Abstract
Salvinorin A (1) is a hallucinogenic neoclerodane diterpene isolated from the widely available psychoactive plant Salvia divinorum and is the first example of a non-nitrogenous opioid receptor ligand. At present, there is little information available as to why this compound is selective for κ opioid receptors. One approach to better understanding the mode of binding of 1 at κ receptors is to systematically alter the structure of 1 and examine the effects on opioid receptor affinity and activity. Currently, there is a paucity of methods described for the preparation of analogues derived from 1. Here, we report the investigation of several chemical transformations of 1 isolated from S. divinorum. In particular, this work provides a semisynthesis of salvinicins A (2) and B (3) and has identified 10a as the first neoclerodane diterpene with δ opioid antagonist activity.
Keywords: salvinorin A, salvinicin A, salvinicin B, neoclerodane diterpenes, and Salvia divinorum
Extracts of the opium poppy, Papaver somniferum, have been used for centuries to relieve pain and to induce sleep.1 Among the most important constituents in opium are the alkaloids morphine and codeine. Many of the agonists and antagonists derived from these alkaloids are essential for the practice of modern medicine. While many potent agonists are effective analgesics, they have undesirable side effects, such as tolerance, dependence, and respiratory depression.2
Recently, opioid receptors have been implicated in the actions of salvinorin A (1), the major active ingredient of Salvia divinorum Epling & Játiva (Lamiaceae), a hallucinogenic plant that has been used historically in the traditional practices of the Mazatecs in Oaxaca, Mexico.3–6 This finding is unique because 1 represents the only known lipid-like small molecule that selectively and potently activates a peptidergic G-protein coupled receptor (GPCR).7, 8 Diterpene 1 was found to be a high efficacy agonist for κ opioid receptors (κOR).6, 9 Thus, 1 provides a truly unique template for the development of novel agents to attenuate pain with a potential for reduced abuse liability.
Recently, we10, 11 and others12–15 have described the isolation and synthesis of several novel neoclerodane diterpenes with opioid receptor activity. Among these compounds were salvinicin A (2) and salvinicin B (3).11 Diterpene 2 was identified as a partial κOR agonist, whereas, 3 was found to be the first neoclerodane diterpene with μ antagonist activity. There is a growing body of information as to why 1 and related analogues have activity at opioid receptors.10, 11, 13–17 However, there are few synthetic methods described for related analogues of 1. Based on previous reports,10, 11, 13,14 the C-2 position and furan ring appear to be important sites for the opioid activity of 1. Thus, we set out to develop synthetic transformations of 1 to selectively modify these sites. Here we describe a concise synthesis of 2 and 3 from 1, as well as, several selective synthetic transformations of 1.
Results and Discussion
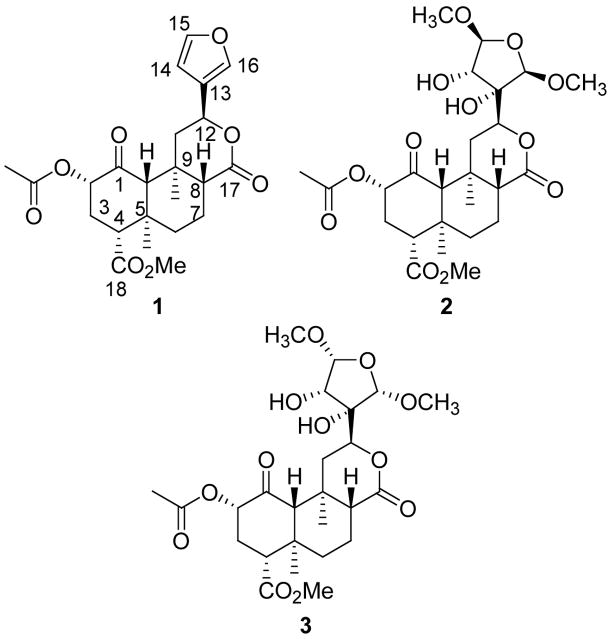
Salvinorin A (1) was isolated from S. divinorum as described previously.18 With 1 in hand, our attention focused on the preparation of salvinicins A (2) and B (3). These compounds were needed on larger scale to further assess their biological activity in vivo. The reaction of 1 with bromine in a mixture of CH2Cl2 and MeOH at −30 °C gave 2,5-dimethoxydihydrofuran 4 in 93% yield presumably as a mixture of cis and trans isomers (Scheme 1).19, 20 The selective oxidation of the cis vs. trans isomers of 4 with KMnO4 in a mixture of THF and H2O at −10 °C afforded a mixture of salvinicin A (2) and salvinicin B (3).21 These compounds were readily separated from the unreacted trans isomers of 4, and each other, by flash chromatography using a mixture of EtOAc/hexanes. Interestingly, 3 was preferentially formed over 2 in an approximate 3:2 ratio, whereas, in the naturally occurring plant, 2 was isolated in higher concentrations than 3.11
Scheme 1.
(i) Br2, MeOH, CH2CI2, -30 °C; (ii) KMnO4, THF/H2O, -10 °C; (iii) RuCI3, NalO4, CCI4/CH3CN/H2O; (iv) NBS, CH3CN; (v) Br2, DMF
Having successfully synthesized 2 and 3, we sought to further probe the chemistry associated with neoclerodane diterpenes related to 1. The reaction of 1 with NaIO4 and a catalytic amount of RuCl3•3H2O in a mixture of CCl4, acetonitrile, and H2O afforded acid 5 in 74% yield.22 This transformation allows for the selective removal of the furan ring.
The treatment of 1 with N-bromosuccinimide in acetonitrile at room temperature afforded bromofuran 6a in 38% yield. In an HMBC experiment, H-12 showed correlations to non-protonated carbons at δ 121.3 and δ 122.7, providing evidence for placement of the bromo group at C-16 as proposed in Scheme 1. Longer reaction times led to decomposition of 1.
