INTRODUCTION
The sensitivity of the mammalian inner ear is extraordinary. At the threshold of hearing, the incoming sound produces vibrations inside the organ of Corti that are of the same order of magnitude or less than the thermal noise motion (Hudspeth, 1989, 1997; Dallos, 1996). Such sensitivity is achieved through an energy-dependent process commonly referred to as “cochlear amplifier,” i.e., the cycle-by-cycle amplification of the intracochlear vibrations that neutralizes viscous losses by positive mechanical feedback (Patuzzi and Robertson, 1988; Ashmore and Kolston, 1994; Kolston, 1995). The mechanical feedback force that makes the amplification possible is thought to be provided by cochlear outer hair cells (OHCs), a class of specialized sensorimotor cells hosted within the organ of Corti (Lim and Kalinec, 1998; Nobili et al., 1998). Direct support to the feedback hypothesis comes from the fact that OHCs posses a unique ability to change significantly their shape in response to electrical stimulation (Brownell et al., 1985; Kachar et al., 1986), a phenomenon called electromotility.
VIDEO MOVIES
Movie 1: OHC Electromotility in an External Electric Field
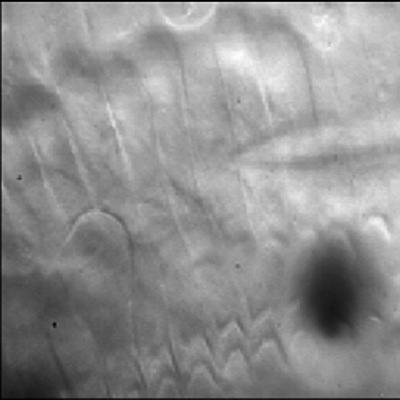
This movie shows contraction and elongation of a guinea pig’s OHC placed in an external alternating electric field (Figure 1). Isolated OHCs were obtained by mechanical dissociation from strips of the organ of Corti after mild enzymatic treatment (Collagenase type IV; Life Technologies, Grand Island, NY; 1 mg/ml, 10–15 min). Two Ag-AgCl electrodes were placed ∼2 mm apart along the cell body. Alternating voltage steps applied to the electrodes (2 V, 2 Hz) produced an electric field approximately parallel to the cell body. Under these experimental conditions the electrically evoked length changes could be as large as ∼1 μm for 70- to 90-μm-long cells.
Figure 1.
OHC electromotility in an external electric field.
A variety of stimuli, chemical (Zenner et al., 1985; Zenner, 1986), thermal (Gitter, 1992), electrical (Brownell et al., 1985), mechanical (Canlon et al., 1988; Brundin et al., 1989; Brundin and Russel, 1994) and UV light (Dulon et al., 1989), have been reported to affect OHC length. Among them, electromotility attracted particular attention, because electrically evoked changes in OHC length are sufficiently fast to follow stimulation on a cycle-by-cycle basis at acoustic frequencies (Kachar et al., 1986; Ashmore, 1987; Reuter et al., 1992, Gale and Ashmore, 1997). Thorough investigation of this phenomenon became possible with the application of the whole-cell patch-clamp recording technique to OHC studies (Ashmore, 1987).
