Abstract
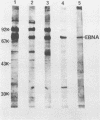
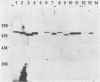
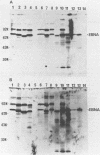
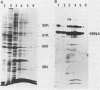
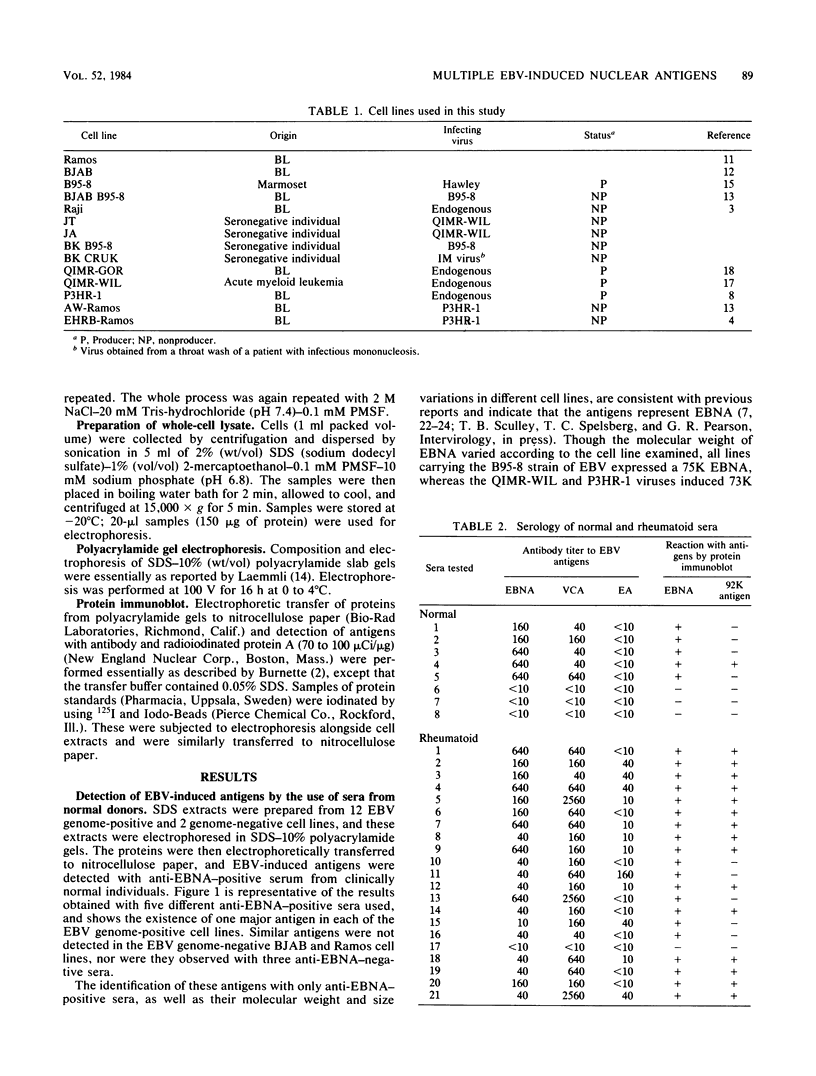
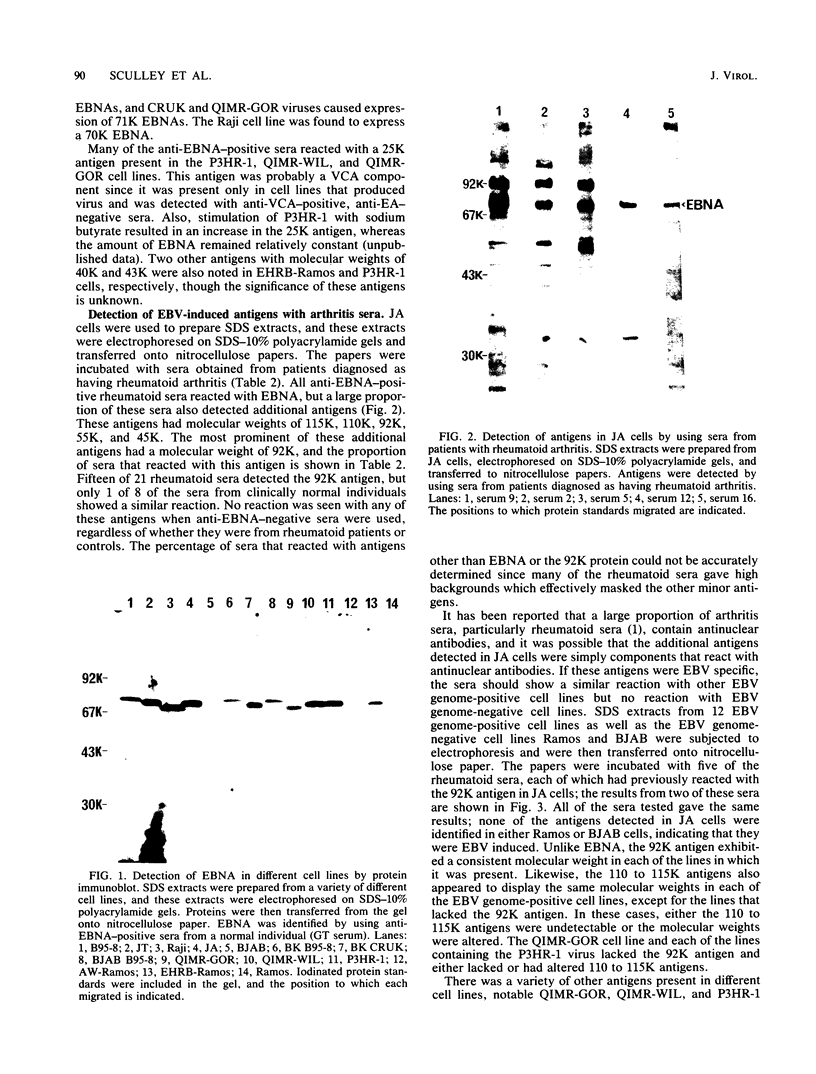
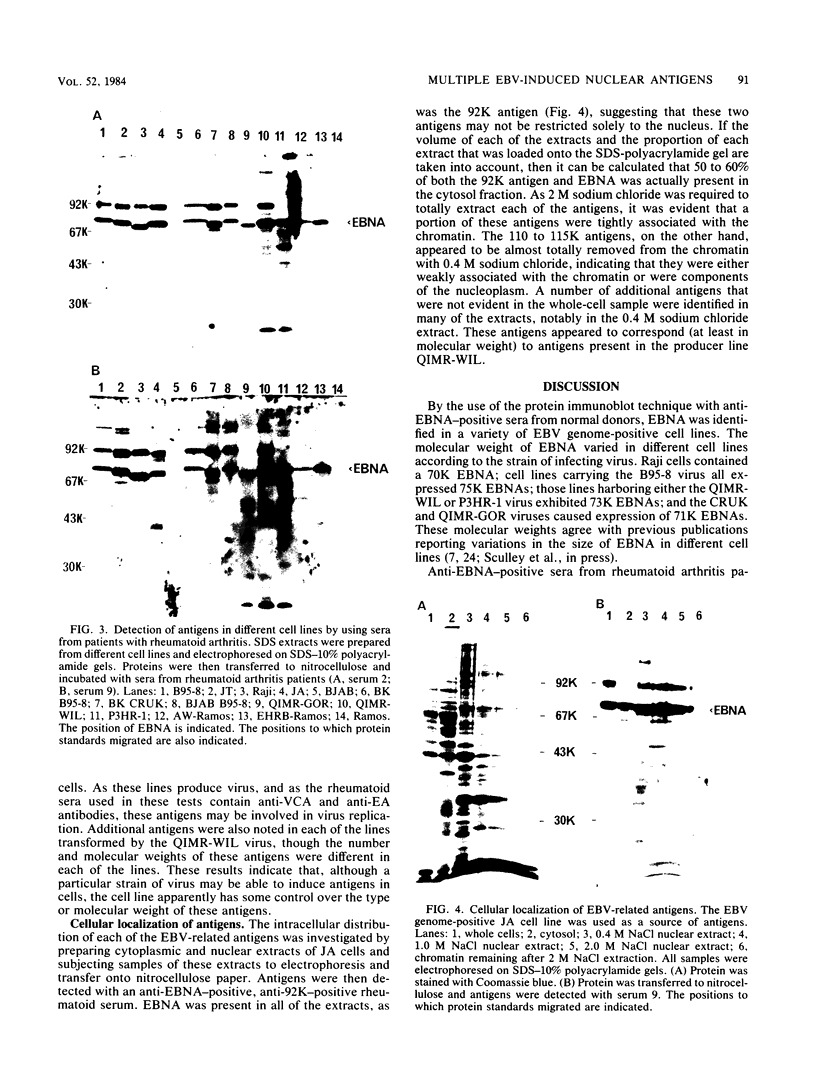
By means of the protein immunoblot technique, the Epstein-Barr virus (EBV) nuclear antigen (EBNA) could be identified in a variety of EBV-transformed cell lines with anti-EBNA-positive sera from normal donors. The molecular weight of EBNA expressed in each of the cell lines varied between 70,000 and 75,000 and was dependent upon the strain of infecting virus. In contrast, 15 of 21 sera from patients with rheumatoid arthritis identified antigens in addition to EBNA. The most prominent of these antigens had molecular weights of 110,000 to 115,000 and 92,000. All of the EBV genome-positive cell lines except for QIMR-GOR and cell lines containing the P3HR-1 virus expressed these antigens. The antigens were not present in the EBV genome-negative Ramos and BJAB cell lines, nor were they identified with EBV seronegative sera, indicating that they were EBV related. There was no direct correlation between the presence of antibodies in sera to EBNA, viral capsid antigen or early antigen, and reaction with the 92,000-molecular-weight antigen in immunoblots, indicating that this antigen was distinct from previously described EBV-related antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitcheson C. T., Peebles C., Joslin F., Tan E. M. Characteristics of antinuclear antibodies in rheumatoid arthritis. Reactivity of rheumatoid factor with a histone-dependent nuclear antigen. Arthritis Rheum. 