Interestingly, when 1 was reacted with bromine in DMF at 0 °C 6a was not formed. Rather, dihydrofuran 6b was formed as a mixture of C-15 α and β isomers. Isomers were readily separated by flash chromatography followed by recrystallization. In attempts to separate the C-15 epimers by column chromatography followed by recrystallization, it was observed that one epimer (the C-15 α epimer) rapidly re-converted to a mixture of C-15 α and β epimers in solution. The structure proposed is based on spectral analysis of the purified epimer (the C-15 β epimer), 6b which was the more stable of the two isomers in solution.
The structure of 6b was elucidated on the basis of mass spectral and spectroscopic techniques. The HRESIMS of 6b (m/z 527.0918. [M+H]+) indicated a molecular formula of C23H27O9Br requiring ten double bond equivalents. The 1H NMR spectrum displayed methyl singlets typical of the salvinorin A core: δ 1.11 (H-19), 1.44 (H-20), 2.19 (-CH3CO) and 3.73 (-CO2CH3). In addition, typical signals for H-2 (δ 5.13, dd, J = 8.0, 12.0 Hz), H-4 (δ 2.74, dd, J = 4.6, 12.2 Hz) and H-11β (δ 2.52, dd, J = 5.8, 13.3 Hz) were observed. As compared to 1, the aromatic region of the spectrum showed significant differences. Only two protons signals were observed which was suggestive of substitution on the furan ring. A COSY spectrum indicated that these two protons were vicinal (δ 6.86 and 7.46, Jvic = 1.4 Hz). Inspection of the 13C NMR spectrum in conjunction with HMBC and HSQC analysis indicated that the furan ring was no longer present. However, signals appeared which were characteristic for an α, β-unsaturated γ-lactone substructure (δ 167.7, 149.7, 132.2). The relative downfield shift observed for H-14 (δH 7.46) indicated that this proton was β to the lactone carbonyl group at δC 167.7. The stereochemical assignment of the C-15 bromo group as β came from a ROESY experiment in which cross-peaks were observed from H-15 to H-12. Thus, the structure of 6b is proposed as depicted in Scheme 1.
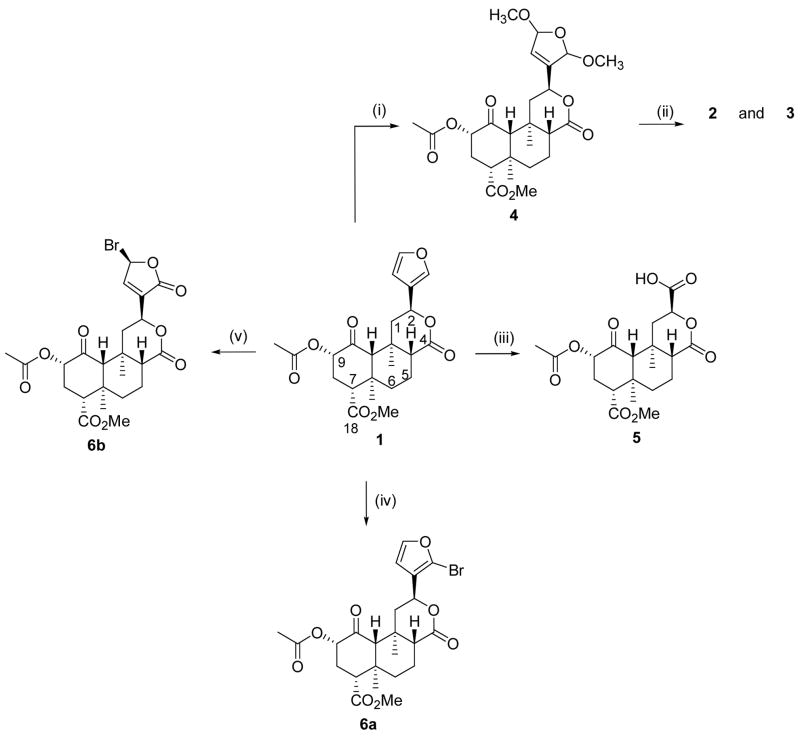
Our additional synthetic efforts are described in Scheme 2. The reaction of salvinorin B (7) (prepared from 1 using previously described methodology)18 with 1,1-thiocarbonyldiimidazole (CDI) and a catalytic amount of DMAP afforded imidazole 8 in 63% yield. The treatment of imidazole 8 with 2,2′-azobis(2-methylpropionitrile) (AIBN) and tributyltin hydride in toluene afforded an epimeric mixture of 9a and 9b in 13 and 28% yield, respectively. Other attempts to remove the 2-acetoxy group in 1 using either samarium iodide23 or Zn and acetic acid were unsuccessful.24 To circumvent this problem, we sought to prepare the C-2 epimer of 1. It was envisioned that perhaps the (β-acetoxy group might be more readily cleaved using these conditions given the structural similarities to the diterpene taxol.23 Thus, the reaction of 7 with acetic acid under modified Mitsunobu conditions25 afforded acetate 10a in 80% yield. To confirm the inversion of stereochemistry at C-2, the 1H NMR spectrum of 10a was compared to 1. The change in chemical shift of H-2 for 10a versus 1 (δ 4.85 vs. 5.14) and the coupling constants observed (dd, J = 3.2, 3.2 Hz vs. dd, J = 10.0, 10.0 Hz) indicated that epimerization had occurred at C-2. Based on previous work,11, 26 the absolute stereochemistry of 10a is as depicted in Scheme 2. Using similar methodology benzoate 10b was also prepared in good yield. However, the reaction of 10a with either samarium iodide or Zn and acetic acid in attempts to prepare 9b were also unsuccessful.
Scheme 2.