Movie 2: OHC Electromotility in Whole-Cell Patch-Clamp Conditions
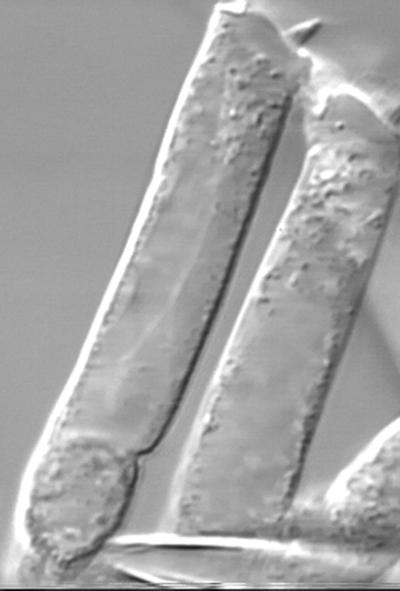
This movie shows OHC motility evoked by changes of intracellular potential under conditions whereby intracellular voltage was imposed on the cell by the patch-clamp amplifier (Figure 2). The potential of the cell was held at −70 mV, and square depolarizing pulses of 60 mV amplitude were delivered at the rate of one per second (stimulation mark is on the bottom left of the frame). Depolarization produced contraction, whereas hyperpolarization resulted in elongation of the cell. Reduction or reversal of transmembrane current in these conditions by various manipulations did not interfere with OHC mechanical response, which led to the suggestion that OHC motility is driven by transmembrane voltage rather than current (Santos-Sacchi and Dilger, 1988). This conclusion has been confirmed by the observation that OHC mechanical response in an external electric field disappeared after membrane permeabilization (Iwasa and Kachar, 1989). Later it was shown that the driving stimulus is indeed a local transmembrane voltage drop and that the cellular motor consists of independent elements, distributed along the lateral cell membrane and its associated cortical structures (Holley and Ashmore, 1988b; Dallos et al., 1991; Kalinec et al., 1992). OHCs respond to this local stimulus with local changes of membrane area (Kalinec et al., 1992).
Figure 2.
OHC electromotility in whole-cell patch-clamp conditions.
The transitions between contracted and elongated states shown in Movie 2 are too fast to be resolved at a standard TV frame rate. Indeed, it was shown that the speed of the OHC motile response in these conditions is limited only by the time constant of the whole-cell recording, which is set essentially by the time required to charge the plasma membrane capacitance through the access resistance of the patch pipette (typically <1 ms; Santos-Sacchi, 1992). The real speed limit of the OHC motor is even higher than can be obtained in whole-cell patch-clamp experiments and does approach the frequency limit of mammalian hearing (tens of kilohertz; Gale and Ashmore, 1997).
In vivo, the intracellular potential is assumed to change after the influx of ionic current through the mechanoelectric transduction channels located at the OHC stereocilia. The channels are opened by displacement of the stereocilia bundles toward the tallest one when the organ of Corti oscillates in response to incoming sound vibrations (Ashmore and Kolston, 1994). Nevertheless, this way of changing the intracellular potential has essentially the same frequency limitations as the experimental whole-cell patch-clamp technique. To overcome this problem, Dallos and Evans (1995) suggested that extracellular potential variations resulting from transduction current changes could be a driving force for high-frequency OHC motility in vivo.
Movie 3: Bending of the OHC in an External Electric Field
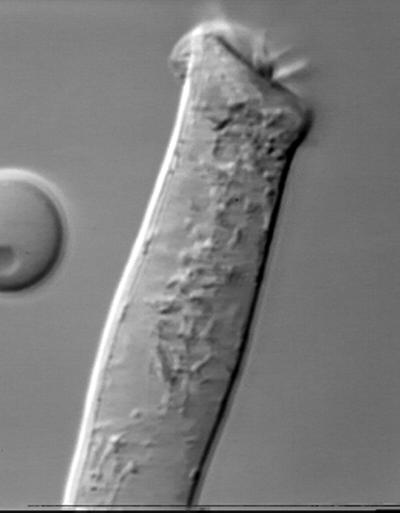
An extracellular electric field is able to drive OHC motility at high frequencies (Reuter et al., 1992; Dallos and Evans, 1995). It may also modify significantly the OHC motile response if there is a component of the voltage gradient across the cell diameter. This movie shows OHC shape changes in an external electric field oriented perpendicularly to the cell axes (Figure 3). Stimulation is the same as in Movie 1. Because of the relative axial symmetry, the OHC may be considered as a conductive liquid compartment enclosed by a membrane with relatively high resistance (Frolenkov et al., 1997). In an external electric field this compartment undergoes charge separation, producing depolarization on one side of the cell and hyperpolarization on the other side. The result is the bending of the cell toward the negative electrode.
