Abstract
The RNA ligase and polynucleotide kinase of bacteriophage T4 are nonessential enzymes in most laboratory Escherichia coli strains. However, T4 mutants which do not induce the enzymes are severely restricted in E. coli CTr5X, a strain derived from a clinical E. coli isolate. We have mapped the restricting locus in E. coli CTr5X and have transduced it into other E. coli strains. The restrictive locus seems to be a gene, or genes, unique to CTr5X or to be an altered form of a nonessential gene, since deleting the locus seems to cause loss of the phenotypes. In addition to restricting RNA ligase- and polynucleotide kinase-deficient T4, the locus also restricts bacteriophages lambda and T4 with cytosine DNA. When lambda or T4 with cytosine DNA infect strains with the prr locus, the phage DNA is injected, but phage genes are not expressed and the host cells survive. These phenotypes are unlike anything yet described for a phage-host interaction.
Full text
PDF
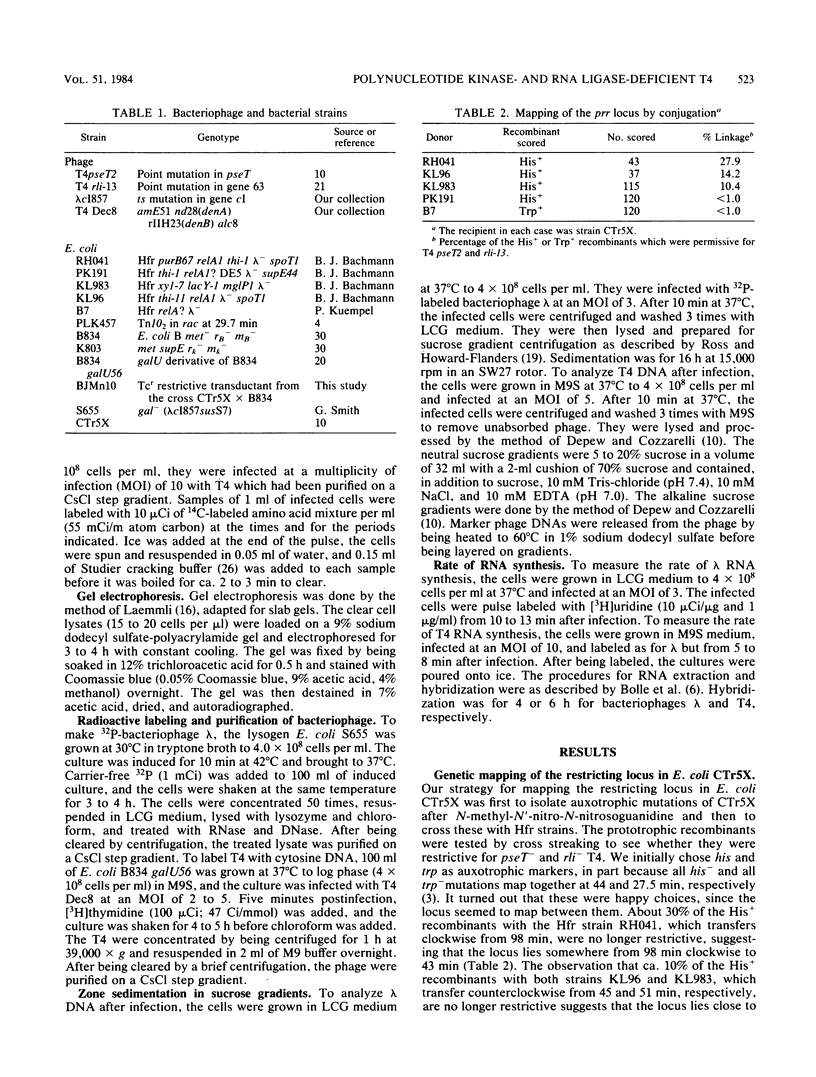

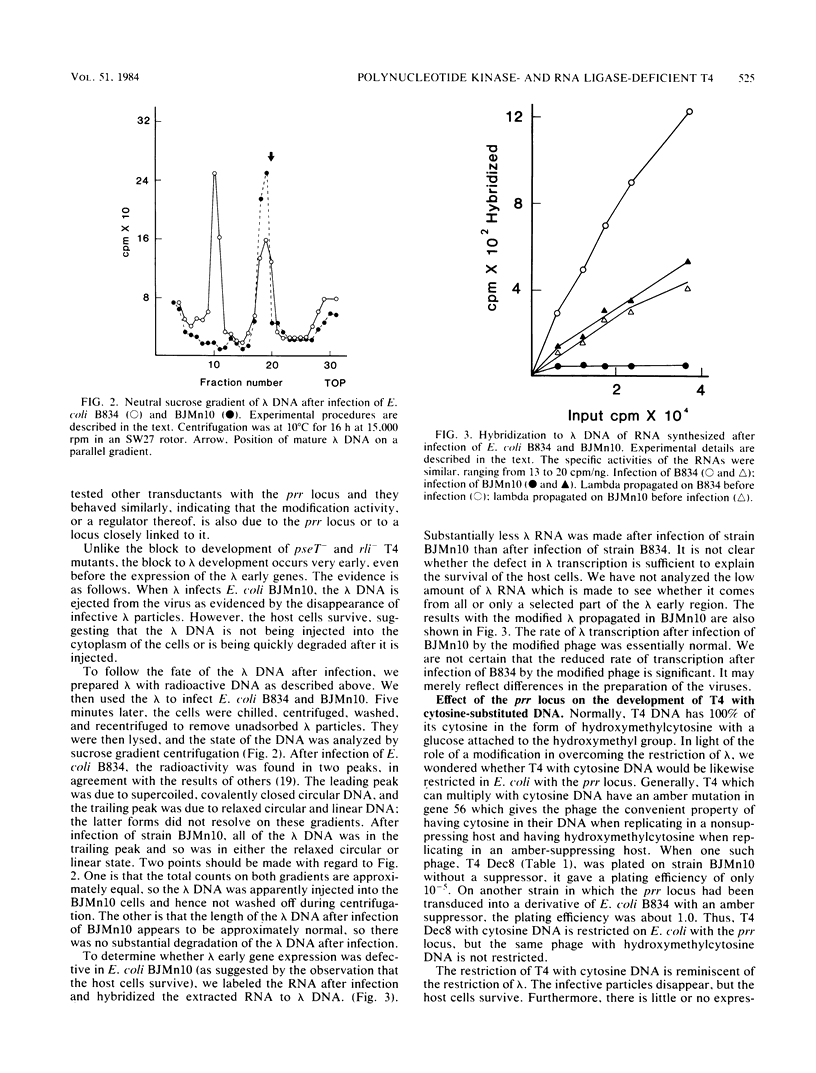
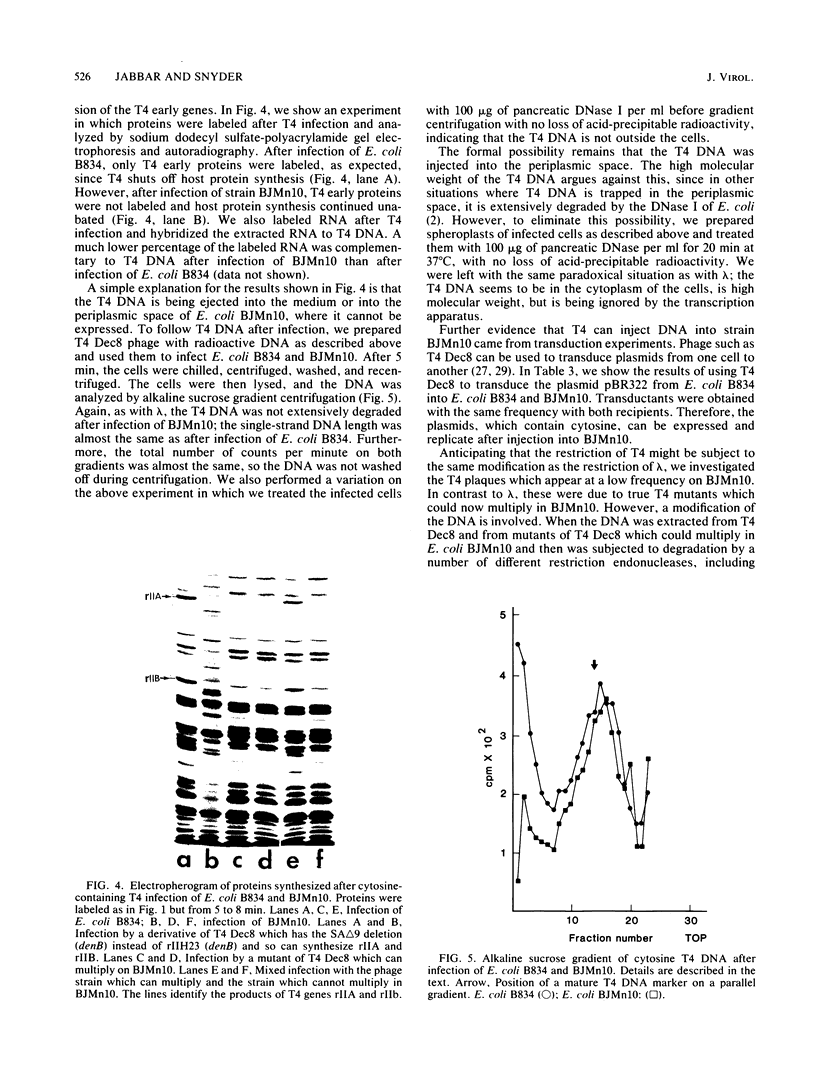


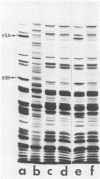
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binding R., Romansky G., Bitner R., Kuempel P. Isolation and properties of Tn10 insertions in the rac locus of Escherichia coli. Mol Gen Genet. 1981;183(2):333–340. doi: 10.1007/BF00270637. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Cooley W., Sirotkin K., Green R., Synder L. A new gene of Escherichia coli K-12 whose product participates in T4 bacteriophage late gene expression: interaction of lit with the T4-induced polynucleotide 5'-kinase 3'-phosphatase. J Bacteriol. 1979 Oct;140(1):83–91. doi: 10.1128/jb.140.1.83-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Borasio G. D., Kaufmann G. Bacteriophage T4-induced anticodon-loop nuclease detected in a host strain restrictive to RNA ligase mutants. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7097–7101. doi: 10.1073/pnas.79.23.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Borasio G. D., Kaufmann G. T4 bacteriophage-coded polynucleotide kinase and RNA ligase are involved in host tRNA alteration or repair. Virology. 1982 Dec;123(2):480–483. doi: 10.1016/0042-6822(82)90284-7. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Cozzarelli N. R. Genetics and physiology of bacteriophage T4 3'-phosphatase: evidence for involvement of the enzyme in T4 DNA metabolism. J Virol. 1974 Apr;13(4):888–897. doi: 10.1128/jvi.13.4.888-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C. L., Javor B., Abelson J. RNA ligase in bacteria: formation of a 2',5' linkage by an E. coli extract. Cell. 1983 Jul;33(3):899–906. doi: 10.1016/0092-8674(83)90032-6. [DOI] [PubMed] [Google Scholar]
- Harayama S., Palva E. T., Hazelbauer G. L. Transposon-insertion mutants of Escherichia coli K12 defective in a component common to galactose and ribose chemotaxis. Mol Gen Genet. 1979 Mar 20;171(2):193–203. doi: 10.1007/BF00270005. [DOI] [PubMed] [Google Scholar]
- Hattman S., Goradia M., Monaghan C., Bukhari A. I. Regulation of the DNA-modification function of bacteriophage Mu. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):647–653. doi: 10.1101/sqb.1983.047.01.076. [DOI] [PubMed] [Google Scholar]
- Kahmann R. Methylation regulates the expression of a DNA-modification function encoded by bacteriophage Mu. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):639–646. doi: 10.1101/sqb.1983.047.01.075. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Bradley D., Schenck R., Guttman B. S., Laiken R. Bacteriophage T4 alc gene product: general inhibitor of transcription from cytosine-containing DNA. J Virol. 