Abstract
Recruitment of intracellular proteins to the plasma membrane is a commonly found requirement for the initiation of signal transduction events. The recently discovered pleckstrin homology (PH) domain, a structurally conserved element found in ∼100 signaling proteins, has been implicated in this function, because some PH domains have been described to be involved in plasma membrane association. Furthermore, several PH domains bind to the phosphoinositides phosphatidylinositol-(4,5)-bisphosphate and phosphatidylinositol-(3,4,5)-trisphosphate in vitro, however, mostly with low affinity. It is unclear how such weak interactions can be responsible for observed membrane binding in vivo as well as the resulting biological phenomena. Here, we investigate the structural and functional requirements for membrane association of cytohesin-1, a recently discovered regulatory protein of T cell adhesion. We demonstrate that both the PH domain and the adjacent carboxyl-terminal polybasic sequence of cytohesin-1 (c domain) are necessary for plasma membrane association and biological function, namely interference with Jurkat cell adhesion to intercellular adhesion molecule 1. Biosensor measurements revealed that phosphatidylinositol-(3,4,5)-trisphosphate binds to the PH domain and c domain together with high affinity (100 nM), whereas the isolated PH domain has a substantially lower affinity (2–3 μM). The cooperativity of both elements appears specific, because a chimeric protein, consisting of the c domain of cytohesin-1 and the PH domain of the β-adrenergic receptor kinase does not associate with membranes, nor does it inhibit adhesion. Moreover, replacement of the c domain of cytohesin-1 with a palmitoylation–isoprenylation motif partially restored the biological function, but the specific targeting to the plasma membrane was not retained. Thus we conclude that two elements of cytohesin-1, the PH domain and the c domain, are required and sufficient for membrane association. This appears to be a common mechanism for plasma membrane targeting of PH domains, because we observed a similar functional cooperativity of the PH domain of Bruton’s tyrosine kinase with the adjacent Bruton’s tyrosine kinase motif, a novel zinc-containing fold.
INTRODUCTION
Intracellular signal transduction pathways are often initiated by recruitment of cytoplasmic proteins into specific cellular compartments, e.g., the inner leaflet of the plasma membrane. Prominent examples are the initial steps of the mitogenic signaling cascade: the induced binding of the grb2-SOS1 complex to plasma membrane-resident, tyrosine-phosphorylated growth factor receptors triggers a second recruitment event, the interaction of the raf kinase with the activated ras protein, and thereby activates downstream events. Specific interaction domains present in the recruited factors, e.g., the Src homology 2 domain and the recently discovered pleckstrin homology (PH) domain, are thought to be responsible for the tethering of cytosolic proteins to the membrane compartment.
PH domains are structural modules present in ∼100 proteins, which play known or postulated roles in signal transduction or cytoskeletal organization (Musacchio et al., 1993). It is now known that PH domains may aid in membrane recruitment of proteins through their interactions with phosphorylated ligands present in cellular membranes (Pawson, 1995; Lemmon et al., 1996, 1997). Although a subgroup of PH domains is capable of interacting with tyrosine-phosphorylated proteins (Lemmon et al., 1996), much reminiscent of the Src homology 2 domain function, several isolated PH domains have been shown to bind to phosphoinositides such as phosphatidylinositol-(4,5)-bisphosphate (PIP2) in vitro (Harlan et al., 1994, 1995; Ferguson et al., 1995; Garcia et al., 1995; Hyvönen et al., 1995; Lemmon et al., 1995; Pitcher et al., 1995; Touhara et al., 1995; Wang and Shaw, 1995; Miki et al., 1996; Salim et al., 1996; Zheng et al., 1996; Chen et al., 1997; Frech et al., 1997; Kubiseski et al., 1997). Interestingly, certain PH domains show in vitro binding preference to lipid compounds that are in vivo phosphorylation products of phosphoinositol (PI) 3-kinase (Salim et al., 1996; Franke et al., 1997a,b; Klarlund et al., 1997).
Cytohesin-1 is a 47-kDa intracellular protein that interacts specifically in several systems with the cytoplasmic domain of the leukocyte integrin αLβ2 (CD11a/18, leukocyte functional antigen-1 [LFA-1]) (Kolanus et al., 1996). Cytohesin-1 bears a short amino-terminal domain, which may aid in oligomerization, an extended central homology region, which is similar to the yeast Sec7 protein, and a carboxyl-terminal PH domain, followed by the c domain. Overexpression of cytohesin-1 or subdomain constructs in the Jurkat T cell line E6 was shown to have pronounced effects on the binding of αLβ2 to its ligand, the intercellular adhesion molecule 1 (ICAM-1). Overexpression of full-length cytohesin-1 or of the isolated Sec7 domain in Jurkat cells resulted in a constitutive adhesion of αLβ2, whereas the expression of a cytohesin-1 PH domain construct, which still contained the c domain, specifically inhibited the activation of LFA-1 in a dominant negative manner. Because the PH domain was not found to be mediating the interaction with the integrin cytoplasmic domain, it has been postulated that its unidentified cellular ligand may be an upstream component of the inside-out signaling pathway of αLβ2 (Kolanus et al., 1996).
We have recently shown that an intact PH domain of cytohesin-1 is required for its association with the plasma membrane and that membrane localization of cytohesin-1 can be regulated by PI 3-kinase (Nagel et al., 1998). Furthermore, the PH domains of cytohesin-1 and general receptor for phosphoinositides-1, a close homologue of cytohesin-1, have both been demonstrated to bind phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) (Klarlund et al., 1997). However, affinities of PH domains for their ligands are often in the micromolar range, which appears rather low and leaves open the question of whether these rather weak interactions are sufficient or compatible with observed biological activities in vivo (Shpetner et al., 1996; Patki et al., 1997).