Figure 3.

Bending of the OHC in an external electric field
Movie 4: OHC Electromotile Force Is Sufficient to Produce Movement inside the Organ of Corti
This animation was built from a set of four images of the cochlear partition viewed from scala media at an angle bringing the full length of OHCs into focus (Figure 4 ) (data acquired in Prof. Jonathan F. Ashmore’s laboratory at the Department of Physiology, University of Bristol, Bristol, United Kingdom; see Mammano et al., 1995). The cell’s stereocilia are visible, through the intact tectorial membrane, near the frame bottom. A patch pipette (entering from the right) was attached to a third-row OHC, incrementing cell potential by 25 mV between successive frames. The cell responded by shortening visibly when depolarized within the intact organ of Corti. Both the synaptic end and the stereociliary end of the OHC moved toward the patch pipette, in accord with the distributed nature of the molecular motors along the cell wall (Holley and Ashmore, 1988b; Dallos et al., 1991). The movement of the synaptic end, cupped by a supporting Deiters’ cell, could be as large as 1.5 μm when the cell membrane potential was stepped positive by 100 mV from rest. However, the movement of the stereociliary end was comparatively reduced as the cell cuticular plate was firmly held within the reticular lamina. The mean potential sensitivity of the motile responses in situ was ∼12 nm/mV. Cell movement caused appreciable displacement of neighboring cells in the organ of Corti, indicating that OHC forces are large enough to alter cochlear mechanics by distorting the cochlear partition.
Figure 4.
OHC electromotile force is sufficient to produce movement inside the organ of Corti.
Movie 5: OHC Electromotility and Intracellular Pressure
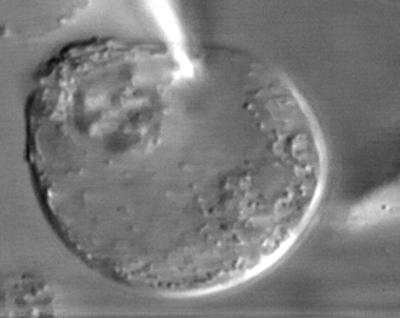
Normal intracellular pressure is essential for maintaining good mechanical responses in OHCs (Brownell, 1990; Chertoff and Brownell, 1994). This video clip demonstrates some significant features of the relationship between electromotility and intracellular pressure in an OHC from a ground squirrel (Figure 5). This particular cell lost some internal pressure after the rupture of the membrane patch under the pipette. Changes of transmembrane potential were still able to produce length changes, but of reduced amplitude. The surface of the cell behaved like a rubber balloon, inflating during depolarization and deflating during hyperpolarization. Two important conclusions may be drawn from such observations. First, deflating the cell did not disrupt the electromotile mechanism per se. Second, the OHC responded to intracellular potential variations with the shrinkage and expansion of its surface area, which in turn produced changes in intracellular pressure. Electromotility models predict pressure changes as large as 0.5 kPa for ∼0.5-μm-length changes (Dallos et al., 1993).
Figure 5.
OHC electromotility and intracellular pressure.
Movie 6: Effect of Salicylate on the OHC Electromotility
This movie shows the mechanical response of a guinea pig’s OHC in patch-clamp conditions before and during puff application of 20 mM salicylic acid (Figure 6). The animation in the top left corner of the frame shows command voltage. The motile response almost disappeared within 5–10 sec after salicylate application.
Figure 6.
Effect of salicylate on the OHC electromotility.
Salicylic acid is one of a few reagents that are known to block OHC electromotility, the other known blockers being some sulfhydryl reagents (Kalinec and Kachar, 1993) and lanthanides (Santos-Sacchi, 1991; Kakehata and Santos-Sacchi, 1996). All these inhibitors are not specific because they affect a variety of other functions in the cell. No specific inhibitor of OHC electromotility has been reported so far.