(i) (lm)2CS, DMAP, CH2CI2; (ii) AIBN, Bu3SnH, Toluene; (iii) Appropriate acid, (2-Py)PPh2, DBAD, THF; (iv) CrO3, Pyridine
We next focused on oxidizing 7 to the corresponding diketone using a mixture of chromium trioxide and pyridine in CH2Cl2. To our surprise, the corresponding diketone was not formed. Instead, dilactone 11 was formed in 37% yield. In the 1H NMR spectrum, signals characteristic of the salvinorin core were observed. Thus, signals at δH 7.43, 7.41 and 6.39 were ascribed to the furan ring. In addition, signals for H-8 (2.15, dd, J = 1.6, 6.1 Hz), H-10 (2.40, s), H-12 (5.59, dd, J = 2.7, 5.7 Hz), two saturated methyl groups (δH 1.33 and 1.45) and the methyl ester group (δH 3.76) were also observed. Based on this data it was inferred that rings B, C and the furan ring had remained intact during the oxidation. Unexpectedly however, a signal at δ 7.32 (1H, s) was present in the spectrum.
In the 13C spectrum, signals were seen for the furan ring (δ 108.5, 125.5, 139.6, 144.0), the ring C lactone carbonyl group (δ 170.8) and the C-18 ester group (δ 164.5). Additionally, signals at δ 123.6 and 148.1 indicative of a double bond were also observed. A signal characteristic of an ester carbonyl group was also observed at δ 166.4. This signal showed HMBC correlations to H-10 and to the proton at δ 7.32. Signals at δC 164.5 and 123.6 also showed HMBC correlations to the proton at δH 7.32. Further HMBC analysis allowed for assignment of the proposed structure. An alternate structure with the ester group at δC 166.4 adjacent to the carbon at δC 148.1 was also considered on the basis of the HMBC data, but this was ruled out on the basis of the chemical shifts observed particularly for H-10. Thus, the structure of 11 is proposed as depicted in Scheme 2.
Biological Results
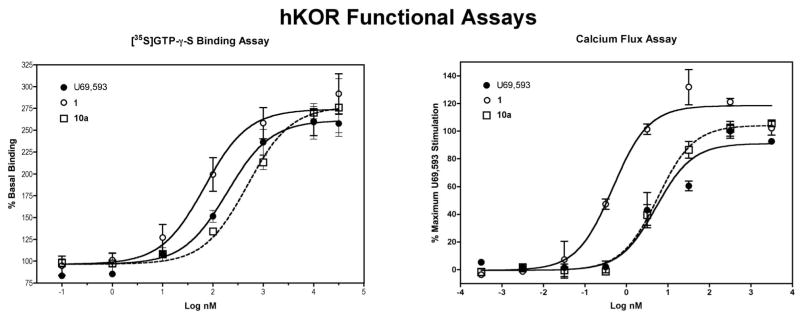
The activity of 1 and 10a at the hκOR was determined using the [35S]GTP-γ-S binding assay in CHO membrane preparations stably expressing the hκOR and these data were compared to those obtained using the Calcium 3 dye homogeneous assay that detects the release of internal calcium stores. For the calcium flux assay, CHO cells stably expressing the promiscuous Gq protein Gα16 were stably transfected with a mammalian vector containing the hκOR resulting in the Gi-coupled hκOR being coupled to the mobilization of internal calcium. In keeping with early results,10 1 was a full agonist at the hκOR and more potent than the κ opioid full agonist U69,593 in the [35S]GTP-γ-S binding assay. Compound 10a was also a full agonist, but with a potency similar to U69,593. The results in the whole cell calcium flux assay paralleled those of the binding assay except that the EC50 values were over 10-fold lower for all three compounds. This probably reflects a higher degree of receptor reserve in the Gα16-hκOR cells (Table 1, Figure 1). The ratios of the EC50 values for 1 and 10a to the U69,593 EC50 were essentially the same in both assays indicating that the nonradioactive calcium flux assay is a viable method for determining the activity of a compound at the hκOR. Of interest is that 1 produced a slight, but significantly higher stimulation of calcium flux than either 10a or U69,593. This change is similar to what has been observed from electrophysiological studies on the activation of hκOR expressed in Xenopus oocytes,8 but significantly higher stimulation of calcium flux than either 10a or U69,593. It is possible that 1 is recognizing conformations of the hκOR not bound by either 10a or U69,593 and not present in appreciable amounts in the CHO cell membranes. Additional experiments are needed to determine if this is the case. The selectivity of 1 and 10a for the hκOR was determined by measuring the ability of test compounds to inhibit stimulated [35S]GTP-γ-S binding produced by the selective agonists DAMGO [(D-Ala2,MePhe4,Gly-ol5)enkephalin] (selective for μ opioid receptor) and DPDPE [cyclo[D-Pen2, D-Pen5]enkephalin] (selective for δ opioid receptor) using membranes prepared from CHO cells expressing the hμOR or hδOR. In keeping with early work,61 was highly selective for the hκOR. In contrast, 10a was found to have antagonist activity at μ (Ke/EC50 = 9.5) and δ (Ke/EC50 = 2.5) opioid receptors. This describes the first neoclerodane diterpene (10a) with δ antagonist activity.
Table 1.
Activity of Compounds at Human Opioid Receptors.
| hκOR [35S]GTP-γ-S | Gα16-hκOR Calcium Flux | Cmpd/U69,593 EC50 Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Cmpd | EC50 (nM)1 | Emax (%)2 | EC50 (nM)3 | Emax (%)4 | [35S]GTP-γ-S | Calcium Flux | hμOR Ks (nM) | hδOR Ke (nM) |
| U69,593 | 207 ± 34a,c | 100±15 | 6.6 ± 0.9a | 100 ± 5a | ||||
| 10a | 385 ± 74a | 104 ± 6 | 6.5 ± 1.9a | 111+2a,b | 1.9 | 1.0 | 3650 ± 1970 | 964 ± 220 |
| 1 | 45 ± 12b,c | 108±4 | 0.81±0.38b | 120 ± 5b | 0.2 | 0.1 | >5000 | >5000 |
The data represent the mean ± SEM from 3–4 independent experiments for the hκ OR data and 2 experiments for the μ and δ receptors. The hκOR data in each column were compared by one-way ANOVA and Tukey’s post hoc test performed only if the ANOVA was significant. Data with different letter superscripts are different from each other.
