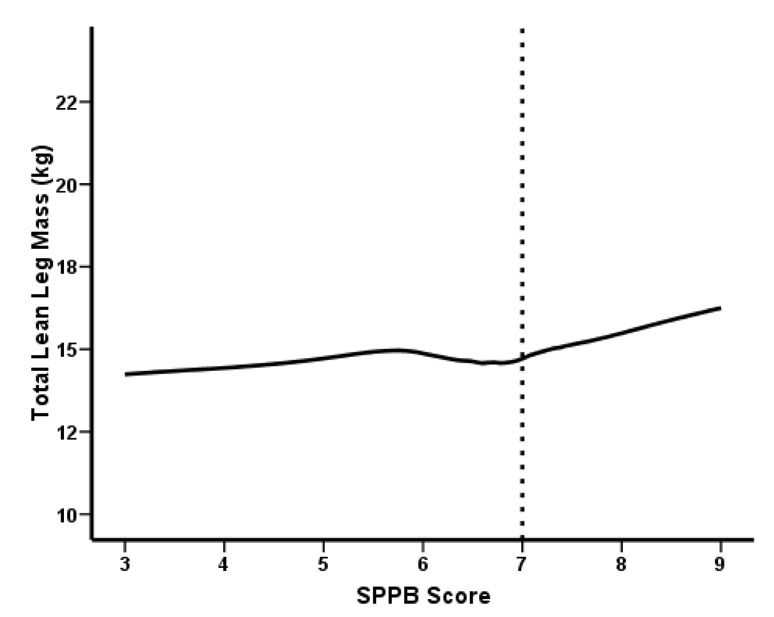
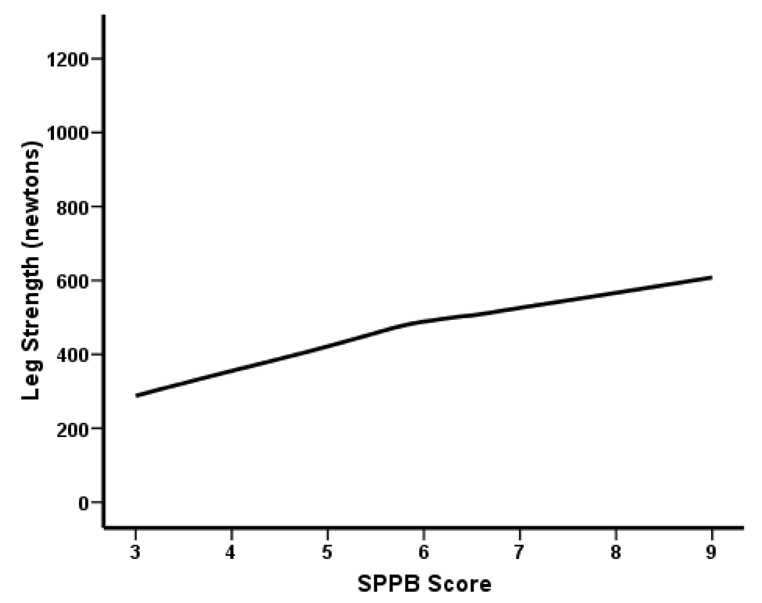
To further examine the relation between SPPB score and TLM we explored potential non-linearity using a generalized additive model (Figures 2a and 2b). As shown in Figure 2a, a SPPB score greater than 7 was associated with a notable increase of TLM, confirming the inverse relationship between TLM and the severity of mobility-disability. Muscle strength appears to be a positive linear function of SPPB score (Figure 2b).
Figure 2.
Figure 2a. Univariate association between SPPB score and TLM threshold suggested by GAM plot (n = 57)
Figure 2b. Univariate association between SPPB score and muscle strength suggested by GAM plot (n = 57)
Discussion
This investigation has demonstrated that lower extremity muscle mass and muscle strength are independent determinants of the severity of mobility-disability in older adults with compromised physical functioning. These findings are important as they suggest that, within this specific population, these two modifiable physiological parameters have stronger predictive capability compared to several other previously reported anthropometric and lifestyle correlates of functional decline. Our findings also confirm a strong interrelationship between lower extremity muscle mass and muscle strength, and extend this association among a group of mobility-impaired elders who exhibited performance-based limitations in physical functioning.