In this study we show by functional and biochemical means, as well as by confocal laser microscopy techniques, that the PH domain of cytohesin-1 specifically mediates membrane association cooperatively with the c domain, a 17-amino-acid stretch that is located carboxyl-terminally adjacent to the PH domain and that is rich in basic residues. The carboxyl terminus is conserved between all members of the cytohesin family that have been described so far (Figure 1). Similar polybasic regions have previously been described to be involved in membrane attachment of cellular or viral proteins (Hancock et al., 1990, 1991; Adamson et al., 1992; Newman et al., 1992; Cadwallader et al., 1994; Mitchell et al., 1994; Ghomashchi et al., 1995; Kwong and Lublin, 1995; Kreck et al., 1996; Soneoka et al., 1997). Both elements, PH domain and c domain, are required to maintain association of cytohesin-1 with the plasma membrane. When the c domain is replaced by the combined isoprenylation–palmitoylation sequence derived from H-ras (Hancock et al., 1991) (termed CAAX motif throughout the article; Figure 2), function is partially retained and membrane association is restored, but targeting specificity is lost, because the fusion protein localizes to a perinuclear membrane compartment in addition to the plasma membrane. Furthermore, an effective membrane association element cannot be generated by grafting the c domain of cytohesin-1 onto the β-adrenergic receptor kinase (βARK) PH domain, thus demonstrating that the plasma membrane localization of cytohesin-1 is achieved by two highly specific plasma membrane interaction elements, the PH domain and the basic c domain. In vitro studies show that the c domain stabilizes the interaction of the PH domain with PIP3 markedly. Although the glutathione S-transferase (GST) fusion protein of the PH domain alone binds to the PIP3 with an affinity of ∼2–3 μM, use of the fusion protein containing the PH domain and the c domain results in high-affinity binding (100 nM).
Figure 1.
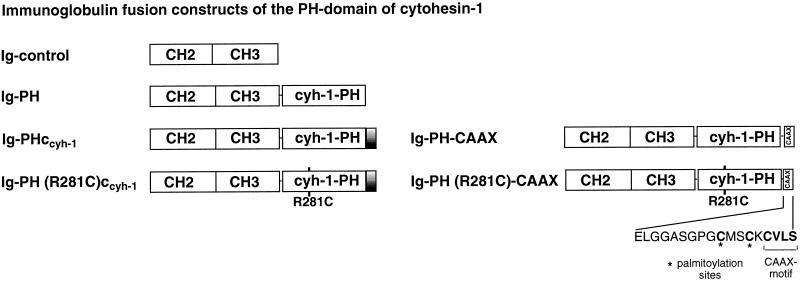
Alignment of the carboxyl-terminal portions (including PH and basic c domains) of human cytohesin-1, cytohesin-2 (also known as ARNO; Chardin et al., 1997), and the homologous murine protein grp-1 (Klarlund et al., 1997). For comparison, the sequence of the carboxyl terminus of the β adrenergic receptor kinase (βARK) is given, which also contains PH and basic domains. The asterisk indicates the position of arginine 281. Arginine and lysine residues of the c domain are shown in bold letters.
Figure 2.
Schematic outline of the cytohesin-1 constructs used in this study.
To further investigate the cooperativity of PH domains with adjacent protein stretches in specific plasma membrane targeting, we introduced the PH domain of Bruton’s tyrosine kinase (Btk) in our study. In this case we could demonstrate as well that the PH domain cooperates with the neighboring protein element, the so-called btk motif in specific plasma membrane association. Interestingly, the zinc-containing btk motif of Btk bears no similarity to the c domain of cytohesin-1.
MATERIALS AND METHODS
Construction of Expression Plasmids
PCR products were derived from existing expression plasmids and inserted into the Mlu1 and NotI sites of the vaccinia expression vector pcIgTkg, subsequently encoding cytoplasmic immunoglobulin (Ig) fusion proteins as described (Kolanus et al., 1996). Specifically, the following primer pairs were used: CGC GGG ACG CGT ACC ATG GGT TTC AAT CCA GAC CGA GAA GGC TGG and CGC GGG GCG GCC GCT TTA GTG TCG CTT CGT GGA GGA GAC CTT (PH); CGC GGG ACG CGT ACC ATG GGT TTC AAT CCA GAC CGA GAA GGC TGG and CGC GGG GCG GCC GCT TTA GTG TCG CTT CGT GGA GGA GAC CTT (PHccyh-1); GCG GGG ACG CGT ACC ATG GAC TAC GCC CTG GGC AAG GAC and GCG GGG GCG GCC GCT TTA CTG CTG GGC CTC GCG GTA GGC GTC (βARK-PH); GCG GGG ACG CGT ACC ATG GAC TAC GCC CTG GGC AAG GAC and GG GCG GGG GCG GCC GCT TTA GAG GCC GTT GGC ACT GCC (βARK-PHcβARK); GCG GGG ACG CGT ACC ATG GAC TAC GCC CTG GGC AAG GAC and GCG GGG GCG GCC GCT TTA GTG TCG CTT CGT GGA GGA GAC CTT CTT TTT CCG TGC TGC GAG CAT TTC GTA CTG CTG GGC CTC GCG GTA GGC GTC (βARK-PH-ccyh-1); CGC GGG ACG CGT ACC ATG GGT TTC AAT CCA GAC CGA GAA GGC TGG and CGC GCG CGG CCG CTT TAG CTC AGC ACG CAC TTG CAG CTC ATG CAG CCG GGG CCG CTG GCG CCC CCG AGC TCG AAA GGG TCC CTG CTG ATG GCT [PH-CAAX and PH (R281C)-CAAX]; CGC GGG ACG CGT GCC ACC ATG GCT GCA GTG ATA CTG GAG AGC and GCG GGG GCG GCC GCT TTA GAT TAC ATT TTT GAG CTG GTG AAT CC (Btk-PH); and CGC GGG ACG CGT GCC ACC ATG GCT GCA GTG ATA CTG GAG AGC and GCG GGG GCG GCC GCT TTA GTT CTC CAA AAT TTG GCA GCC C (Btk-PHbtk motif).
All PCR products were confirmed by double-stranded sequencing. Secreted receptor–globulin fusion proteins of the ICAM-1 extracellular domains were used as described (Kolanus et al., 1996).
Eukaryotic Expression and Adhesion Assay
Vaccinia expression constructs were recombined with wild-type vaccinia virus (WR strain) in CV-1 cells; recombinant plaques were purified; and high-titer virus stocks were generated as described (Romeo and Seed, 1991). The ICAM-1-Rg fusion protein was expressed in COS cells, purified from culture supernatants by protein A-Sepharose, eluted, resuspended in PBS and subsequently coated onto Falcon (Lincoln Park, NJ) 1008 dishes as described (Walz et al., 1990). Jurkat cells (2 × 106) were infected with recombinant viruses and incubated for 4 h at 37°C. After centrifugation cells were resuspended in RPMI medium and incubated for 5 min at 37°C with or without the addition of 40 ng/ml phorbol 12-myristate 13-acetate. Cells were subsequently allowed to adhere to ICAM-1-Rg–coated dishes at 37°C for 10 min, and the bound fraction was determined with the aid of an ocular reticle.