(F(2,8)= 9.99, P<0.01).
(F(2,8) = 0.137, P=NS).
(F(2,6) = 7.09, P<0.05).
(F(2,6) = 5.63, P<0.05)
Figure 1.
Representative data from the [35S]GTP-γ-S binding and Calcium 3 dye assays. All the compounds displayed greater potency in the calcium flux assay suggesting greater receptor/effector coupling in the Gα16-hκOR CHO cells. Both 1 and 10a were full agonists in the [35S]GTP-γ-S binding assay and 1, but not 10a, elicited greater calcium stimulation than U69,593 in the calcium flux assay.
In conclusion, we have developed a practical method for the synthesis of salvinicins A (2) and B (3) from 1 isolated from S. divinorum. In addition, we report useful methodology for the functionalization of 1. We have shown that reduction of the furan to a dihydrofuran is feasible. The furan ring may be selectively removed or halogenated. A three step procedure for removal of the acetoxy group has been developed and inversion of stereochemistry at C-2 has been accomplished. These methods provide access to the compounds described, as well as to related analogues which may be used to further study the structure-activity relationships of neoclerodane diterpenes at opioid receptors. Finally, we have identified 10a as the first neoclerodane diterpene with δ antagonist activity.
Experimental Section
General Experimental Procedures
Unless otherwise indicated, all reagents were purchased from commercial suppliers and were used without further purification. All melting points were determined on a Thomas – Hoover capillary melting apparatus and are uncorrected. The 1H NMR and 13C NMR spectra were recorded at 300 MHz on a Bruker Avance-300 spectrometer or on a Bruker AMX-600 spectrometer using CDC13 as solvent, δ values in ppm (TMS as internal standard), and J (Hz) assignments of 1H resonance coupling. HMBC and HMQC data were collected on the AMX-600 spectrometer. Thin-layer chromatography (TLC) was performed on 0.25 mm Analtech GHLF silica gel plates. Spots on TLC were visualized with vanillin/H2SO4 in EtOH. Column chromatography was performed with Silica Gel (32–63 μ particle size) from Bodman Industries (Atlanta, GA). Elemental analyses were performed by Atlantic Microlabs, Norcross, GA. DAMGO, DPDPE, and U69,593 were obtained via the Research Technology Branch, NIDA, and were prepared by Multiple Peptide Systems (San Diego, CA). [35S]GTP-γ-S was obtained from Perkin-Elmer Inc., (Boston, MA) and GTP-γ-S and GDP were obtained from Sigma Chemical Company (St. Louis, MO). The systematic name for salvinorin A (1) is (2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-2-(furan-3-yl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester.
Plant Material
Dried S. divinorum leaves, were purchased in May 2004 from Ethnogens.com (Berkeley, CA). Voucher specimens were deposited at the Ada Hayden Herbarium, Iowa State University, Ames, Iowa, Voucher # 437081.
Isolation of 1
Dried S. divinorum leaves (1.5 kg), obtained commercially from ethnogens.com, were ground to a fine powder and percolated with acetone. The acetone extract was concentrated under reduced pressure to afford a crude green gum, which was subjected to repeated column chromatography on silica gel with elution using a mixture of EtOAc/hexanes to afford 1 (TLC) and other minor diterpenes. The melting point, 1H NMR and 13C spectra of 1 were in agreement with previously reported data.3, 27
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-2-(2,5-dimethoxy-2,5-dihydrofuran-3-yl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (4)
A solution of bromine (0.075 mL, 1.46 mmol) in MeOH (1 mL) was added in a drop wise manner to a solution of 1 (0.25 g, 0.59 mmol) in a mixture of CH2C12 (50 mL) and MeOH (2 mL) at −30 °C. The mixture was allowed to stir at −30 °C for 1h and was quenched by the addition of saturated NaHCO3 (100 mL). The layers were separated and the organic layer was collected, washed with saturated NaHCO3 (50 mL) and H2O (70 mL), and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a white foam. The foam was purified by flash column chromatography (eluent: EtOAc/hexanes, 60%) to give 0.27 g (93%) of 4 as a colorless oil: 1H NMR (CDCl3): δ 1.10 (3H, s); 1.40 (3H, s); 1.56 (2H, m); 1.68 (1H, dd, J= 2.7, 12.6); 1.78 (1H, dd, J = 2.7, 9.9); 2.03 (1H, dd, J = 2.7, 11.4); 2.13 (1H, m); 2.17 (3H, s); 2.17 (1H, s); 2.30 (2H, m); 2.47 (1H, m); 2.75 (1H, dd, J= 5.6 10.9); 3.39 (3H, dd, J = 1.4, 2.0); 3.44 (3H, dd, m); 3.73 (3H, s); 5.14 (2H, m); 5.59 (1H, m); 5.85 (2H, m); HRESIMS m/z [M + Na]+551.2080, (calcd for C25H34O12Na, 551.2104).
