Abstract
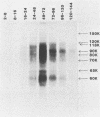
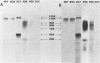
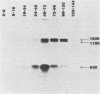
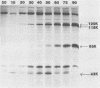
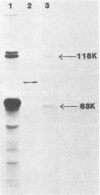
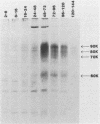
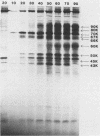
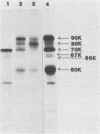
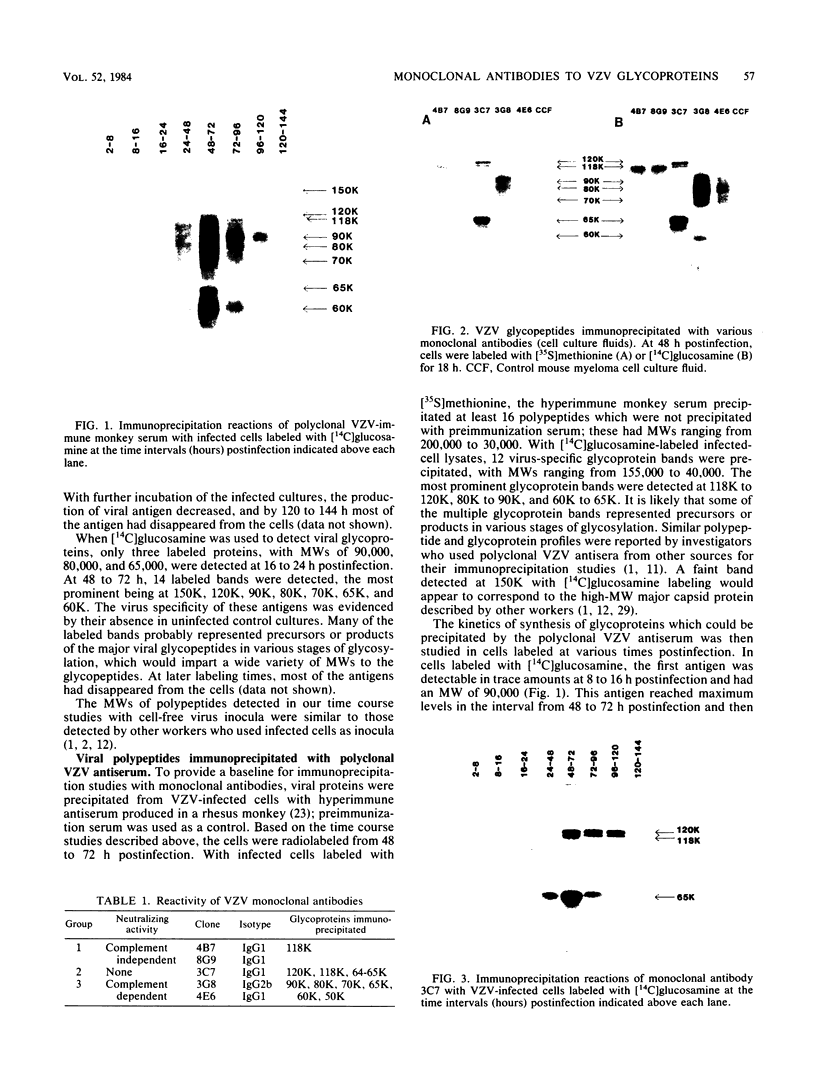
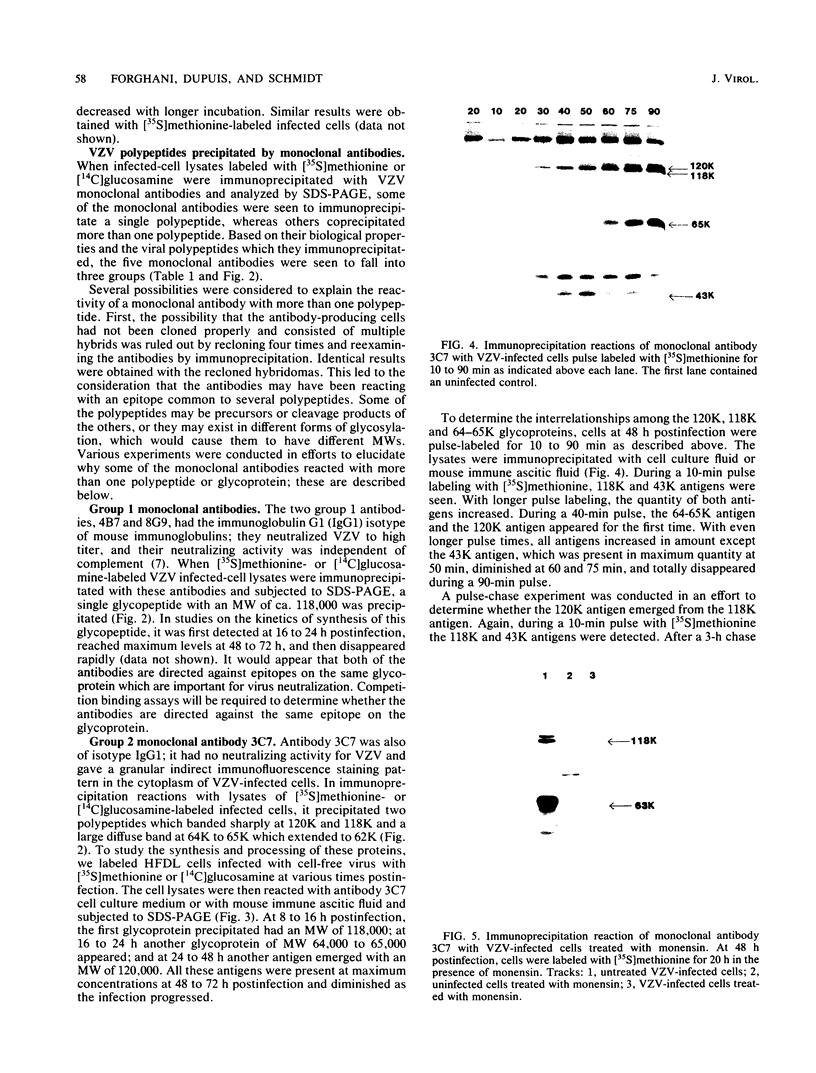
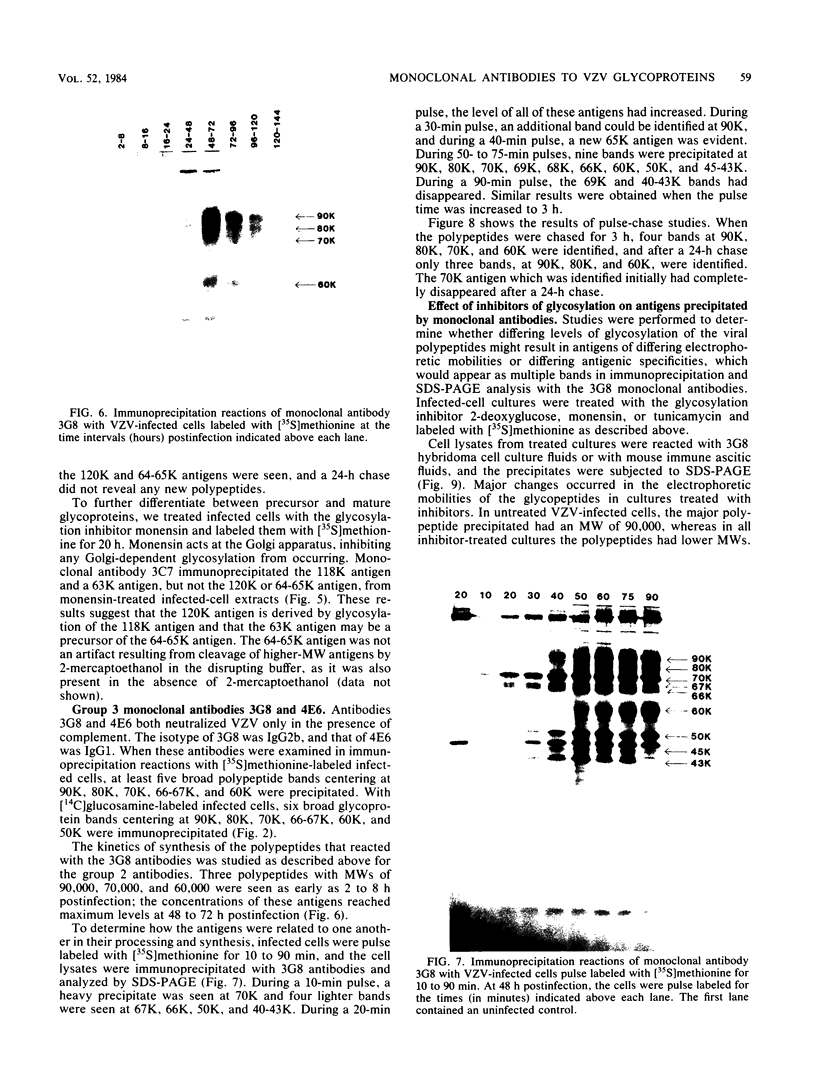
Monoclonal antibodies to varicella-zoster virus were used to study viral glycoproteins by immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Based on the viral glycoproteins immunoprecipitated, the five monoclonal antibodies fell into three groups. Two antibodies, 4B7 and 8G9 (group 1), immunoprecipitated a single glycoprotein of molecular weight (MW) 118,000 (118K glycoprotein) and had high neutralizing activity in the absence of complement. One antibody, 3C7 (group 2), which lacked neutralizing activity, immunoprecipitated two glycoproteins of MWs 120,000 and 118,000 and a glycoprotein giving a diffuse band in the region of 64,000 to 65,000. Pulse-chase experiments and experiments with monensin as an inhibitor of glycosylation suggested that the 120K polypeptide was derived by glycosylation of the 118K polypeptide and that a 43K antigen was processed into the 64 to 65K glycoprotein. Two antibodies, 3G8 and 4E6 (group 3), both had neutralizing activity only in the presence of complement, and both immunoprecipitated at least five polypeptides, with MWs ranging from 50,000 to 90,000. Antibody 3G8 was isotype immunoglobulin G2b (IgG2b), and its immunoprecipitating activity was stronger than that of 4E6, which was isotype IgG1. Pulse-chase experiments with antibody 3G8 showed that lower-MW glycopeptides chased into three polypeptides of MWs 90,000, 80,000, and 60,000 by 24 h. Immunoprecipitation experiments with antibody 3G8 on infected cells treated with glycosylation inhibitors 2-deoxyglucose, monensin, and tunicamycin, suggested that a prominent, early-appearing 70K polypeptide may have been processed into the glycoproteins of higher MWs and that the 60K polypeptide may have been derived by glycosylation of polypeptides of lower MWs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Takahashi M. Studies on the polypeptides of varicella-zoster (V-Z) virus. 1. Detection of varicella-zoster virus polypeptides in infected cells. Biken J. 1979 Sep;22(3):81–89. [PubMed] [Google Scholar]