The loss of muscle mass with advancing age, or sarcopenia, is hypothesized as being closely associated with the deterioration of physical function (26). From the published literature, this relationship has not been firmly established, as studies describing the relation between low muscle mass and physical function have provided inconsistent results (12, 13, 15, 27, 28). There may, however, be several plausible explanations for this discordance. Many of the previous large-scale observational studies have attempted to characterize this relationship in healthy, well-functioning older adults with relatively narrow age ranges (27). This may have resulted in an underestimation of any observed associations, as mobility-impaired elders are more likely to have lower muscle mass and reduced muscle strength when compared to elders of high functional status (6, 14, 26). Furthermore, many of the previous studies have also used self-report methods to assess functional status. Incident mobility limitations based on subjective self-report measures have been shown to be influenced by factors such as cognitive status, perceived mastery, and depressive symptoms, which may lead to a potential misclassification bias in these studies (15). In addition, there is also the strong likelihood that the sarcopenia-functional decline relationship is influenced by other inevitable and concurrent age-related changes in body composition and muscle quality, which may include an increase in fat tissue, a loss of bone mass, and alterations in neural activation (26).
The current study confirms that the age-related loss of lean muscle mass and strength are important determinants of mobility-disability in old age. We sought to overcome many of the limitations in previous studies by incorporating a robust, objective and well-validated method to ascertain functional status. After controlling for several other physiological and lifestyle factors associated with decreased function, logistic regression analyses revealed that for every kilogram increase in total lean leg mass, the odds of having severe functional limitations decreased by 53% (Table 4). The relation between muscle mass and mobility-disability was also supported by the generalized additive model analysis, which showed graphically that a SPPB score > 7 was associated with a marked increase in lean leg muscle mass. Given this relationship, together with the finding that muscle mass accounted for over 60% of the total variance in muscle strength in this population of frail elders, it was not surprising that muscle strength was also a significant independent predictor of the severity of mobility-disability in a separate regression model. It is also highly likely that additional indices of muscle quality such as neuromuscular function and skeletal muscle fat infiltration would explain a portion of the total variance in muscle strength and contribute to the severity of mobility-disability in these participants (29). Unfortunately, these additional factors were not assessed in the current study.
The findings described in this study support the view that intervention strategies designed to preserve both skeletal muscle mass and muscle strength should be initiated among older adults. Resistance exercise may be particularly beneficial because it has been shown to attenuate or reverse the age-related decrements in muscle mass and strength (30–33). However, other factors have also been reported to contribute to the age-associated decline in skeletal muscle mass. These include declining levels of steroid hormones, reduced dietary protein intake, and decreased levels of physical activity (14). Additional studies are still necessary in order to develop optimal interventions designed to maintain muscle mass, strength and function among older adults.
Despite the robust association between muscle mass and function, there are limitations associated with this study. The cross-sectional nature of the analysis precludes definitive causal inferences about the relationship between muscle mass, strength and the level of mobility-disability. It is also possible that the decline in functional performance may have preceded the reductions in both muscle mass and strength in this population. Although DXA assessment of muscle mass is reproducible and has been validated against other body composition assessment methodologies, it does not capture important intrinsic changes in muscle quality (e.g. intermuscular fat infiltration) that accompany aging (34).
Conclusions
The findings of this study suggest that preservation of skeletal muscle mass may be an important intervention in the disablement pathway among mobility-impaired older adults. Such findings may have important implications for the design of suitable strategies to maintain functional independence, and indicate that efforts to preserve and increase muscle mass are likely to have a significant effect on preserving strength and function in this population. Additional studies are warranted to assess the efficacy of exercise, lifestyle or pharmacologic interventions for increasing muscle mass in functionally-limited elders. Further prospective research is also needed to delineate the relative contributions of muscle mass and muscle strength, together with the role of other neuromuscular, anthropometric, and lifestyle factors that potentially contribute to the onset and progression of mobility-disability with advancing age.
Acknowledgments
This work was supported by the National Institute on Aging grant number AG18844 and the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. The authors wish to acknowledge the support of the Boston University Medical Center General Clinical Research Center (M01 RR00533). Subjects were recruited from a Volunteer Registry supported by the Hebrew SeniorLife Institute for Aging Research.
