Abstract
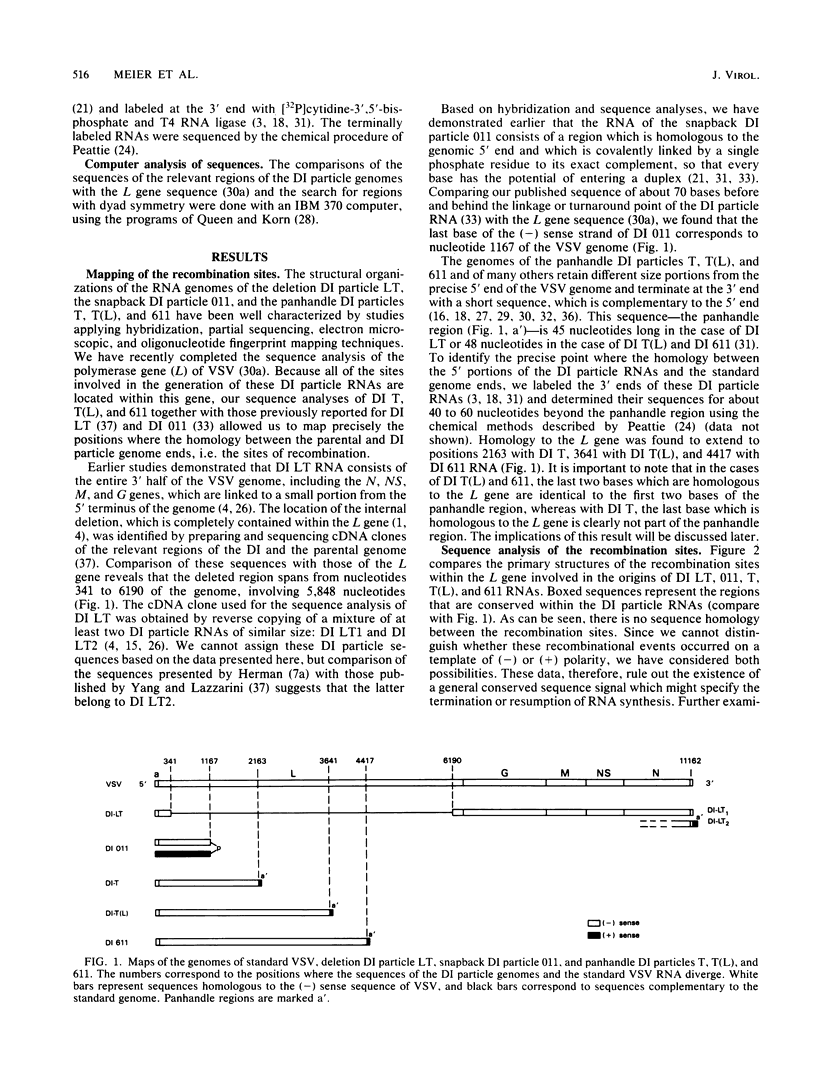
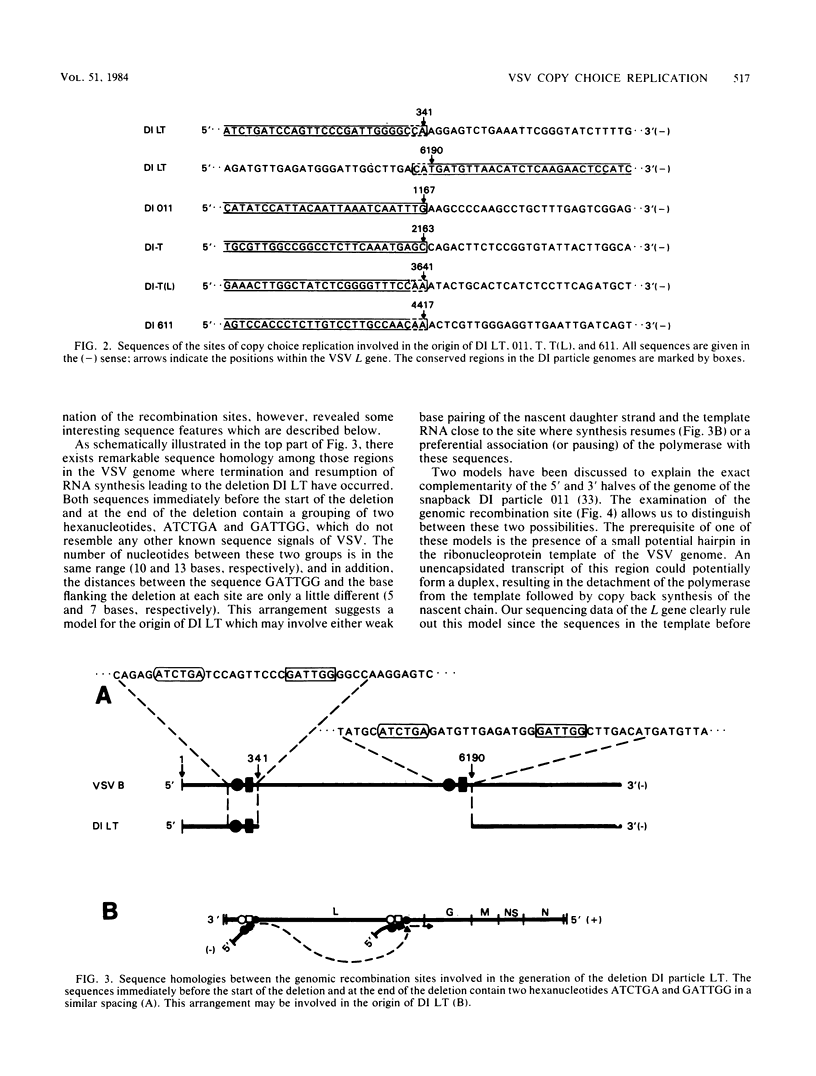
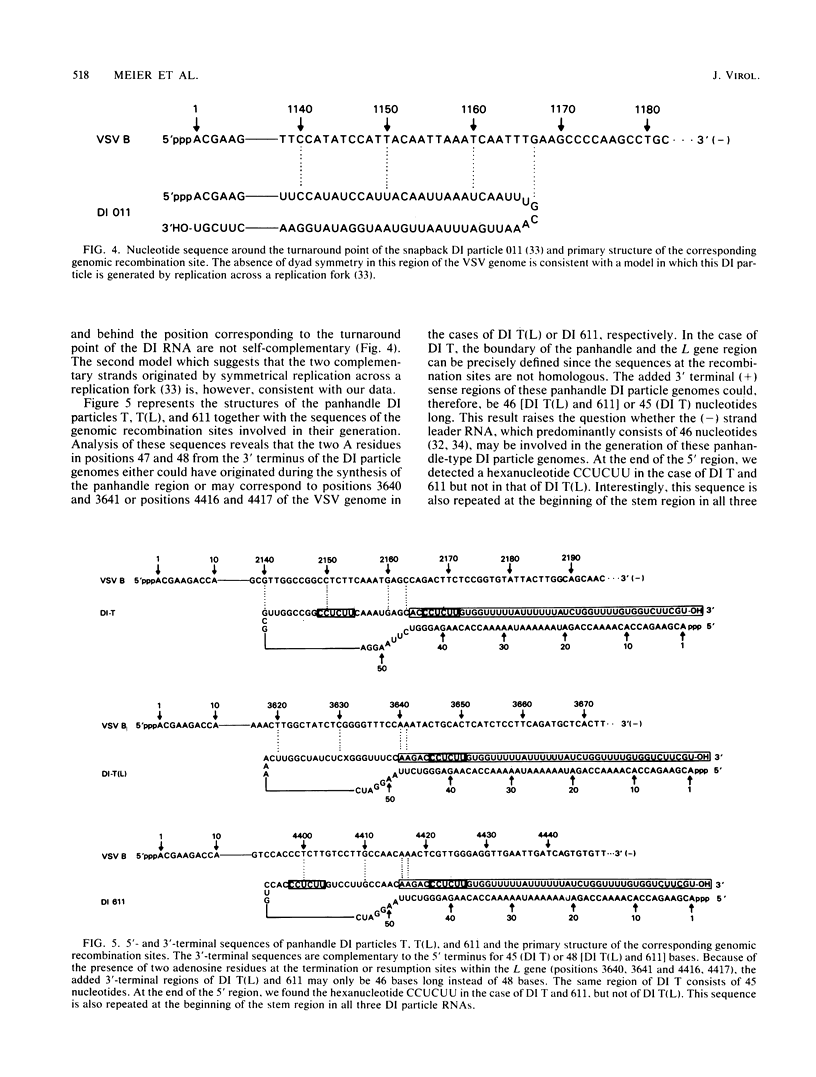
The copy choice model for the generation of defective interfering (DI) particles of vesicular stomatitis virus suggests that during replication the polymerase prematurely terminates, moves with the nascent daughter strand to another site on the same or a different template molecule, and resumes elongation of the nascent chain. We have analyzed the sites where premature termination or resumption of replication has occurred during the generation of the deletion DI particle LT, the snapback DI particle 011, and the panhandle DI particles T, T(L), and 611. The recombination sites were identified by comparing the nucleotide sequences of the relevant regions of these DI particle RNAs to those of the vesicular stomatitis virus L gene (Schubert et al., J. Virol. 51:505-514, 1984). Sequence homology was not detected between these sites, which rules out the existence of a general terminator or promoter sequence involved in copy choice replication. In several cases, however, premature termination or resumption of RNA replication may be favored by specific signal sequences. The sequences immediately before the start and at the end of the deletion in DI LT contain two hexanucleotides, ATCTGA and GATTGG, in a similar spacing. In these case of DI T and 611, but not of DI T(L), the end of the 5'-terminal region bears the hexanucleotide CCUCUU. This sequence is also repeated in the stem region in all three DI particle genomes. In addition, we present data that the added 3'-terminal regions of the panhandle DI particle RNAs may differ by only one base and are 46 [DI T(L) and 611] or 45 (DI T) bases long. We suggest that each site of the vesicular stomatitis virus genome has the potential to give rise to DI particle RNAs. Specific sequences, however, may modulate this process in a quantitative way, and they favor the generation of certain types of DI particle genomes like those of the panhandle type.