Measurement of Phosphatidylinositol Binding of Various GST-PH Domain Constructs by IAsys Biosensor Technology
PH domains of cytohesin -1 were expressed as (GST) fusion proteins as described (Nagel et al., 1998). An optical evanescence resonant mirror cuvette system (IAsys, Affinity Sensors, Cambridge, United Kingdom) was used to measure interaction of GST fusion proteins with phosphatidylinositol-(3,4,5)-triphosphate (PIP3). A lipid monolayer containing 70% (wt/wt) β-palmitoyl-γ-oleoyl-l-α-phosphatidylcholine (POPC), 30% (wt/wt) dioleoyl-l-α-phosphatidyl-dl-glycerol, or a lipid mixture of 60% (wt/wt) palmitoyl-γ-oleoyl-l-α-phosphatidylcholine and 30% (wt/wt) and 10% (wt/wt) PIP3 (Mantreya, Pleasant Gap, PA) was mounted on an IAsys hydrophobic sensor surface (FCH-0601) at 0.1 mg/ml lipid. The cuvette was subsequently washed with 0.1 M HCl, PBS, and 10 mM NaOH. After the final wash with PBS, the cuvette was equilibrated in binding buffer (PDI: PBS, 2 mM DTT, 0.001% [vol/vol] Igepal CA-630, Sigma), and affinity-purified GST fusion proteins dissolved in binding buffer were added. The binding of various concentrations of the GST-PH domain fusion proteins were monitored for 5 min. Dissociation was initiated by adding PDI to the cuvette. Determination of the association equilibrium constant was done by equilibrium titration. The interaction profiles for each protein concentration were analyzed using FASTfit kinetics analysis software supplied with the instrument.
Cellular Fractionation
Cells that had been infected with recombinant vaccinia viruses were collected by centrifugation and resuspended on ice in 0.5 ml ice-cold hypotonic solution (HS: 10 mM HEPES, pH 7.5, 10 mM KCl, 10 mM MgCl2, 0.5 mM DTT) containing 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF. Fractionation of cells was performed as described (Meller et al., 1996). Briefly, cells were sheared, the nuclei were removed by centrifugation at 1000 × g for 10 min, and the supernatant cytosol was collected. The cytosolic fraction was brought to a final concentration of 1% (vol/vol) Igepal CA-630 (Sigma, St. Louis, MO; identical to Nonidet P-40) and 150 mM NaCl and used directly for immunoprecipitation. The pellet was resuspended, washed with 0.5 ml HS, and centrifuged at 15,000 × g for 15 min. The resulting pellet was resuspended in HS containing 1% (vol/vol) Igepal CA-630 and 150 mM NaCl, and centrifuged again, and the supernatant representing the particulate fraction was subjected to immunoprecipitation. For precipitation of the Ig fusion (Ig) proteins fractions were incubated with protein A-Sepharose 6 MB beads (Pharmacia, Piscataway, NJ) for 2 h at 4°C. Then beads were washed with 1 ml HS containing 1% (vol/vol) Igepal CA-630 and 150 mM NaCl, and immunoprecipitates were resolved by 10% SDS-PAGE and analyzed by standard Western blot techniques. Specifically, proteins were blotted onto nitrocellulose and detected by a primary mouse polyclonal antibody preparation that had been raised against the intracellular CH2 and CH3 domains (Kolanus, unpublished data). A peroxidase-conjugated anti-mouse IgG antibody (Jackson ImmunoResearch, West Grove, PA) and subsequent ECL reaction (Amersham, Arlington Heights, IL) were used for visualization of the bands. To assess that cytoplasmic contents were not trapped in the particulate fraction, lactate dehydrogenase activities were monitored as described (Ma et al., 1997).
Confocal Laser Scanning Microscopy
Six hours after infection of Jurkat E6 cells with recombinant vaccinia viruses, cells were placed on poly-l-lysine–covered microscope slides for 1 h in a humidified chamber at 37°C. Then nonadherent cells were washed off with HBSS, and adherent cells were fixed and immobilized with freshly prepared 3% (wt/vol) paraformaldehyde in PBS overnight at 4°C. Subsequently, cells were permeabilized for 15 min with 0.2% (vol/vol) Triton X-100 in PBS, blocked with 2% (wt/vol) glycine in PBS, and incubated with an FITC-labeled goat anti-human IgG (Fcγ specific; Jackson ImmunoResearch) antibody at 1:100 dilution in PBS for 2 h at room temperature. After the final wash with PBS, slides were mounted on a 9:1 mixture of glycerol and PBS (pH 9.0) containing n-propyl-gallate at 20 mg/ml as an antifading reagent. Cells were examined using a confocal laser scanning microscope (TCS-NT system; Leica, Nussloch, Germany) attached to a Leica DMIRB inverted microscope with a plane apochromatic objective 63×, 1.32 oil immersion objective. Confocal images were collected as 512 × 512 pixel files and processed with the help of the Photoshop program (Adobe Systems, San Jose, CA).
RESULTS
PH Domain and C Domain of Cytohesin-1 Are Both Required for Membrane Association and Dominant Negative Inhibition of T Cell Adhesion
We have previously shown that the PH domain of cytohesin-1 is required for its association with the plasma membrane, because a point mutant of the PH domain (R281C) abrogated both membrane association as well as in vitro binding to PIP3 (Nagel et al., 1998). Furthermore, overexpression of a recombinant PH domain construct that retained the 17-amino acid carboxyl terminus of cytohesin-1 resulted in a dominant negative abrogation of LFA-1-mediated adhesion to its counter-receptor ICAM-1 (Kolanus et al., 1996). The dominant negative effect has been shown to be correlated with competitive inhibition of the membrane attachment of endogenous cytohesin-1 and therefore serves as a valid correlate of cellular function (Nagel et al., 1998).
In this study, we attempted to dissect the functional relationship between two structural elements of cytohesin-1, the PH domain and the adjacent c domain. Consequently, a construct was made in which the c domain was deleted from the PH domain context (Ig-PH). This fusion protein was subsequently examined with respect to its effect on cell adhesion and membrane association. For comparison, the entire carboxyl-terminal region of cytohesin-1 (Ig-PHccyh-1) or a point mutant derivative thereof [Ig-PH (R281C)ccyh-1], containing the previously described, functionally inactivated PH domain, were used (Nagel et al., 1998). These and the constructs described below were expressed in the Jurkat E6 (T cell leukemia) line with the help of recombinant vaccinia viruses (Figure 2). They all contain amino-terminal Ig domains for convenient immunoprecipitation and detection (Kolanus et al., 1996). LFA-1-mediated adhesion was monitored by specific binding of the cells to an immobilized ICAM-1 Ig fusion protein (Kolanus et al., 1996).