- Asano Y., Takahashi M. Studies on the polypeptides of varicella-zoster (V-Z) virus. II. Syntheses of viral polypeptides in infected cells. Biken J. 1980 Sep;23(3):95–106. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Forghani B., Dennis J., Schmidt N. J. Visual reading of enzyme immunofluorescence assays for human cytomegalovirus antibodies. J Clin Microbiol. 1980 Nov;12(5):704–708. doi: 10.1128/jcm.12.5.704-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Myoraku C. K., Gallo D. Serological reactivity of some monoclonal antibodies to varicella-zoster virus. Arch Virol. 1982;73(3-4):311–317. doi: 10.1007/BF01318084. [DOI] [PubMed] [Google Scholar]
- Glorioso J., Szczesiul M. S., Marlin S. D., Levine M. Inhibition of glycosylation of herpes simplex virus glycoproteins: identification of antigenic and immunogenic partially glycosylated glycopeptides on the cell surface membrane. Virology. 1983 Apr 15;126(1):1–18. doi: 10.1016/0042-6822(83)90458-0. [DOI] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E. Immunoprecipitable polypeptides specified by varicella-zoster virus. Virology. 1982 Apr 15;118(1):86–95. doi: 10.1016/0042-6822(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousoulas K. G., Bzik D. J., Person S. Effect of the ionophore monensin on herpes simplex virus type 1-induced cell fusion, glycoprotein synthesis, and virion infectivity. Intervirology. 1983;20(1):56–60. doi: 10.1159/000149375. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Norrild B., Pedersen B. Effect of tunicamycin on the synthesis of herpes simplex virus type 1 glycoproteins and their expression on the cell surface. J Virol. 1982 Aug;43(2):395–402. doi: 10.1128/jvi.43.2.395-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Yamanishi K., Shiraki K., Takahashi M. Synthesis and processing of glycoproteins of Varicella-Zoster virus (VZV) as studied with monoclonal antibodies to VZV antigens. Virology. 