References
- 1.Espeland MA, Gill TM, Guralnik J, Miller ME, Fielding R, Newman AB, Pahor M. Designing clinical trials of interventions for mobility disability: results from the lifestyle interventions and independence for elders pilot (LIFE-P) trial. J Gerontol A Biol Sci Med Sci. 2007;62:1237–1243. doi: 10.1093/gerona/62.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasch EK, Hochberg MC, Magder L, Magaziner J, Altman BM. Health of community-dwelling adults with mobility limitations in the United States: prevalent health conditions. Part I. Arch Phys Med Rehabil. 2008;89:210–218. doi: 10.1016/j.apmr.2007.08.146. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Penninx BW, Leveille SG, Corti MC, Pahor M, Wallace R, Harris TB, Havlik RJ, Guralnik JM. Characteristics of nondisabled older persons who perform poorly in objective tests of lower extremity function. J Am Geriatr Soc. 2000;48:1102–1110. doi: 10.1111/j.1532-5415.2000.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 7.Buchman AS, Wilson RS, Boyle PA, Tang Y, Fleischman DA, Bennett DA. Physical activity and leg strength predict decline in mobility performance in older persons. J Am Geriatr Soc. 2007;55:1618–1623. doi: 10.1111/j.1532-5415.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson HD, Nevitt MC, Scott JC, Stone KL, Cummings SR. Smoking, alcohol, and neuromuscular and physical function of older women. Study of Osteoporotic Fractures Research Group. JAMA. 1994;272:1825–1831. doi: 10.1001/jama.1994.03520230035035. [DOI] [PubMed] [Google Scholar]
- 9.Coakley EH, Kawachi I, Manson JE, Speizer FE, Willet WC, Colditz GA. Lower levels of physical functioning are associated with higher body weight among middle-aged and older women. Int J Obes Relat Metab Disord. 1998;22:958–965. doi: 10.1038/sj.ijo.0800698. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989;79:703–708. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. JAMA. 1994;271:1093–1098. [PubMed] [Google Scholar]
- 12.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, Williamson JD, Harris TB. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 16.Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:147–150. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 17.Taaffe DR, Simonsick EM, Visser M, Volpato S, Nevitt MC, Cauley JA, Tylavsky FA, Harris TB. Lower extremity physical performance and hip bone mineral density in elderly black and white men and women: cross-sectional associations in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2003;58:M934–M942. doi: 10.1093/gerona/58.10.m934. [DOI] [PubMed] [Google Scholar]
- 18.Reid KF, Carabello RJ, Phillips EM, Frontera WR, Fielding RA. Lower Extremity Power Training in Elderly Subjects with Mobility Limitations: A Randomized Controlled Trial. Aging Clin Exp Res. 2008 doi: 10.1007/bf03324865. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality in nursing home admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 20.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 21.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Macera CA, Blair SN, Brill PA, Kohl HW, 3rd, Kronenfeld JJ. Physical fitness, physical activity, and functional limitation in adults aged 40 and older. Med Sci Sports Exerc. 1998;30:1430–1435. doi: 10.1097/00005768-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Successful aging: predictors and associated activities. Am J Epidemiol. 1996;144:135–141. doi: 10.1093/oxfordjournals.aje.a008900. [DOI] [PubMed] [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Generalized additive models. New York, NY: Chapman & Hall; 1990. [Google Scholar]
- 26.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 27.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 28.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 30.Evans WJ. Reversing sarcopenia: how weight training can build strength and vitality. Geriatrics. 1996;51:46–47. 51-43; quiz 54. [PubMed] [Google Scholar]
- 31.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 32.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 33.Tseng BS, Marsh DR, Hamilton MT, Booth FW. Strength and aerobic training attenuate muscle wasting and improve resistance to the development of disability with aging. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:113–119. doi: 10.1093/gerona/50a.special_issue.113. [DOI] [PubMed] [Google Scholar]
- 34.Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Skeletal muscle mass, fat mass, and hip bone mineral density in elderly women with hip fracture. J Bone Miner Metab. 2007;25:237–242. doi: 10.1007/s00774-007-0752-1. [DOI] [PubMed] [Google Scholar]