Preparation of Salvinicin A (2) and Salvinicin B (3) from 4
To a solution of 4 (0.27 g, 0.54 mmol) in THF (30 mL) at −10 °C under nitrogen was added with vigorous stirring KMnO4 (0.09 g, 0.54 mmol) in H2O (10 mL). The resulting mixture was stirred at −10 °C for 30 min and was then allowed to warm to room temperature and stirred for a further 16 h. The mixture was filtered and the residue was washed with THF (2 × 30 mL). Solvent was removed under reduced pressure affording a crude oil. The crude oil was dissolved in EtOAc (50 mL) and the solution was washed with H2O (70 mL) and dried (Na2SO4). Removal of solvent under reduced pressure afforded a crude mixture. The mixture was subjected to flash chromatography eluting with 50% – 70% EtOAc/hexanes to afford 2 (0.03 g, 17%), 3 (0.04 g, 27%) and trans-4 (0.11 g). Compounds 2 and 3 showed [α]D, IR, 1H NMR, 13C NMR and MS identical with those previously reported for the natural diterpenoids previously found in S. divinorum.11
(2S ,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-2,7-dicarboxylic acid 7-methyl ester (5)
A solution of 1 (0.10 g, 0.23 mmol), in CCl4/CH3CN/H2O (2:2:3, 7 mL) was stirred at room temperature. To the solution was added NaIO4 (0.75 g, mmol) followed by a catalytic amount of RuCl3•3H2O. The mixture was stirred vigorously at room temperature for 1 h and then filtered through a pad of celite. The celite pad was washed with EtOAc (50 mL) and the organic layer was collected. The organic layer was washed with saturated NaHCO3 (30 mL) and the aqueous layer was collected. The aqueous layer was then acidified with 2 N HCl (50 mL) and extracted with EtOAc (50 mL). The organic extract was washed with H2O (2 × 30 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure to yield 0.70 g (74%) of 5 as a white solid, mp 144–146.5 °C : 1H NMR (CDC13): δ 1.07 (3H, s); 1.37 (3H, s); 1.62 (4H, m); 2.13 (2H, m); 2.19 (3H, s); 2.30 (2H, m); 2.38 (1H, s); 2.59 (1H, dd, J= 6.6, 13.5); 2.85 (1H, dd, J= 6.0, 11.4); 3.72 (3H, s); 5.01 (1H, dd, J = 6.9, 10.2); 5.28 (1H, dd, J = 9.3, 10.8); 5.78 (1H, br.s); 13C NMR (CDCl3): δ 15.9, 16.5, 18.3, 20.9, 30.8, 35.4, 37.8, 38.8, 42.2, 50.1, 52.2, 53.2, 63.7, 73.6, 75.7, 170.8, 171.1, 171.9, 172.9, 202.3 ; HRESIMS m/z [M + H]+ 411.1672, (calcd for C20H27O9, 411.1655).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-2-(2-bromofuran-3-yl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (6a)
A solution of 1 (0.10 g, 0.23 mmol) and N-Bromosuccinimide (0.46 g, 0.25 mmol) in acetonitrile (40 mL) was stirred at room temperature for 2.5 h. Solid Na2CO3 (0.30 g) was added and the mixture stirred at room temperature for 5 minutes. The reaction mixture was filtered to remove solid Na2CO3 and concentrated under reduced pressure. The crude product was purified by flash column chromatography (eluent: hexanes/EtOAc, 2:3) in 40% EtOAc/hexanes to yield 0.45 g (38%) of 6a as a white solid, mp 171–174 °C: 1H NMR (CDCl3): δ 1.14 (3H, s); 1.49 (3H, s); 1.62 (3H, m); 1.82 (1H, dd, J = 3.5, 10.6); 2.17 (3H, s); 2.18 (2H, m); 2.31 (3H, m); 2.41 (1H, dd, J= 5.1, 13.5); 2.78 (1H, dd, J= 8.4, 8.4); 3.75 (3H, s); 5.14 (1H, dd, J= 9.6, 10.5); 5.45 (1H, dd, J= 5.4, 12.0); 6.40 (1H, d, J= 2.1); 7.44 (1H, d, J= 2.4); 13C NMR (CDCl3): δ 15.0, 16.4, 18.1, 20.5, 30.7, 35.5, 38.2, 42.2, 42.8, 51.6, 52.0, 53.6, 63.9, 71.9, 75.0, 110.75, 121.3, 122.7, 144.6, 170.0, 170.9, 171.5, 201.8; HRESIMS m/z [M + H]+ 511.0947, (calcd for C23H28O8Br, 511.0968).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-2-(5-bromo-2-oxo-2,5-dihydrofuran-3-yl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (6b)
A solution of 1 (0.10 g, 0.23 mmol) in DMF (40 mL) was cooled to 0 °C in an ice bath. A solution of Br2 in DMF was prepared by dissolving 1 mL of Br2 in 9 mL of DMF. A 0.1 mL portion of this solution was added over 10 min. to the stirring solution of 1 and stirred for 20 minutes. An additional 0.05 mL of the Br2 solution was added over 2 minutes and the reaction was allowed to warm to room temperature and stirred for 1 hour. The mixture was diluted with H2O and extracted with Et2O (2 × 20 mL). The combined organic extracts were then dried (Na2SO4), filtered, and concentrated under reduced pressure to yield a crude oil. The oil was purified by flash column chromatography (eluent: EtOAc/hexanes, 1:1) to gave 0.042 g (34.3%) of 6b as a white solid, mp 128–130.5 °C (EtOAc/hexanes): 1H NMR (CDCl3): δ 1.11 (3H, s); 1.44 (3H, s); 1.57 (1H, s); 1.64 (3H, m); 1.80 (1H, m); 2.17–2.20 (2H, m); 2.19 (3H, s); 2.31 (2H, m); 2.52 (1H, dd, J = 5.8, 13.3); 2.74 (1H, dd, J = 4.6, 12.2); 3.73 (3H, s); 5.13 (1H, dd, J= 8.0, 12.0); 5.41 (1H, dd, J = 5.7, 11.7); 6.86 (1H, dd, J= 1.4, 3.9); 7.46 (1H, dt, J = 1.4, 5.1); 13C NMR (CDCl3): δ 14.0, 15.9, 18.4, 20.6., 31.1, 36.0, 38.0, 40.1, 41.7, 51.6, 52.7, 53.3, 64.5, 72.0, 74.0, 75.2, 132.2, 149.7, 167.7, 170.1, 170.5, 171.9, 201.0; HRESIMS m/z [M + Na]+549.0708, (calcd for C23H27O9BrNa, 549.0736).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Imidazole-1-carbothioyloxy)-dodecahydro-6a,10b-dimethy1-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (8)
A solution of 7 (0.14 g, 0.36 mmol), 1,1′-thiocarbonyldiimidazole (0.19 g, 1.07 mmol), and a catalytic amount of DMAP in CH2Cl2 (30 mL) was stirred at room temperature for 3 h. The solvent was removed under reduced pressure to yield a crude solid. The solid was purified by flash column chromatography (eluent: EtOAc/hexanes, from 50 – 65% EtOAc) to gave 0.12 g (64%) of 8 as a white solid, mp 207–208 °C: 1H NMR (CDCl3): δ 1.18 (3H, s); 1.48 (3H, s); 1.66 (3H, m); 1.87 (1H, dd, J= 2.5, 10.0); 2.18 (2H, m); 2.30 (1H, s); 2.53 (3H, m); 2.87 (1H, dd, J= 3.3, 12.3); 3.76 (3H, s); 5.50 (1H, dd, J = 4.3, 10.8); 5.88 (1H, dd, J= 7.2, 10.8); 6.39 (1H, dd, J= 0.9, 1.8); 7.08 (1H, dd, J= 0.6, 1.8); 7.41 (1H, br. d, J= 1.5); 7.44 (1H, m); 7.67 (1H, t, J = 1.2); 8.39 (1H, s); anal. C 59.92%, H 5.67%, O 22.25%, calcd for C25H28N2O7S, C 59.99%, H 5.64%, O 22.37%.