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clerx-Van Haaster C. M., Clewley J. P., Bishop D. H. Oligonucleotide sequence analyses indicate that vesicular stomatitis virus large defective interfering virus particle RNA is made by internal deletion: evidence for similar transcription polyadenylation signals for the synthesis of all vesicular stomatitis virus mRNA species. J Virol. 1980 Feb;33(2):807–817. doi: 10.1128/jvi.33.2.807-817.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Dierks P. M., Parsons J. T. In vitro synthesis of a unique RNA species by a T particle of vesicular stomatitis virus. J Virol. 1977 Sep;23(3):708–716. doi: 10.1128/jvi.23.3.708-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Epstein D. A., Herman R. C., Chien I., Lazzarini R. A. Defective interfering particle generated by internal deletion of the vesicular stomatitis virus genome. J Virol. 1980 Feb;33(2):818–829. doi: 10.1128/jvi.33.2.818-829.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B., Kolakofsky D. Sequence determination of the (+) leader RNA regions of the vesicular stomatitis virus Chandipura, Cocal, and Piry serotype genomes. J Virol. 1983 Apr;46(1):125–130. doi: 10.1128/jvi.46.1.125-130.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Huang A. S. Comparison of ribonucleotide sequences from the genome of vesicular stomatitis virus and two of its defective interfering particles. J Virol. 1981 Jan;37(1):363–371. doi: 10.1128/jvi.37.1.363-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C. Nucleotide sequence of an aberrant glycoprotein mRNA synthesized by the internal deletion mutant of vesicular stomatitis virus. J Virol. 1984 May;50(2):524–528. doi: 10.1128/jvi.50.2.524-528.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P. A., Finch J. T., Winter G., Robertson J. S. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983 Sep;34(2):619–627. doi: 10.1016/0092-8674(83)90394-x. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Glimp T., Clewley J. P., Bishop D. H. Studies on the generation of vesicular stomatitis virus (indiana serotype) defective interfering particles. Virology. 