1983 Sep;129(2):357–368. doi: 10.1016/0042-6822(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Blomberg J., Lycke E. O-glycosidic carbohydrate-peptide linkages of Herpes simplex virus glycoproteins. Arch Virol. 1981;70(4):321–329. doi: 10.1007/BF01320247. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Improved yields of cell-free varicella-zoster virus. Infect Immun. 1976 Sep;14(3):709–715. doi: 10.1128/iai.14.3.709-715.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Neutralizing antibody responses to varicella-zoster virus. Infect Immun. 1975 Sep;12(3):606–613. doi: 10.1128/iai.12.3.606-613.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Woodie J. D., Ho H. H. Immunofluorescent staining in the laboratory diagnosis of varicella-zoster virus infections. J Lab Clin Med. 1965 Sep;66(3):403–412. [PubMed] [Google Scholar]
- Shemer Y., Leventon-Kriss S., Sarov I. Isolation and polypeptide characterization of varicella-zoster virus. Virology. 1980 Oct 15;106(1):133–140. doi: 10.1016/0042-6822(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Okuno T., Yamanishi K., Takahashi M. Polypeptides of varicella-zoster virus (VZV) and immunological relationship of VZV and herpes simplex virus (HSV). J Gen Virol. 1982 Aug;61(Pt 2):255–269. doi: 10.1099/0022-1317-61-2-255. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Takahashi M. Virus particles and glycoprotein excreted from cultured cells infected with varicella-zoster virus (VZV). J Gen Virol. 1982 Aug;61(Pt 2):271–275. doi: 10.1099/0022-1317-61-2-271. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983 Oct;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- Wolff M. H. The proteins of varicella-zoster-virus. Med Microbiol Immunol. 1978 Nov 17;166(1-4):21–28. doi: 10.1007/BF02121130. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Neff B. J. Immune response after exposure to varicella zoster virus: characterization of virus-specific antibodies and their corresponding antigens. Infect Immun. 1981 Jan;31(1):436–444. doi: 10.1128/iai.31.1.436-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]