Abstract
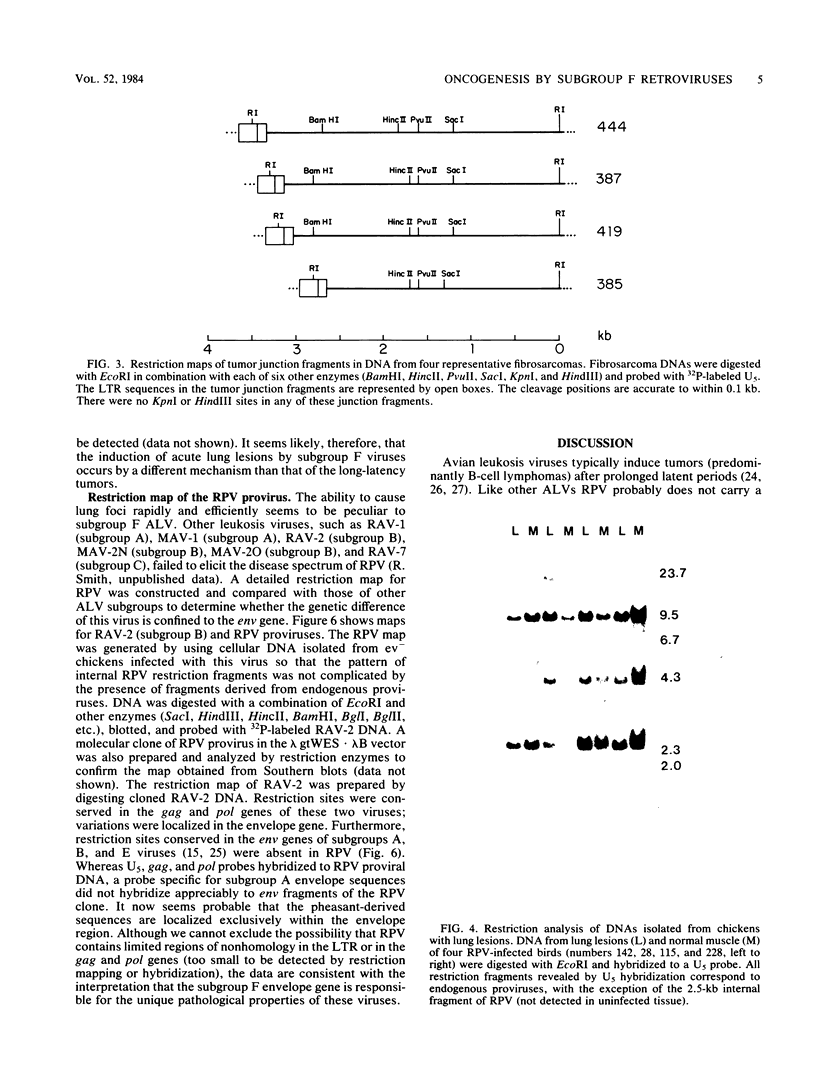
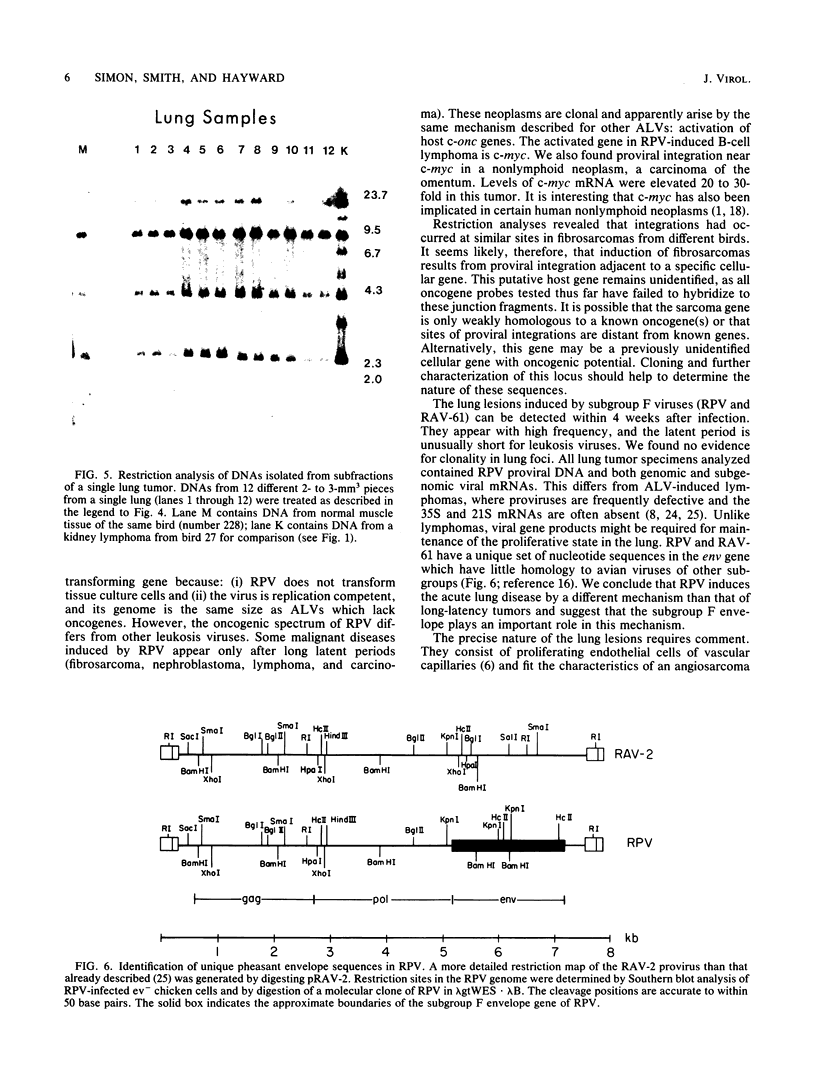
Subgroup F avian leukosis viruses, such as RAV-61 and ring-necked pheasant virus, are recombinants between exogenous chicken retroviruses and endogenous pheasant viruses and contain new envelope (env) genes. Chickens infected as 10-day-old embryos with subgroup F viruses develop fibrosarcomas, nephroblastomas, osteopetrosis, B-cell lymphomas, and a high incidence of a proliferative disorder involving the lung. Fibrosarcomas, nephroblastomas, and lymphomas appear after long latent periods (3 to 12 months). They contain discrete virus-cell junction fragments and are therefore clonal outgrowths of a single infected cell. Two ring-necked pheasant virus-induced B-cell lymphomas and an adenocarcinoma of the abdomen contained proviruses integrated at the c-myc locus and elevated levels of myc mRNA. At least four of the fibrosarcomas appeared to contain proviruses integrated at a common site, suggesting that a specific cellular gene may be involved in these tumors. The host gene has not been identified, however; 16 different oncogene probes failed to hybridize to fibrosarcoma junction fragments. In contrast to these neoplasms, lung lesions appeared rapidly (4 to 5 weeks), showed no evidence of clonality, and lacked long terminal repeat-initiated transcripts other than viral 35S and 21S mRNA. We conclude, therefore, that subgroup F retroviruses induce the proliferative disorder of the lung by a different mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- BEARD J. W. VIRAL TUMORS OF CHICKENS WITH PARTICULAR REFERENCE TO THE LEUKOSIS COMPLEX. Ann N Y Acad Sci. 1963 Nov 4;108:1057–1085. doi: 10.1111/j.1749-6632.1963.tb13436.x. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. II. Evidence for a strand displacement mechanism in plus-strand synthesis. J Virol. 1981 Jan;37(1):117–126. doi: 10.1128/jvi.37.1.117-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. K., Proctor S. J., Smith R. E. Induction of angiosarcomas by ring-necked pheasant virus. Infect Immun. 1983 Apr;40(1):310–319. doi: 10.1128/iai.40.1.310-319.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Chen Y. C., Friis R. R., Vogt P. K. RNA tumor viruses of pheasants: characterization of avian leukosis subgroups F and G. Virology. 