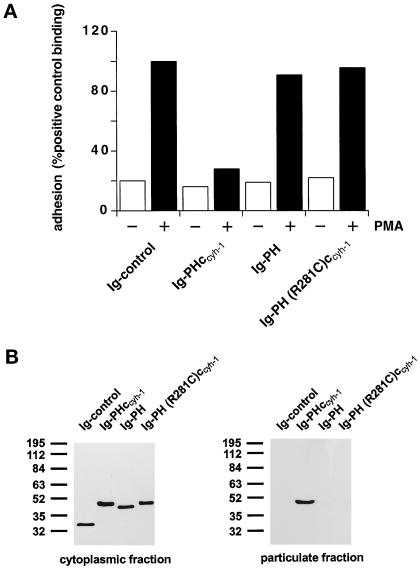
Figure 3 confirms that an intact PH domain is necessary for membrane association (Figure 3B) and dominant negative inhibition of LFA-1 adhesion to ICAM-1 (Figure 3A), because the R281C mutation abrogates both functions. Thus, the PH domain alone is not sufficient for both membrane association and function, because it requires the presence of the c domain (Figure 3, A and B). The c domain alone, used in the context of an inactive PH domain [Ig-PH (R281C)ccyh-1], was not sufficient for membrane association and had no effect on T cell adhesion (Figure 3, A and B). It therefore appears that the PH domain and c domain are both responsible for membrane association of cytohesin-1.
Figure 3.
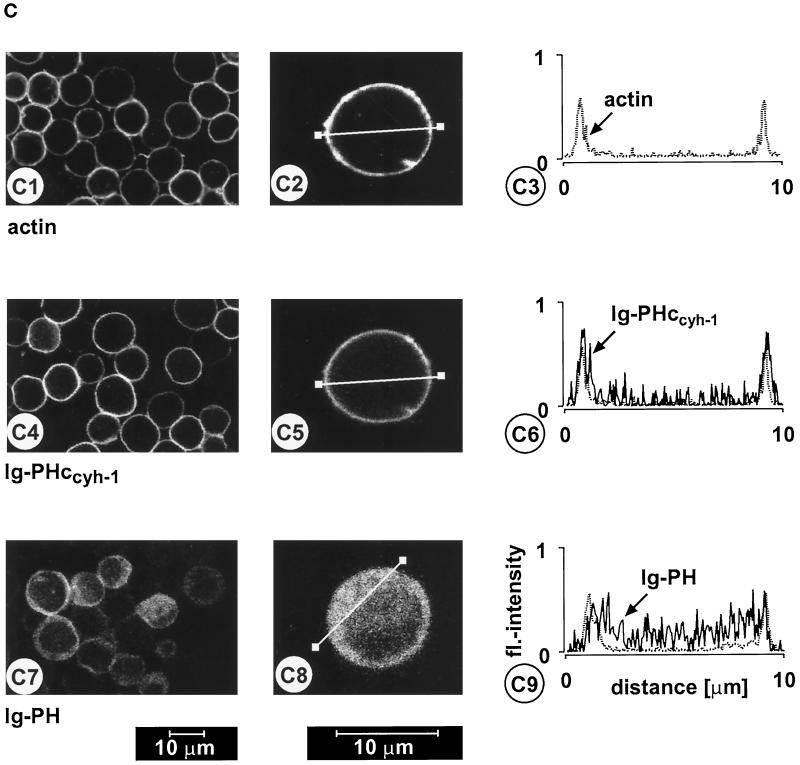
(A) Adhesion assay. The intact PH domain and the adjacent c domain of cytohesin-1 are both required for dominant inhibition of Jurkat cell adhesion to ICAM-1. Cytoplasmic Ig fusion constructs were expressed using recombinant vaccinia viruses, and the adhesion assay was performed as described in MATERIALS AND METHODS. Normalization was performed against the positive control (Ig-control + PMA). (B) Cellular fractionation assay. The intact PH domain and the adjacent c domain of cytohesin-1 are both required for particulate association in Jurkat cells. “Particulate fraction” denotes a crude lysate of cellular membranes. The Ig-control, which is exclusively expressed in the cytoplasm, serves as an internal control for cellular fractionation. (C facing page) Confocal laser scans of the subcellular localization of cytohesin-1 subdomain constructs Ig-PH (C7–C9) and Ig-PHccyh-1 (C4–C6), respectively. Ig fusion proteins were visualized with an FITC-conjugated anti-human IgG Fcγ-specific antibody. For quantitation of subcellular localization, cells were double stained with FITC-labeled anti-Ig (Fcγ) for the respective fusion protein and with TRITC-labeled phalloidin for the visualization of actin. Actin only is shown in C1–C3. Staining intensities measured according to pixel brightness were quantified along cell transects for each construct in a representative positively stained cell. The large, central unstained region visible in C8 is due to the nucleus.
PH Domain and C Domain of Cytohesin-1 Determine Predominant Targeting Specificity to the Plasma Membrane
We and others have shown that cytohesin-1 PH domain binds to the phosphoinositide PIP3 in vitro (Klarlund et al., 1997; Nagel et al., 1998) and, consistent with this notion, that its association with membranes is possibly regulated by PI 3-kinase in vivo. Figure 3C shows that PH and c domains are simultaneously required for predominant plasma membrane association in Jurkat cells. Confocal laser scanning microscopy was used to show that the PH domain alone was localized in the cytoplasm, whereas the PHccyh construct colocalized very well with actin, a cytoskeletal protein that resides at the inner leaflet of the plasma membrane.