The mechanism of OHC electromotility is clearly different from most other forms of cell motility. It does not depend on the presence of ATP (Kachar et al., 1986; Holley and Ashmore, 1988b) and is resistant to the drugs affecting cycles of polymerization and depolymerization of actin and tubulin (Holley and Ashmore, 1988a). Inhibition by sulfhydryl reagents indicates the involvement of proteins (Kalinec and Kachar, 1993), whereas the dependence on the local transmembrane voltage locates the electromotility mechanism somewhere in plasma membrane and underlying structures (Dallos et al., 1991).
Movie 7: Disruption of the OHC Cytoskeleton with Diamide Does Not Affect Electromotility
The self-supporting shape of OHC is maintained by its cortical structures and in particular by the cortical lattice, a two-dimensional cytoskeleton that lines the inner aspect of the lateral plasma membrane (Holley and Ashmore, 1988a, 1990). Circumferential actin filaments with longitudinal spectrin cross-links determine the greater mechanical compliance of the cortical lattice in the longitudinal direction (Holley et al., 1992; Tolomeo et al., 1996), providing a mechanical frame for the OHC motile response that is oriented mainly longitudinally (Kalinec and Kachar, 1995). This cortical lattice frame seems to be mechanically passive, although the possibility exists in principle that spectrin may generate forces relevant for OHC length changes.
This movie shows electromotile response of an OHC incubated with 1 mM diamide and attached to the glass coverslip at its apical pole (Figure 7). Diamide is a bifunctional thiol reagent that oxidizes the sulfhydryl group of cysteine to the disulfide and affects spectrin but not actin (Becker et al., 1986). After diamide application, the cell became less rigid and was easily stretched by displacing the patch pipette. The persistence of electromotile responses even after the cell body has been stretched extensively indicates clearly that the cell electromotility does not require an intact cytoskeleton.
Figure 7.

Disruption of the OHC cytoskeleton with diamide does not affect electromotility
Movie 8: Electromotile Mechanism Requires Only Plasma Membrane Integrity
The most reasonable suggestion is that the electromotility mechanism is located in the plasma membrane itself. To test this hypothesis the patch pipette was filled with solution containing trypsin (150 μg/ml) to produce enzymatic digestion of the cell content. This movie shows that the electromechanical responses were still prominent several minutes after the start of intracellular dialysis with trypsin (Figure 8). These results indicate that electromotility depends on a mechanism secluded from the action of trypsin and therefore residing within the plasma membrane (Kalinec et al., 1992). The dramatic disruption of subplasmalemmal structures by trypsin was confirmed by electron microscopy controls (Huang and Santos-Sacchi, 1994).
Figure 8.
Electromotile mechanism requires only plasma membrane integrity.
Freeze-fracture images of the lateral plasma membrane of OHCs revealed an unusually high concentration of intramembrane particles that were never observed in inner hair cells (Gulley and Reese, 1977). Freeze-etching of the membranes after partial extraction with detergent revealed that these intramembrane particles are organized in regular arrays (Kalinec et al., 1992; Kalinec and Kachar, 1995). The center-to-center distance between particles in these arrays is ∼13 nm (Kachar and Frolenkov, unpublished results), corresponding to a density of ∼6000 per square micrometer. Such dense arrays of intramembrane particles could form the basis for a force-generating mechanism in OHCs.
Each of these large intramembrane particles may represent a sensorimotor protein complex that detects voltage changes across the membrane and undergoes either conformational changes by itself or changes in the packing within the arrays. Conformational changes in voltage-gated ion channels are associated with gating currents, which represent a total effective electrical charge moving across the membrane (Bezanilla and White, 1982). Similar voltage-dependent charge movement closely associated with the electromotile responses can be recorded in OHCs (Santos-Sacchi, 1991; Iwasa, 1993; Gale and Ashmore, 1997). If the maximal gating charge and the later surface of OHCs are known, it is possible to estimate the effective number of elementary charges that move across the membrane. Recent estimates gave values of 7558 e−/μm2 (Huang and Santos-Sacchi, 1993), 4200–8400 e−/μm2 (Gale and Ashmore, 1997), and 12,900 ± 400 e−/μm2 (Frolenkov and Kachar, unpublished results) which are of the same order of magnitude as the density of intramembrane particles.