1978 Jan;84(1):142–152. doi: 10.1016/0042-6822(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Chien I. M., Lazzarini R. A. Vesicular stomatitis virus defective interfering particle containing a muted internal leader RNA gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2090–2094. doi: 10.1073/pnas.78.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Intervening sequence between the leader region and the nucleopcapsid gene of vesicular stomatitis virus RNA. J Virol. 1980 Feb;33(2):789–794. doi: 10.1128/jvi.33.2.789-794.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kolakofsky D. Effect of defective interfering particles on plus- and minus- strand leader RNAs in vesicular stomatitis virus-infected cells. J Virol. 1980 Sep;35(3):704–709. doi: 10.1128/jvi.35.3.704-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Repik P., Bishop D. H. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J Virol. 1973 Nov;12(5):969–983. doi: 10.1128/jvi.12.5.969-983.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. The size and the cistronic origin of defective vesicular stomatitis virus particle RNAs in relation to homotypic and heterotypic interference. J Mol Biol. 1976 Mar 5;101(3):307–325. doi: 10.1016/0022-2836(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. Structure and origin of a snapback defective interfering particle RNA of vesicular stomatitis virus. J Virol. 1981 Feb;37(2):661–672. doi: 10.1128/jvi.37.2.661-672.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamminger G. M., Lazzarini R. A. RNA synthesis in standard and autointerfered vesicular stomatitis virus infections. Virology. 1977 Mar;77(1):202–211. doi: 10.1016/0042-6822(77)90418-4. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Yang F., Lazzarini R. A. Analysis of the recombination event generating a vesicular stomatitis virus deletion defective interfering particle. J Virol. 1983 Feb;45(2):766–772. doi: 10.1128/jvi.45.2.766-772.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