In Vitro Binding of the Carboxyl-Terminal Domains of Cytohesin-1 to Phosphatidylinositol-(3,4,5)-Triphosphate
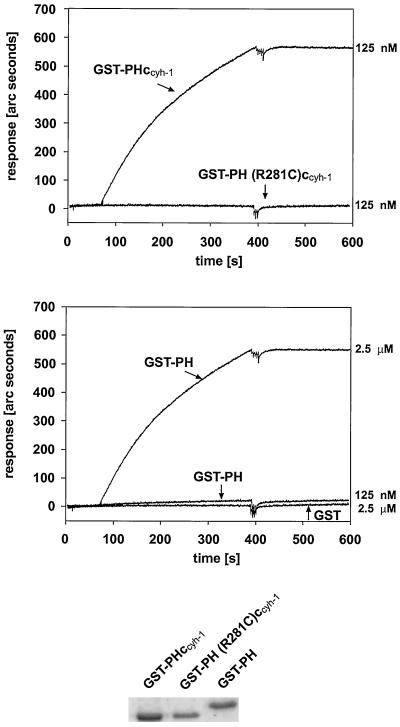
How does the c domain aid the PH domain in plasma membrane association? Although PH domains alone can bind to inositol-(1,3,4,5)-tetrakisphosphate or PIP3, it is possible that the positively charged c domain stabilizes this interaction. We used IAsys interaction measurement technology to determine the relative affinities of the PH domain or of PHccyh-1 for PIP3, which had been mounted on a hydrophobic sensor surface. Figure 4 shows that GST fusion proteins containing either the PH domain alone or the PH domain including the c domain can both bind to PIP3, confirming that the PH domain is sufficient for phosphoinositide binding in vitro. Quantitative analyses showed that there are considerable differences in affinity. Although a 125 nM preparation of GST-PHccyh-1 bound to PIP3 with a fast on-rate and very slow off-rate, there was basically no binding of GST-PH at that concentration. At 2.5 μM, however, GST-PH bound to PIP3 with similar kinetics as GST-PHccyh-1. It therefore appears that the c domain enhances the on-rate of the interaction. Once the proteins were bound to the phospholipid they dissociated very slowly. We then determined the affinities of either GST-PHccyh-1 or GST-PH for PIP3. This was done by measuring binding to PIP3 at various protein concentrations (our unpublished results). We found half-maximal binding to PIP3 using 100 nM GST-PHccyh-1 or 3 μM GST-PH, respectively. Although the number for GST-PH is less exact, we conclude that there is at least one order of magnitude difference between the affinites of the two fusion proteins for PIP3, which may well account for the observed biological effects.
Figure 4.
Affinities of GST-PHccyh-1 and GST-PH for immobilized PIP3 were measured using IAsys biosensor technology. The top panel shows high-affinity binding of a 125 nM solution of GST-PHccyh-1, whereas the middle panel demonstrates that GST-PH has to be used at micromolar concentrations to detect binding. The bottom part shows Coomassie stains of the purified fusion proteins on 10% SDS-PAGE. GST-PH (R281C)ccyh-1 or GST, respectively, does not bind to PIP3 at any concentration. None of the fusion proteins used bind to control cuvette surfaces containing the lipid moiety without PIP3 (our unpublished results).
The c Domain Does Not Support the Membrane Association of a Heterologous PH Domain
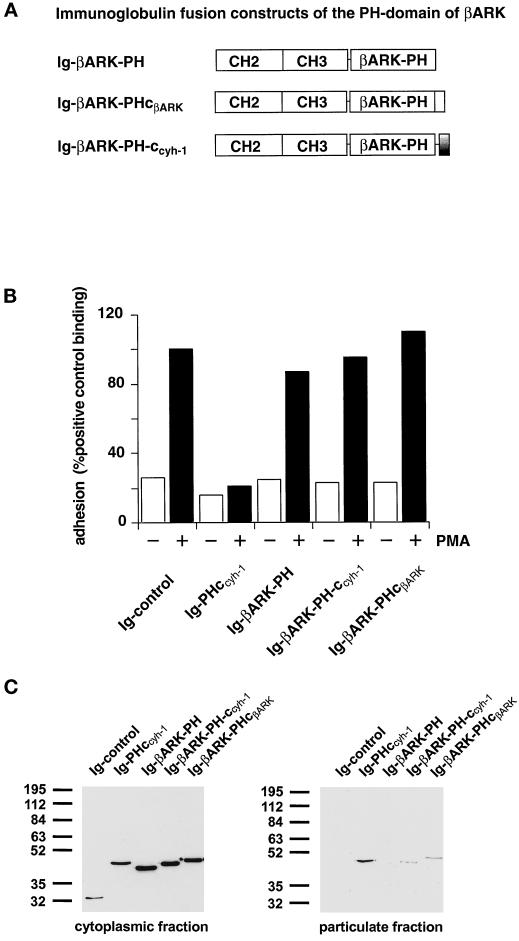
We went on to determine whether the cytohesin-1 c domain specifically complements the cytohesin-1 PH domain, or whether it would also cooperate with a different PH domain in membrane association. The rationale behind this is that the c domain is positively charged and may therefore nonspecifically aid PH domains in binding negatively charged membrane phospholipids. A similar, nonspecific auxiliary contribution to membrane association has been suggested for charged elements in phospholipase Cβ1, which resemble the c domain (Kim et al., 1996). The PH domain of βARK was chosen because it has been described to bind PIP2 in vitro (Pitcher et al., 1995) and has also therefore been implicated in membrane association. Moreover, wild-type βARK also contains a polybasic stretch, carboxyl-terminally adjacent to the PH domain (Figures 1 and 5A). Consequently, the c domain of cytohesin-1 was grafted onto the βARK PH domain, and the resulting fusion protein (Ig-βARK-PH-ccyh-1) was compared with the wild-type βARK constructs (Ig-βARK-PH and Ig-βARK-PHcβARK) for its ability to interfere with LFA-1-mediated adhesion and for its capacity to associate with membranes. Figure 5C shows that the wild-type PH domain of βARK does not associate with membranes. Addition of either the wild-type c domain of βARK or of the cytohesin-1 PH domain supports membrane association of the respective fusion proteins to a minor but reproducible extent. However, confocal laser scanning microscopy confirmed that both proteins were predominantly expressed in the cytoplasm (Figure 5D). As expected from these data, neither fusion protein had a significant effect on Jurkat cell adhesion compared with the cytohesin-1 Ig-PHccyh-1 construct (Figure 5B). Thus, it appears that the carboxyl-terminal elements of cytohesin-1 cooperate specifically in membrane association and cellular function.
Figure 5.
(A) Outline of the βARK-PH domain constructs used in this part of the study. In the Ig-βARK-PH-ccyh-1 construct, the c domain of cytohesin-1 has been fused to the PH domain of βARK. (B) Adhesion assay of βARK-PH fusion proteins. Neither the wild-type βARK-PH domain and polybasic c-terminus nor a fusion protein of the βARK-PH domain with the c domain of cytohesin-1 (Ig-βARK-PH-ccyh-1) interferes with induced adhesion of Jurkat cells to ICAM-1. (C) Cellular fractionation of βARK-PH fusion proteins. Polybasic elements of either βARK or cytohesin-1 do not cooperate significantly with the βARK-PH domain in membrane association. (D facing page) Confocal laser scans of the subcellular distribution of βARK-PH fusion proteins. All tested fusion proteins are expressed in the cytoplasm.