Molecular Identity of the OHC Motor
The challenge for the future remains the identification of sensorimotor protein structures at the molecular level. Although the motor is unlikely to be an ion channel, because no ionic current is associated with OHC electromotility, there exists the possibility that it is a modified channel with an integral voltage sensor but no conducting pore. Such molecular structures have been described previously (Olcese et al., 1997), but attempts to use a variety of ion channel blockers to inhibit electromotility gave negative results (see Table 1). The other possibility is that the motor is a transporter protein linked with underlying cytoskeleton. There are reasons to suspect that the OHC motor could be a modified version of an anion exchanger protein (Kalinec et al., 1993, 1997; Knipper et al., 1995; Lim and Kalinec, 1998), but a complete molecular and functional characterization of this protein in OHCs is still lacking.
Table 1.
Ion channel blockers tested on OHC electromotility
| Blocker | Concentration |
|---|---|
| K+ voltage-gated channel | |
| Agiotoxin | 10 nM |
| α-Dendrotoxin | 500 nM |
| β-Dendrotoxin | 100 nM |
| γ-Dendrotoxin | 100 nM |
| δ-Dendrotoxin | 100 nM |
| Dendrotoxin-I | 100 nM |
| Margatoxin | 10 nM |
| MCD peptide | 1 μM |
| Stichodactila toxin | 500 nM |
| Tityustoxin Kα | 50 nM |
| L-type Ca2+ channel | |
| Calcicludine | 10 nM |
| Calciseptine | 14 nM |
| FS-2 | 10 nM |
| TaiCai toxin | 500 nM |
| Waglerine-I | 6 μM |
| Ca2+-activated K+ channel | |
| Apamin | 1 μM |
| Charibdotoxin | 100 nM |
| Iberiotoxin | 50 nM |
| Kaliotoxin | 100 nM |
| Noxiustoxin | 100 nM |
| Paxilline | 1 μM |
| Penitrem | 1 μM |
| Non-L-type Ca2+ channel | |
| ω-Agatoxin TK | 1 μM |
| ω-Conotoxin GVIA | 33 nM |
| ω-Conotoxin MVIIA | 18 nM |
| ω-Conotoxin MVIIC | 10 nM |
| ω-Conotoxin SVIB | 18 nM |
| FTX-3.3 | 1 μM |
| sFTX-3.3 | 10 μM |
| PLTX-II | 50 nM |
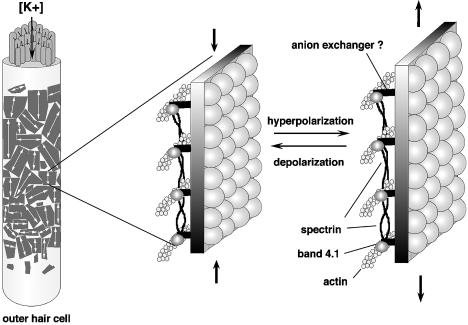
Movie 9: Schematics of OHC Force Generation Unit
The lateral plasma membrane of the OHCs of the organ of Corti contain “force generation units” composed of small domains of a semicrystalline array of motor proteins (Figure 9). Pillars, connecting these motor proteins to an actin–spectrin meshwork inside the membrane, convey the forces generated in the plane of the membrane to the cell’s interior. We propose that the pillars are composed of the anion exchanger protein and 4.1-band proteins, but it is unclear whether these proteins are just force conveyors or are themselves the motor proteins.
Figure 9.
Schematics of OHC force generation unit.