Preparation of 9a and 9b from 8
A solution of 8 (0.30 g, 0.60 mmol), AIBN (0.02 g, 0.12 mmol), Bu3SnH (0.52 g, 1.78 mmol), in toluene (30 mL) was heated at reflux overnight. The reaction mixture was concentrated under reduced pressure to yield a crude solid. The solid was purified by flash column chromatography (eluent: EtOAc/hexanes, 10:3) to give 0.03 g (13%) of 9a as a white solid and 0.09 g (28%) of 9b as a white solid.
(2S,4aR,6aR,7R,9S,10aS,10bR)-Dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]-pyran-7-carboxylic acid methyl ester (9a)
mp 224–226 °C (EtOAc/hexanes); 1H NMR (CDCl3): δ 1.13 (3H, s); 1.41 (3H, s); 1.52 (2H, m); 1.69 (2H, m); 2.03 (2H, m); 2.14 (1H, s); 2.27 (3H, m); 2.47 (1H,m);2.68 (2H, m); 3.71 (3H, s); 5.54 (1H, dd, J = 4.8, 11.7); 6.39 (1H, dd, J= 0.6, 1.5); 7.40 (1H, dd, J = 1.5, 1.8); 7.42 (1H, dd, J = 0.6, 1.8); 13C NMR (CDCl3): δ 15.5, 17.7, 24.6, 25.2, 34.0, 34.7, 41.7, 41.9, 45.7, 48.8, 51.7, 55.0, 66.3, 70.3, 108.7, 123.9, 139.8, 143.7, 173.5, 174.0, 208.7; HRESIMS m/z [M + H]+ 375.1805, (calcd for C21H27O6, 375.1808).
(2S,4aS,6aR,7R,9S,10aS,10bR)-Dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]-pyran-7-carboxylic acid methyl ester (9b)
mp 158–160 °C (EtOAc/hexanes); 1H NMR (CDCl3): δ 1.10 (3H, s); 1.44 (2H, m); 1.58 (3H, s); 1.84 (1H, m); 1.98 (2H, m); 2.15 (1H, m); 2.19 (1H, s); 2.27 (2H, m); 2.44 (3H, m); 2.67 (1H, dd, J = 3.3, 12.6); 3.68 (3H, s); 5.27 (1H, dd, J = 1.8, 12.0); 6.38 (1H, dd, J = 0.6, 1.8); 7.39 (1H, dd, J = 1.5, 1.8); 7.44 (1H, dd, J = 0.6, 1.5); 13C NMR (CDCl3): δ 15.2. 16.6, 18.3, 25.3, 35.5, 38.3, 41.6, 41.8, 43.9, 51.7, 51.9, 55.9, 66.3, 72.2, 108.5, 125.8, 139.4, 143.8, 171.7, 173.2, 208.6; HRESIMS m/z [M + H]+375.1760, (calcd for C21H27O6, 375.1808).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetoxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (10a)
A mixture of 7 (0.20 g, 0.51 mmol), diphenyl-2-pyridylphosphine (0.20 g, 0.77 mmol) and acetic acid (0.12 mL, 2.05 mmol) was dissolved in anhydrous THF (15 mL) under an argon atmosphere. To this solution was added di-tert-butylazodicarboxylate (DEAD) (0.18 g, 0.77 mmol) in one portion and the mixture was stirred for 18 hours at 60 °C. The solvent was removed under reduced pressure and the residue was dissolved in dichloromethane (25 mL). The CH2Cl2 portion was washed with 4 M HCl (2 × 25 mL), saturated NaCl (25 mL), and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a crude residue. Flash column chromatography (eluent: EtOAc/hexanes, 3:7) gave 0.18 g (80%) of 10a as a white crystalline solid, mp 215–216 °C; 1H NMR (CDCl3): δ 1.10 (3H, s); 1.28 (1H, dd, J= 6.0, 14.1); 1.45 (1H, m); 1.46 (3H, s); 1.64 (2H, m); 1.80 (1H, dd, J= 3.0, 9.9); 2.10 (1H, m); 2.13 (3H, s); 2.18 (1H, ddd, J = 3.3, 3.3, 15.0); 2.34 (1H, ddd, J = 3.3, 13.0, 15.6); 2.45 (1H, dd, J = 5.1, 13.5); 2.54 (1H, s); 2.90 (1H, dd, J= 3.6, 13.2); 3.71 (3H, s); 4.85 (1H, dd, J= 3.2, 3.2); 5.54 (1H, dd, J= 5.1, 11.7); 6.38 (1H, dd, J= 1.4, 1.7); 7.40 (2H, m); 13C NMR (CDCl3): δ 15.4, 16.3, 18.3, 21.2, 31.1, 35.2, 38.8, 43.2, 43.6, 51.1, 51.5, 52.1, 61.9, 72.3, 76.6, 108.5, 125.7, 139.4, 143.9, 169.6, 171.4, 172.4, 204.6; anal. C 63.63%, H 6.59%, O 29.70%, calcd for C23H28O8, C 63.88%, H 6.53%, O 29.60%.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Benzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (10b)
10b was synthesized as described for 10a from 7 using benzoic acid to afford 0.19 g (75 %) of 10b as a white crystalline solid, mp 223–225 °C; 1H NMR (CDCl3): δ 1.13 (3H, s); 1.40 (1H, dd, J = 11.6, 13.1); 1.50 (3H, s); 1.64 (2H, m); 1.84 (1H, dd, J= 2.9, 11.3); 2.10 (2H, m); 2.37 (1H, ddd, J= 3.0, 4.2, 12.3); 2.45 (1H, m); 2.50 (1H, m); 2.62 (1H, s); 3.01 (1H, dd, J= 4.4, 12.5); 3.73 (3H, s); 5.00 (1H, dd, J= 3.0, 3.0); 5.52 (1H, dd, J = 4.7, 11.4); 6.32 (1H, dd, J = 0.8, 2.0); 7.32 (1H, m); 7.36 (1H, dd, J = 1.8, 1.8); 7.50 (2H, m); 7.65 (1H, tt, J = 1.6, 7.5); 8.03 (2H, m); 13C NMR (CDCl3): δ 15.6, 16.2, 18.3, 31.3, 35.2, 38.9, 43.4, 43.6, 51.4, 51.4, 52.1, 61.8, 72.2, 77.5, 108.5, 125.7, 129.0, 129.0, 129.9, 134.1, 139.3, 143.8, 165.4, 171.4, 172.2, 204.8; anal. C 67.85%, H 6.14%, O 25.70%, calcd for C28H3oO8, C 68.00%, H 6.11%, O 25.88%.