1980 May;23(5):528–538. doi: 10.1002/art.1780230503. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Grogan E. A., Summers W. P., Dowling S., Shedd D., Gradoville L., Miller G. Two Epstein-Barr viral nuclear neoantigens distinguished by gene transfer, serology, and chromosome binding. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7650–7653. doi: 10.1073/pnas.80.24.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch I., Kuchlerová L., Brichácek B., Suchánková A., Vonka V. Blocking of acid-fixed nuclear binding of Epstein-Barr virus nuclear antigen (EBNA) by different DNA species. J Gen Virol. 1979 Sep;44(3):849–852. doi: 10.1099/0022-1317-44-3-849. [DOI] [PubMed] [Google Scholar]
- Klein G., Dombos L. Relationship between the sensitivity of EBV-carrying lymphoblastoid lines to superinfection and the inducibility of the resident viral genome. Int J Cancer. 1973 Mar 15;11(2):327–337. doi: 10.1002/ijc.2910110210. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Terasaki P., Billing R., Honig R., Jondal M., Westman A., Clements G. Inducibility of the Epstein-Barr virus (EBV) cycle and surface marker properties of EBV-negative lymphoma lines and their in vitro EBV-converted sublines. Int J Cancer. 1976 Nov 15;18(5):639–652. doi: 10.1002/ijc.2910180513. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med. 1973 Dec 1;138(6):1398–1412. doi: 10.1084/jem.138.