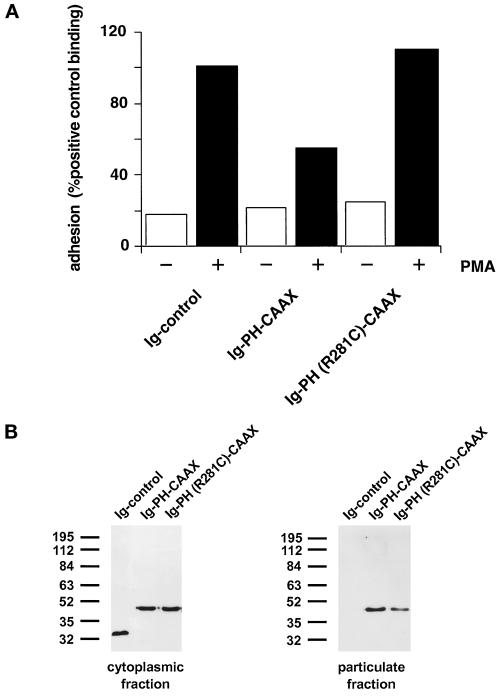
Substitution of the c Domain by a Heterologous Membrane-targeting Element (CAAX Motif) Leads to Unspecific Association with Various Intracellular Membranes and Therefore Results in Incomplete Functional Complementation
We replaced the c domain of cytohesin-1 with the CAAX motif from H-ras, known to be sufficient for membrane association in cells, and tested the respective constructs [Ig-PH-CAAX and Ig-PH (R281C)-CAAX] in both systems. Biochemical analyses showed that both CAAX constructs retained the ability to associate with cellular membranes, even if the PH domain was inactivated (Figure 6B). Although the Ig-PH (R281C)-CAAX construct did readily partition into the particulate (membrane) fraction (Figure 6B), it did not exert dominant negative inhibition of Jurkat cell adhesion to ICAM-1 (Figure 6A). This suggests that the cellular function of the cytohesin-1 PH domain requires proper in vivo ligand binding and is not only dependent on its expression in the membrane fraction per se. The intact Ig-PH-CAAX fusion protein, on the other hand, did partially retain in vivo activity with respect to dominant negative inhibition of Jurkat cell adhesion to ICAM-1, thus demonstrating that the Ig-PH-CAAX fusion protein was functional in principle (Figure 6A).
Figure 6.
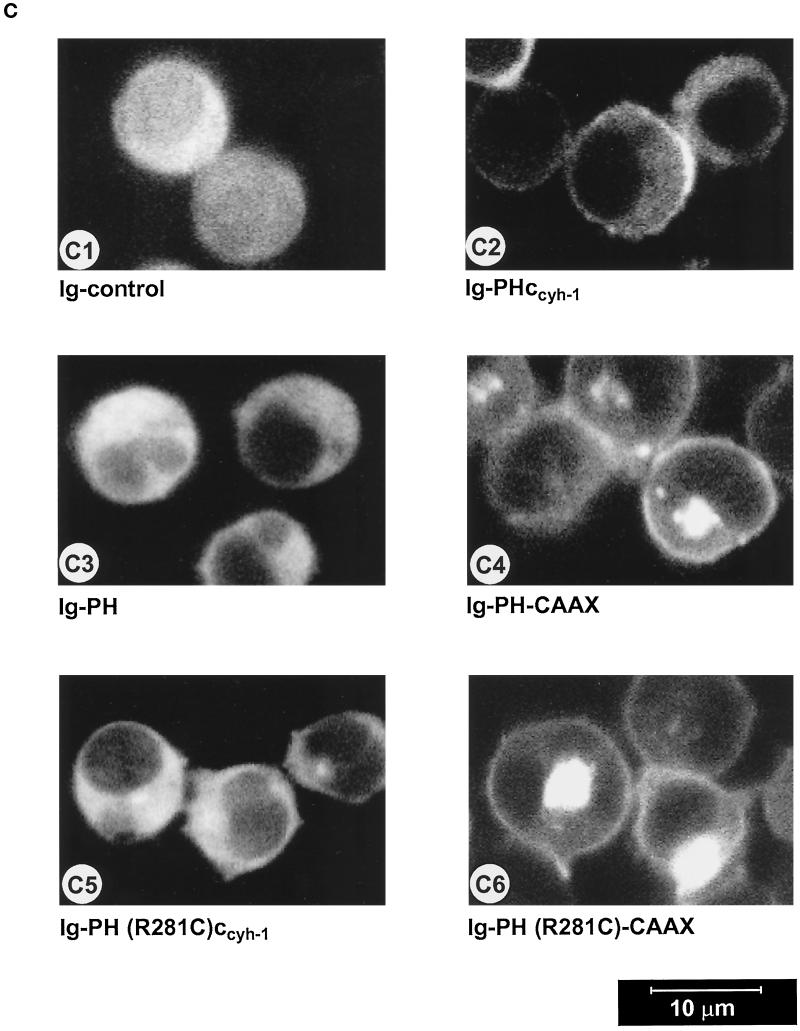
(A) Adhesion assay of cytohesin-1 PH-CAAX chimeras. Replacement of the c domain with a CAAX motif partially restores the dominant inhibition of Jurkat cell adhesion by the PH domain of cytohesin-1. (B) Cellular fractionation of cytohesin-1 PH-CAAX chimeras. The CAAX motif is sufficient for particulate association of cytoplasmic Ig fusion proteins in Jurkat cells. (C facing page) Confocal laser scans of the subcellular distribution of various intracellular Ig fusion proteins derived from cytohesin-1. C1 (Ig control), C3, (Ig-PH), and C5 [Ig-PH (R281C)ccyh-1] show diffuse cytoplasmic localization of the Ig chimeras. In addition to some cytoplasmic staining, C2 (Ig-PHccyh1) exhibits pronounced plasma membrane staining of the respective chimera due to intact PH and c domains. PH domain chimeras containing the CAAX motif Ig-PH-CAAX (C4) and Ig-PH (R281C)-CAAX (C6) appear both in the plasma membrane and within a perinuclear compartment. Ig fusion proteins were visualized with an FITC-conjugated anti-human IgG Fcγ-specific antibody.
Confocal laser scanning microscopy experiments were conducted to elucidate the subcellular localization of the fusion proteins. Figure 6C2 shows that the Ig-PHccyh-1 construct is both found in the cytoplasm and associated with the plasma membrane. On the other hand, Ig-PH (Figure 6C3) as well as Ig-PH (R281C)ccyh-1 (Figure 6C5) are only detected in the cytoplasm, consistent with all functional and biochemical analyses above. The CAAX constructs (Figure 6, C4 and C6) were present in multiple membrane compartments, predominantly perinuclear membranes and also plasma membrane, appearing very different in cellular distribution if compared with the wild-type domains. It is apparent that the CAAX motif targets the fusion proteins to various cellular membrane compartments in a nonspecific manner. Although it was initially described that the combined palmitoylation–isoprenylation sequence is sufficient for plasma membrane association (Hancock et al., 1991), a different report showed that fusion proteins containing this element can also be detected in the Golgi apparatus, the latter finding being in concordance with our results (D’Souza and Stahl, 1995).