(4aR,6aR,9S,10aS,10bR)-9-(3-Furyl)-4a,10a-dimethyl-1,7-dioxo-5,6,6a,7,9,10,10a,10b-octahydro-1H,4aH-pyrano[3,4,f]isochromene-4-carboxylic acid methyl ester (11)
A solution of chromium trioxide (0.15 g, 1.53 mmol) and pyridine (0.25 mL) in CH2Cl2 (30 mL) was stirred at room temperature for 15 minutes. A solution of 7 (0.10 g, 0.26 mmol) in CH2Cl2 (10 mL) was added in one portion and stirred at room temperature for 48 h. A second portion of CrO3 (0.30 g, mmol) and pyridine (0.5 mL), prepared as before, was then added to the mixture and stirred at room temperature for 16 hours and filtered. The filtrate was washed with 2N NaOH (20 mL), 2 N HCl (30 mL) and water (30 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure to yield a brown solid. The solid was purified by flash column chromatography (eluent: EtOAc/hexanes, 3:10) to give 0.03 g (37%) of 11 as a white solid, mp 271–273 °C (EtOAc/hexanes): 1H NMR (CDCl3): δ 1.33 (3H, s); 1.45 (3H, s); 1.56 (1H, s); 1.69 (2H, m); 2.15 (1H, dd, J= 1.6, 6.1); 2.20 (2H, m); 2.43 (1H, s); 2.72 (1H, dt, J = 3.0, 13.8); 2.93 (1H, dd, J= 5.4, 13.5); 3.76 (3H, s); 5.59 (1H, dd, J= 2.7, 5.7); 6.39 (1H, dd, J= 0.6, 1.8); 7.32 (1H, s); 7.41 (1H, dd, J = 1.5, 1.8); 7.43 (1H, dd, J= 0.6, 1.5); 13C NMR (CDCl3): δ 14.7, 18.1, 21.2, 34.2, 35.7, 37.2, 43.8, 51.3, 52.0, 56.3, 72.0, 108.5, 123.6, 125.5, 139.6, 144.0, 148.1, 164.5, 166.4, 170.8; HRESIMS m/z [M + H]+ 375.1455, (calcd for C20H23O7, 375.1444).
Intrinsic activity at human κ opioid receptors
Test compounds were assayed for their ability to stimulate [35S]GTP-γ-S binding in CHO cell membrane homogenates expressing the human κOR, or to stimulate the mobilization of internal calcium via κ opioid receptors coupled to the promiscuous Gq protein Gα16 expressed in CHO cells (Molecular Devices Corporation, Sunnyvale, CA) (see below). For [35S]GTP-γ-S binding, 7 to 8 different concentrations of test compound were assayed in triplicate in 1.4 mL polypropylene tubes (Marix Technologies, Hudson, NH) in 96-well format. The subtype selective agonists (D-Ala2,MePhe4,Gly-ol5)enkephalin (DAMGO, μOR), (D-Pen2,D-Pen5)enkephalin (DPDPE, δOR) or U69,593 (κOR) were run as positive controls as appropriate. The membranes were incubated with positive control or test compound, 0.1 nM [35S]GTP-γ-S and 1 μM GDP in 50 mM HEPES buffer (pH 7.4) at room temperature for one hour, after which bound radioligand was separated from free via rapid vacuum filtration over GF-B filters with a Brandel Scientific (Gaithersburg, MD) 96-well harvester. Bound radioactivity was determined using a TopCount 12-detector instrument (Packard Instruments) using standard scintillation counting techniques. The data were normalized to samples containing vehicle (basal binding).
Apparent affinity (Ke) at human μ and δ opioid receptors
The ability of a single concentration of test compound to shift the agonist dose response curve to the right was used to determine its Ke. Assay conditions were identical to that for the determination of intrinsic activity except that the final GDP concentration was 10 μM. The EC50s were calculated from a three-parameter logistic curve fit to the data with Prism (version 4.0, GraphPad Software, Inc., San Diego, CA). Agonist dose response curves were run in the presence or absence of a single concentration of test compound. The Ke values were calculated using the formula: Ke = [L]/[(A′/A)-1)], where [L] is the concentration of antagonist and A′ and A the agonist EC50 value in the presence or absence of antagonist, respectively.