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Klestov A., Burrows S., Kane R. G. A comparison of Epstein-Barr virus-specific T-cell immunity in rheumatoid arthritis and osteoarthritis patients. Aust J Exp Biol Med Sci. 1983 Oct;61(Pt 5):509–516. doi: 10.1038/icb.1983.48. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Achong B. G., Epstein M. A., Biddulph J. Burkitt lymphoma in New Guinea: establishment of a line of lymphoblasts in vitro and description of their fine structure. J Natl Cancer Inst. 1967 Nov;39(5):933–945. [PubMed] [Google Scholar]
- Pope J. H. Establishment of cell lines from Australian leukaemic patients: presence of a herpes-like virus. Aust J Exp Biol Med Sci. 1968 Oct;46(5):643–645. doi: 10.1038/icb.1968.171. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Powell A. L., King W., Kieff E. Epstein-Barr virus-specific RNA. III. Mapping of DNA encoding viral RNA in restringent infection. J Virol. 1979 Jan;29(1):261–274. doi: 10.1128/jvi.29.1.261-274.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Sculley T. B., Kreofsky T., Pearson G. R., Spelsberg T. C. Partial purification of the Epstein-Barr virus nuclear antigen(s). J Biol Chem. 1983 Mar 25;258(6):3974–3982. [PubMed] [Google Scholar]
- Spelsberg T. C., Sculley T. B., Pikler G. M., Gilbert J. A., Pearson G. R. Evidence for two classes of chromatin-associated Epstein-Barr virus-determined nuclear antigen. J Virol. 1982 Aug;43(2):555–565. doi: 10.1128/jvi.43.2.555-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]