1974 Aug;60(2):558–571. doi: 10.1016/0042-6822(74)90350-x. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Isolation of leukosis-type virus from pheasant embryo cells: possible presence of viral genes in cells. Virology. 1973 Jan;51(1):247–251. doi: 10.1016/0042-6822(73)90388-7. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Nucleotide sequences derived from pheasant DNA in the genome of recombinant avian leukosis viruses with subgroup F specificity. J Virol. 1977 Nov;24(2):505–513. doi: 10.1128/jvi.24.2.505-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Kabat D. Reduced leukemogenicity caused by mutations in the membrane glycoprotein gene of Rauscher spleen focus-forming virus. J Virol. 1984 Feb;49(2):394–402. doi: 10.1128/jvi.49.2.394-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Gasic G. P., Rogler C. E., Skalka A. M., Ju G., Hishinuma F., Papas T., Astrin S. M., Hayward W. S. Molecular analysis of the c-myc locus in normal tissue and in avian leukosis virus-induced lymphomas. J Virol. 1982 Oct;44(1):158–166. doi: 10.1128/jvi.44.1.158-166.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. V., Keene J. D., Linial M., Smith R. E. Association of 3' terminal RNA sequences with avian leukosis viruses causing a high incidence of osteopetrosis. Virology. 1982 Jan 15;116(1):163–180. doi: 10.1016/0042-6822(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Homology between avian oncornavirus RNAs and DNA from several avian species. J Virol. 1975 Dec;16(6):1492–1502. doi: 10.1128/jvi.16.6.1492-1502.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Ribonucleotide sequence homology among avian oncornaviruses. J Virol. 1975 Jan;17(1):106–113. doi: 10.1128/jvi.17.1.106-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Avian leukosis viruses of different subgroups and types isolated after passage of Rous sarcoma virus-Rous-associated virus-0 in cells from different ring-necked pheasant embryos. J Virol. 1976 Aug;19(2):302–312. doi: 10.1128/jvi.19.2.302-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S. Retrovirus lymphomagenesis: relationship of normal immune receptors to malignant cell proliferation. Curr Top Microbiol Immunol. 1982;98:103–112. doi: 10.1007/978-3-642-68369-5_8. [DOI] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]