Calcium flux assay
κOR activation was also assayed in CHO cells stably expressing Gα16 (Molecular Devices, Sunnyvale, CA) and the hκOR (University of Missouri cDNA Resource Center). The Calcium 3 dye assay (Molecular Devices) was used and carried out according to manufacturer’s specifications. Briefly, the cells were plated in HAM’s F-12 medium with 10% FBS, 200 μg/ml hygromycin and 400 μg/ml geneticin at 30,000 cells/well in a 96-well black clear-bottom plates (Corning, Corning, NY) and incubated at 37 °C, 5% CO2 overnight. Activation of the hκOR via test compounds was assessed the next day. On the day of assay, the culture medium was removed and the cells washed once in 100 μL of HBSS buffer containing 0.78 mg/mL probenicid, followed by the addition of 100 μL HBSS buffer plus probenicid and 100 μL of Calcium 3 dye (diluted 1:1 with HBSS). The cells were incubated with the dye at 37 °C for 1 hr. Test compounds were evaluated using 7 different concentrations run in duplicate. These were added to cells at 5× final concentration in HBSS/probenicid containing 1.25% DMSO (0.25% final concentration). The effect of test compound on internal calcium mobilization was determined with a FlexStation II 384 (Molecular Devices) set for bottom read with 485 nm excitation and 525 nm emission wavelengths with a 515 nm emission cutoff. Data were captured using SoftmaxPro (Molecular Devices) and max-min used to calculate the change in fluorescence intensity.
Data Analysis
Data are reported as the mean ± SEM from at least three separate experiments. Prism was also used to run the one-way ANOVA and post hoc tests on the hκOR data.
Acknowledgments
The CHO cells expressing human opioid receptors were kind gifts of Dr. Lawrence Toll (SRI, Menlo Park, CA; hμOR and hδOR) and Dr. Lee-Yuan Liu-Chen (Temple University, Philadelphia, PA; hκOR). The authors also thank Vic Parcell and Lynn Teesch of the High Resolution Mass Spectrometry Facility, University of Iowa for mass spectral analysis and Kathleen Kitsopoulos and Keith Warner for their valuable technical assistance. This work was supported by National Institutes of Health, National Institute on Drug Abuse Grant DA-018151-2 (to TEP) and in part by the Intramural Research Program of the NIH, NIDA.
Footnotes
This work was presented in part at the American Society of Pharmacognosy 46th Annual Meeting, July 23 – July 27, 2005.
References
- 1.Casy AF, Parfitt RT. Opioid analgesics : chemistry and receptors. Plenum Press; New York: 1986. pp. xv–518. [Google Scholar]
- 2.Stein C, Schafer M, Machelska H. Nat Med. 2003;9:1003–8. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 3.Valdes LJ, III, Butler WM, Hatfield GM, Paul AG, Koreeda MJ. Org Chem. 1984;49:4716–4720. [Google Scholar]
- 4.Valdes LJ, III, Diaz JL, Paul AGJ. Ethnopharmacol. 1983;7:287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 5.Siebert DJJ. Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 6.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheffler DJ, Roth BL. Trends Pharmacol Sci. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 8.Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BLJ. Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LYJ. Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 10.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TEJ. Med Chem. 2005;8:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 11.Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. Org Lett. 2005;7:3017–3020. doi: 10.1021/ol0510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munro TA, Rizzacasa MAJ. Nat Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
- 13.Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan FJ. Med Chem. 2005;48:345–348. doi: 10.1021/jm049438q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beguin C, Richards MR, Wang Y, Chen Y, Liu-Chen LY, Ma Z, Lee DY, Carlezon WA, Jr, Cohen BM. Bioorg Med Chem Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- 15.Lee DY, Ma Z, Liu-Chen LY, Wang Y, Chen Y, Carlezon WA, Jr, Cohen B. Bioorg Med Chem. 2005;13:5635–5639. doi: 10.1016/j.bmc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 16.Lee DYW, Karnati VVR, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C. Bioorg Med Chem Lett. 2005;15:3744–3474. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Lee DY, He M, Kondaveti L, Liu-Chen LY, Ma Z, Wang Y, Chen Y, Li JG, Beguin C, Carlezon WA, Jr, Cohen B. Bioorg Med Chem Lett. 2005;15:4169–4173. doi: 10.1016/j.bmcl.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 18.Tidgewell K, Harding WW, Schmidt M, Holden KG, Murry DJ, Prisinzano TE. Bioorg Med Chem Lett. 2004;14:5099–5102. doi: 10.1016/j.bmcl.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 19.Fakstorp J, Raleigh D, Schniepp LEJ. Am Chem Soc. 1950;72:869–874. [Google Scholar]
- 20.Gagnaire D, Vottero P. Bull Soc Chim Fr. 1963:2779–2884. [Google Scholar]
- 21.Honel M, Mosher HSJ. Org Chem. 1985;50:4386–4388. [Google Scholar]
- 22.Aprile C, Gruttadauria M, Amato ME, D’Anna F, Lo Meo P, Riela S, Noto R. Tetrahedron. 2003;59:2241–2251. [Google Scholar]
- 23.Dutta D, Hadd H, Vander Velde DG, Georg GI. Bioorg Med Chem Lett. 1999;9:3277–8. doi: 10.1016/s0960-894x(99)00609-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Kawada K, Gross RS, Watt DSJ. Org Chem. 1990;55:504–511. [Google Scholar]
- 25.Kiankarimi M, Lowe R, McCarthy JR, Whitten JP. Tetrahedron Lett. 1999;40:4497–4500. [Google Scholar]
- 26.Koreeda M, Brown L, Valdes LJ., III Chem Lett. 1990:2015–2018. [Google Scholar]
- 27.Ortega A, Blount JF, Manchand PS. J Chem Soc Perkin Trans 1. 1982:2505–2508. [Google Scholar]