Abstract
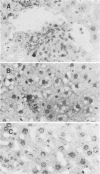
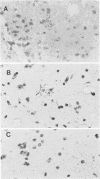
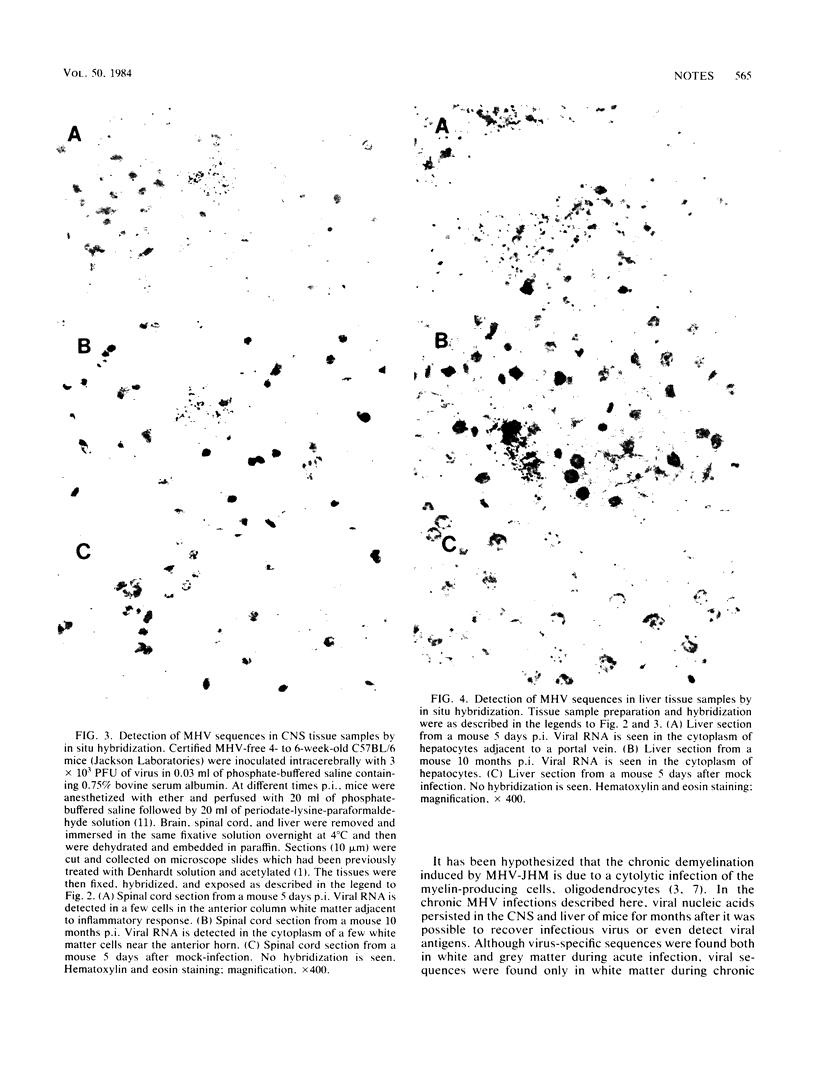
Mouse hepatitis virus strain A59 produces chronic central nervous system demyelination in rodents. As late as 6 months after intracerebral inoculation of mice 4 to 6 weeks old, when infectious virus cannot be recovered and viral antigens cannot be detected in the central nervous systems and livers of these animals, primary demyelination is still evident. Using cloned virus-specific DNAs and the highly sensitive and specific technique of in situ hybridization, we have detected low levels of mouse hepatitis virus A59 RNA in the central nervous systems and livers of mice 10 months after inoculation. We suggest that viral persistence may play a role in mouse hepatitis virus A59-induced chronic demyelination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Fleury H. J., Sheppard R. D., Bornstein M. B., Raine C. S. Further ultrastructural observations of virus morphogenesis and myelin pathology in JHM virus encephalomyelitis. Neuropathol Appl Neurobiol. 1980 May-Jun;6(3):165–179. doi: 10.1111/j.1365-2990.1980.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Webb E. A., Cory S., Adams J. M. Molecular cloning of seven mouse immunoglobulin kappa chain messenger ribonucleic acids. Biochemistry. 1980 Jun 10;19(12):2702–2710. doi: 10.1021/bi00553a026. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Swoveland P., Stowring L., Ventura P., Johnson K. P., Norrby E., Gibbs C. J., Jr Measles virus genome in infections of the central nervous system. J Infect Dis. 1981 Aug;144(2):154–160. doi: 10.1093/infdis/144.2.154. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Lampert P. W., Oldstone M. B. Virus persistence and recurring demyelination produced by a temperature-sensitive mutant of MHV-4. Nature. 1982 Jul 15;298(5871):279–280. doi: 10.1038/298279a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Highkin M. K., Weiss S. R. MHV-A59 pathogenesis in mice. Adv Exp Med Biol. 1984;173:237–245. doi: 10.1007/978-1-4615-9373-7_24. [DOI] [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Wroblewska Z., Rorke L. B., Weiss S. R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984 May;34(5):597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Dayan A. D., Allison A. C. Neuropathological effects of persistent infection of mice by mouse hepatitis virus. Infect Immun. 1975 Nov;12(5):1127–1140. doi: 10.1128/iai.12.5.1127-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Leibowitz J. L. Characterization of murine coronavirus RNA by hybridization with virus-specific cDNA probes. J Gen Virol. 1983 Jan;64(Pt 1):127–133. doi: 10.1099/0022-1317-64-1-127. [DOI] [PubMed] [Google Scholar]