Supplementary Material
Footnotes
REFERENCES
- Ashmore JF. A fast motile response in guinea pig outer hair cells: the cellular basis for the cochlear amplifier. J Physiol. 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Kolston PJ. Hair cell based amplification in the cochlea. Curr Opin Neurobiol. 1994;4:503–508. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Becker PS, Cohen CM, Lux SE. The effect of mild diamide oxidation on the structure and function of human erythrocyte spectrin. J Biol Chem. 1986;261:4620–4628. [PubMed] [Google Scholar]
- Bezanilla F, White MM. Gating currents associated with potassium channel activation. Nature. 1982;296:657–659. doi: 10.1038/296657a0. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Brundin L, Flock Å, Canlon B. Sound-induced motility of isolated cochlear outer hair cells is frequency-specific. Nature. 1989;342:814–816. doi: 10.1038/342814a0. [DOI] [PubMed] [Google Scholar]
- Brundin L, Russell I. Tuned phasic and tonic motile responses of isolated outer hair cells to direct mechanical stimulation of the cell body. Hear Res. 1994;73:35–45. doi: 10.1016/0378-5955(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Canlon B, Brundin L, Flock Å. Acoustic stimulation causes tonotopic alterations in the lenght of isolated outer hair cells from guinea pig hearing organ. Proc Natl Acad Sci USA. 1988;85:7033–7035. doi: 10.1073/pnas.85.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertoff ME, Brownell WE. Characterization of cochlear outer hair cell turgor. Am J Physiol. 1994;266:C467–C479. doi: 10.1152/ajpcell.1994.266.2.C467. [DOI] [PubMed] [Google Scholar]
- Dallos P. Overview: cochlear neurobiology. In: Dallos P, Popper AN, editors. The Cochlea. Vol. 8. R.R. Fay, New York: Springer-Verlag; 1996. pp. 1–43. [Google Scholar]
- Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- Dallos P, Evans BN, Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991;350:155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dallos P, Hallworth R, Evans BN. Theory of electrically driven shape changes of cochlear outer hair cells. J Neurophysiol. 1993;70:299–323. doi: 10.1152/jn.1993.70.1.299. [DOI] [PubMed] [Google Scholar]
- Dulon D, Zajic G, Schacht J. Photo-induced irreversible shortening and swelling of isolated cochlear outer hair cells. Int J Radiat Biol. 1989;55:1007–1014. doi: 10.1080/09553008914551031. [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Kalinec F, Tavartkiladze GA, Kachar B. Cochlear outer hair cell bending in an external electrical field. Biophys J. 1997;73:1665–1672. doi: 10.1016/S0006-3495(97)78198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF. An intrinsic frequency limit to the cochlear amplifier. Nature. 1997;389:63–66. doi: 10.1038/37968. [DOI] [PubMed] [Google Scholar]
- Gitter AH. The length of isolated outer hair cells is temperature dependent. ORL J Otorhinolaryngol Relat Spec. 1992;54:121–123. doi: 10.1159/000276279. [DOI] [PubMed] [Google Scholar]
- Gulley RL, Reese TS. Regional specialization of the hair cell plasmalemma in the organ of Corti. Anat Rec, 1977;189:109–124. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- Holley MC, Ashmore JF. A cytoskeletal spring in cochlear outer hair cells. Nature. 1988a;335:635–637. doi: 10.1038/335635a0. [DOI] [PubMed] [Google Scholar]
- Holley MC, Ashmore JF. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988b;232:413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley MC, Ashmore JF. Spectrin, actin and the structure of the cortical lattice in mammalian cochlear outer hair cells. J Cell Sci. 1990;96:283–291. doi: 10.1242/jcs.96.2.283. [DOI] [PubMed] [Google Scholar]
- Holley MC, Kalinec F, Kachar B. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci. 1992;102:569–580. doi: 10.1242/jcs.102.3.569. [DOI] [PubMed] [Google Scholar]