1981 Dec;40(3):822–829. doi: 10.1128/jvi.40.3.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P., Howard-Flanders P. Initiation of recA+-dependent recombination in Escherichia coli (lambda). I. Undamaged covalent circular lambda DNA molecules in uvrA+ recA+ lysogenic host cells are cut following superinfection with psoralen-damaged lambda phages. J Mol Biol. 1977 Nov 25;117(1):137–158. doi: 10.1016/0022-2836(77)90028-6. [DOI] [PubMed] [Google Scholar]
- Runnels J. M., Soltis D., Hey T., Snyder L. Genetic and physiological studies of the role of the RNA ligase of bacteriophage T4. J Mol Biol. 1982 Jan 15;154(2):273–286. doi: 10.1016/0022-2836(82)90064-x. [DOI] [PubMed] [Google Scholar]
- Runnels J., Snyder L. Isolation of a bacterial host selective for bacteriophage T4 containing cytosine in its DNA. J Virol. 1978 Sep;27(3):815–818. doi: 10.1128/jvi.27.3.815-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Cooley W., Runnels J., Snyder L. R. A role in true-late gene expression for the T4 bacteriophage 5' polynucleotide kinase 3' phosphatase. J Mol Biol. 1978 Aug 5;123(2):221–233. doi: 10.1016/0022-2836(78)90322-4. [DOI] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Saito H. Mechanism of pBR322 transduction mediated by cytosine-substituting T4 bacteriophage. Mol Gen Genet. 1982;186(4):497–500. doi: 10.1007/BF00337955. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Young K. Y., Edlin G. J., Konigsberg W. High-frequency generalised transduction by bacteriophage T4. Nature. 1979 Jul 5;280(5717):80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Conley M. P., Lyle H. L., Dickson R. C. Attachment of tail fibers in bacteriophage T4 assembly. Purification, properties, and site of action of the accessory protein coded by gene 63. J Biol Chem. 1978 Apr 10;253(7):2437–2445. [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]