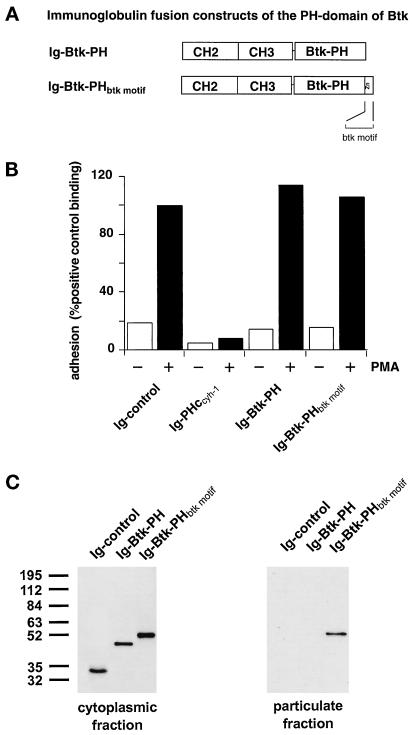
The PH Domain of Bruton’s Tyrosine Kinase Requires the Carboxyl-Terminally Adjacent Btk Motif for Membrane Association
Because many PH domains bind phosphatidylinositols with low affinity, we thought that the functional cooperativity of PH domains with adjacent amino acid stretches might be a common paradigm for PH domain function. Therefore, we investigated the membrane association of the PH domain of Btk in the presence or absence of the adjacent Btk motif. In perfect analogy to the findings with cytohesin-1, we found that the Btk-PHbtk motif structure associated extremely well with the plasma membrane, whereas the Btk-PH domain alone failed to do so (Figure 7, C and D). Interestingly, the Btk-PHbtk motif structure did not block LFA-1-mediated cell adhesion (Figure 7B), despite its predominant plasma membrane localization. This indicates that cytohesin-1 and Btk bind to highly distinct plasma membrane ligands, at least in Jurkat cells. The c domain of cytohesin-1 was also grafted onto the PH domain of Btk, but as in the case of the βARK-PH domain, no restoration of membrane association was observed (our unpublished results). Again, this stresses the notion that the cooperativity of PH domains with intramolecular motifs like the c domain or the btk motif is highly specific and probably dependent on tight structural constraints.
Figure 7.
(A) Construct outline. (B) Adhesion assay. PH domain constructs of Btk do not inhibit Jurkat cell adhesion to ICAM-1. (C) Cellular fractionation. The btk motif is required for membrane association of the Btk-PH domain. (D facing page) Confocal laser scans according to Figure 3C. The PH domain and btk motif are simultaneously required for membrane association.
DISCUSSION
In this paper we show that the PH domain of cytohesin-1 and a carboxyl-terminal, positively charged sequence element coordinately mediate correct subcellular targeting, as well as functional specificity. PH domains have been suggested to participate in membrane recruitment of proteins (Garcia et al., 1995; Wang and Shaw, 1995; Wang et al., 1996, 1997; Chen et al., 1997; Ma et al., 1997; Michiels et al., 1997). This assumption was supported by the finding that several PH domains were found to bind PIP2, other phosphoinositides, and soluble inositol phosphates in vitro (see INTRODUCTION and references therein). However, the same finding led to the obvious question of targeting specificity, because it appeared highly implausible that the majority of proteins that contain PH domains are attached to cellular membranes by PIP2 in vivo. The finding that most—but not all—PH domains bind PIP2 with rather low affinity in vitro also ruled against this structure as a commonly used ligand for PH domains in vivo (Lemmon et al., 1997).
Our work presented here uses genetic fusion protein technology as well as functional, biochemical, and immunofluorescence techniques to show that two elements of cytohesin-1, the PH domain and the basic c domain, cooperate specifically in directing the protein to the plasma membrane compartment. Both domains were shown to be simultaneously required for plasma membrane association. Replacement of the c domain by an isoprenylation motif partially restored the cellular function of the PH domain, but the precise targeting specificity was lost, because the resulting fusion protein was also found in a perinuclear membrane compartment. Moreover, grafting of the c domain onto the βARK-PH domain or onto the Btk-PH domain was not sufficient to confer membrane association of the resulting fusion proteins or interference with β2 integrin function in Jurkat cells. These findings further support the view that cooperation of PH domains with additional membrane recruitment elements can result in a remarkable specificity of cellular localization and function. However, at this point it cannot be ruled out that the c domain of cytohesin-1 may be capable of aiding other PH domains in membrane association. A previous report also showed specific cooperation between a polybasic sequence and myristoylation of K-ras in plasma membrane association (Cadwallader et al., 1994).
Does the c domain bind a distinct ligand, or does it support PIP3 binding by the PH domain? In vitro binding studies revealed enhanced binding of the PH and c domains to PIP3 compared with the isolated PH domain. These findings would argue against a distinct, as of yet unidentified, ligand for the c domain, which is located at the inner leaflet of the plasma membrane, but rather for a stabilization role and maybe a regulatory function of the c domain in PIP3 binding. This does not appear to be a mere charge compensation effect, because at least two other PH domains do not seem to profit from the attachment of the cytohesin-1 c domain to their carboxyl terminus with respect to membrane association inside the cell.
We found that the PH domain of Btk required the presence of the btk motif for membrane association. The btk motif is not homologous to the c domain and is not polybasic. In fact, it shifts the isoelectric point of the protein to more acidic values, again suggesting a structurally defined contribution to ligand binding by the PH domain, a view that is supported by the resolution of Btk-PH and btk motif crystal structure (Hyvönen and Saraste, 1997). This study revealed that the btk motif resides in close contact with the PH domain and may therefore modulate ligand binding. Remarkably, overexpression of the Btk-PH domainbtk motif construct did not inhibit LFA-1 binding to ICAM-1 at all. This finding points to distinct ligand uses of cytohesin-1 and Btk. The PH domain of Btk also binds PIP3 in vitro but apparently binds higher-order phosphorylation products of phosphatidylinositol, too (Fukada et al., 1996).
Taken together, our findings support a general explanation for the in vivo membrane-targeting specificity of signaling proteins: cooperativity of interaction elements.