- Huang G, Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J. 1993;65:2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Santos-Sacchi J. Motility voltage sensor of the outer hair cell resides within the lateral plasma membrane. Proc Natl Acad Sci USA. 1994;91:12268–12272. doi: 10.1073/pnas.91.25.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–950. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Iwasa KH. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993;65:492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa KH, Kachar B. Fast in vitro movement of outer hair cells in an external electric field: effect of digitonin, a membrane permeabilizing agent. Hear Res. 1989;40:247–254. doi: 10.1016/0378-5955(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–367. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F, Holley MC, Iwasa K, Lim DJ, Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci USA. 1992;89:8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F, Jaeger R, Kachar B. Mechanical coupling of the outer hair cell plasma membrane to the cortical cytoskeleton by anion exchanger and 4.1 proteins. In: Duifhuis H, Horst JW, van Dijk P, van Netten SM, editors. Biophysics of Hair Cell Sensory Systems. Singapore: World Scientific; 1993. pp. 175–181. [Google Scholar]
- Kalinec F, Kachar B. Inhibition of outer hair cell electromotility by sulfhydryl specific reagents. Neurosci Lett. 1993;157:231–134. doi: 10.1016/0304-3940(93)90744-6. [DOI] [PubMed] [Google Scholar]
- Kalinec F, Kachar B. Structure of the electromechanical transduction mechanism in mammalian outer hair cells. In: Flock Å, Ottoson D, Ulfendahl M, editors. Active Hearing. Oxford: Elsevier Science; 1995. pp. 179–191. [Google Scholar]
- Kalinec F, Kalinec G, Negrini C, Kachar B. Immunolocalization of anion exchanger 2-alpha in auditory sensory hair cells. Hear Res. 1997;110:141–146. doi: 10.1016/s0378-5955(97)00076-2. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zimmermann U, Köpschall I, Rohbock K, Jüngling S, Zenner H-P. Immunological identification of candidate proteins involved in regulating active shape changes of outer hair cells. Hear Res. 1995;86:100–110. doi: 10.1016/0378-5955(95)00060-h. [DOI] [PubMed] [Google Scholar]
- Kolston PJ. A faster transduction mechanism for the cochlear amplifier. Trends Neurosci. 1995;18:427–429. doi: 10.1016/0166-2236(95)90091-8. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Kalinec F. Cell and molecular basis of hearing. Kidney Int. 1998;53:S104–S113. [PubMed] [Google Scholar]
- Mammano F, Kros CJ, Ashmore JF. Patch clamped responses from outer hair cells in the intact adult organ of Corti. Pflügers Arch. 1995;430:745–750. doi: 10.1007/BF00386170. [DOI] [PubMed] [Google Scholar]
- Nobili R, Mammano F, Ashmore JF. How well do we understand the cochlea. Trends Neurosci. 1998;21:159–167. doi: 10.1016/s0166-2236(97)01192-2. [DOI] [PubMed] [Google Scholar]
- Olcese R, Latorre R, Toro L, Bezanilla F, Stefani E. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J Gen Physiol. 1997;110:579–589. doi: 10.1085/jgp.110.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patuzzi R, Robertson D. Tuning in the mammalian cochlea. Physiol Rev. 1988;68:1009–1079. doi: 10.1152/physrev.1988.68.4.1009. [DOI] [PubMed] [Google Scholar]
- Reuter G, Gitter AH, Thurm U, Zenner HP. High frequency radial movements of the reticular lamina induced by outer hair cell motility. Hear Res. 1992;60:236–246. doi: 10.1016/0378-5955(92)90025-i. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. On the frequency limit and phase of outer hair cell motility: effects of the membrane filter. J Neurosci. 1992;12:1906–1916. doi: 10.1523/JNEUROSCI.12-05-01906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dilger JP. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Tolomeo JA, Steele CR, Holley MC. Mechanical properties of the lateral cortex of mammalian auditory hair cells. Biophys J. 1996;71:421–429. doi: 10.1016/S0006-3495(96)79244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner HP. Motile responses in outer hair cells. Hear Res. 1986;22:83–90. doi: 10.1016/0378-5955(86)90082-1. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Zimmerman U, Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985;18:127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.