ACKNOWLEDGMENTS
We E.-L. Winnacker for continuing support and members of the lab for discussion and advice. In addition, we thank Bob Davies at Affinity Sensors (Cambridge, United Kingdom) for the donation of hydrophobic cuvettes and for technical advice. This work was supported by the Deutsche Forschungsgemeinschaft (Ko-1034/2-2) and the BMBF.
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwallader KA, Paterson H, Macdonald SG, Hancock JF. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol Cell Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, Paris S, Antonny B, Robineau S, Beraud Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1997;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- Chen R-H, Corbalan-Garcia S, Bar-Sagi D. The role of the PH domain in the signal dependent membrane targeting of Sos. EMBO J. 1997;16:1351–1359. doi: 10.1093/emboj/16.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza SC, Stahl PD. Myristoylation is required for the intracellular localization and endocytic function of ARF6. Exp Cell Res. 1995;221:153–159. doi: 10.1006/excr.1995.1362. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell. 1995;83:1037–1046. doi: 10.1016/0092-8674(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997a;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997b;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- Fukada M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the Pleckstrin homology domain of Bruton’s tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5 tetrakisphosphate binding capacity. J Biol Chem. 1996;271:30303–30306. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- Garcia P, Gupta R, Shah S, Morris AJ, Rudge SA, Scarlata S, Petrova V, McLaughlin S, Rebecchi MJ. The pleckstrin homology domain of phospholipase C-delta 1 binds with high affinity to phosphatidylinositol 4,5-bisphosphate in bilayer membranes. Biochemistry. 1995;34:16228–16234. doi: 10.1021/bi00049a039. [DOI] [PubMed] [Google Scholar]
- Ghomashchi F, Zhang X, Liu L, Gelb MH. Binding of prenylated and polybasic peptides to membranes: affinities and intervesicle exchange. Biochemistry. 1995;34:11910–11918. doi: 10.1021/bi00037a032. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- Harlan JE, Yoon HS, Hajduk PJ, Fesik SW. Structural characterization of the interaction between a pleckstrin homology domain and phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1995;34:9859–9864. doi: 10.1021/bi00031a006. [DOI] [PubMed] [Google Scholar]
- Hyvönen M, Macias MJ, Nilges M, Oschkinat H, Saraste M, Wilmanns M. Structure of the binding site for inositol phosphates in a PH domain. EMBO J. 1995;14:4676–4685. doi: 10.1002/j.1460-2075.1995.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvönen M, Saraste M. Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. EMBO J. 1997;16:3396–3404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CG, Park D, Rhee SG. The role of carboxyl-terminal basic amino acids in Gqalpha-dependent activation, particulate association, and nuclear localization of phospholipase C-beta1. J Biol Chem. 1996;271:21187–21192. doi: 10.1074/jbc.271.35.21187. [DOI] [PubMed] [Google Scholar]
- Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Kreck ML, Freeman JL, Abo A, Lambeth JD. Membrane association of Rac is required for high activity of the respiratory burst oxidase. Biochemistry. 1996;35:15683–15692. doi: 10.1021/bi962064l. [DOI] [PubMed] [Google Scholar]
- Kubiseski TJ, Chook YM, Parris WE, Rozakis AM, Pawson T. High affinity binding of the pleckstrin homology domain of mSos1 to phosphatidylinositol (4,5)-bisphosphate. J Biol Chem. 1997;272:1799–1804. doi: 10.1074/jbc.272.3.1799. [DOI] [PubMed] [Google Scholar]
- Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun. 1995;207:868–876. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Falasca M, Ferguson KM, Schlessinger J. Regulatory recruitment of signalling molecules to the cell membrane by pleckstrin homology domains. Trends Cell Biol. 1997;7:237–242. doi: 10.1016/S0962-8924(97)01065-9. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, O’Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- Ma AD, Brass LF, Abrams CS. Pleckstrin associates with plasma membranes and induces the formation of membrane projections: requirements for phosphorylation and the NH2-terminal PH domain. J Cell Biol. 1997;136:1071–1079. doi: 10.1083/jcb.136.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller N, Liu YC, Collins TL, Bonnefoyberard N, Baier G, Isakov N, Altman ARA. Direct interaction between protein kinase c theta (pkc theta) and 14 3 3 tau in t cells: 14 3 3 overexpression results in inhibition of pkc theta translocation and function. Mol Cell Biol. 1996;16:5782–5791. doi: 10.1128/mcb.16.10.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls VSL, Feltkamp CA, Collard JG. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Farh L, Marshall TK, Deschenes RJ. A polybasic domain allows nonprenylated Ras proteins to function in Saccharomyces cerevisiae. J Biol Chem. 1994;269:21540–21546. [PubMed] [Google Scholar]
- Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in structural pathwork of signalling protein. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Nagel W, Zeitlmann L, Schilcher P, Geiger C, Kolanus W. Phosphoinositide 3-OH kinase activates the beta-2 integrin adhesion pathway and induces the recruitment of cytohesin-1. J Biol Chem. 1998;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- Newman CM, Giannakouros T, Hancock JF, Fawell EH, Armstrong J, Magee AI. Post-translational processing of Schizosaccharomyces pombe YPT proteins. J Biol Chem. 1992;267:11329–11336. [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–579. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Salim K, et al. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneoka Y, Kingsman SM, Kingsman AJ. Mutagenesis analysis of the murine leukemia virus matrix protein: identification of regions important for membrane localization and intracellular transport. J Virol. 1997;71:5549–5559. doi: 10.1128/jvi.71.7.5549-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Koch WJ, Hawes BE, Lefkowitz RJ. Mutational analysis of the pleckstrin homology domain of the beta-adrenergic receptor kinase. Differential effects on G beta gamma and phosphatidylinositol 4,5-bisphosphate binding. J Biol Chem. 1995;270:17000–17005. doi: 10.1074/jbc.270.28.17000. [DOI] [PubMed] [Google Scholar]
- Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Wang DS, Deng T, Shaw G. Membrane binding and enzymatic activation of a Dbl homology domain require the neighboring pleckstrin homology domain. Biochem Biophys Res Commun. 1997;234:183–189. doi: 10.1006/bbrc.1997.6589. [DOI] [PubMed] [Google Scholar]
- Wang DS, Miller R, Shaw R, Shaw G. The pleckstrin homology domain of human beta I sigma II spectrin is targeted to the plasma membrane in vivo. Biochem Biophys Res Commun. 1996;225:420–426. doi: 10.1006/bbrc.1996.1189. [DOI] [PubMed] [Google Scholar]
- Wang DS, Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1,4,5 triphosphate binding site. Biochem Biophys Res Commun. 1995;217:608–615. doi: 10.1006/bbrc.1995.2818. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]