Abstract
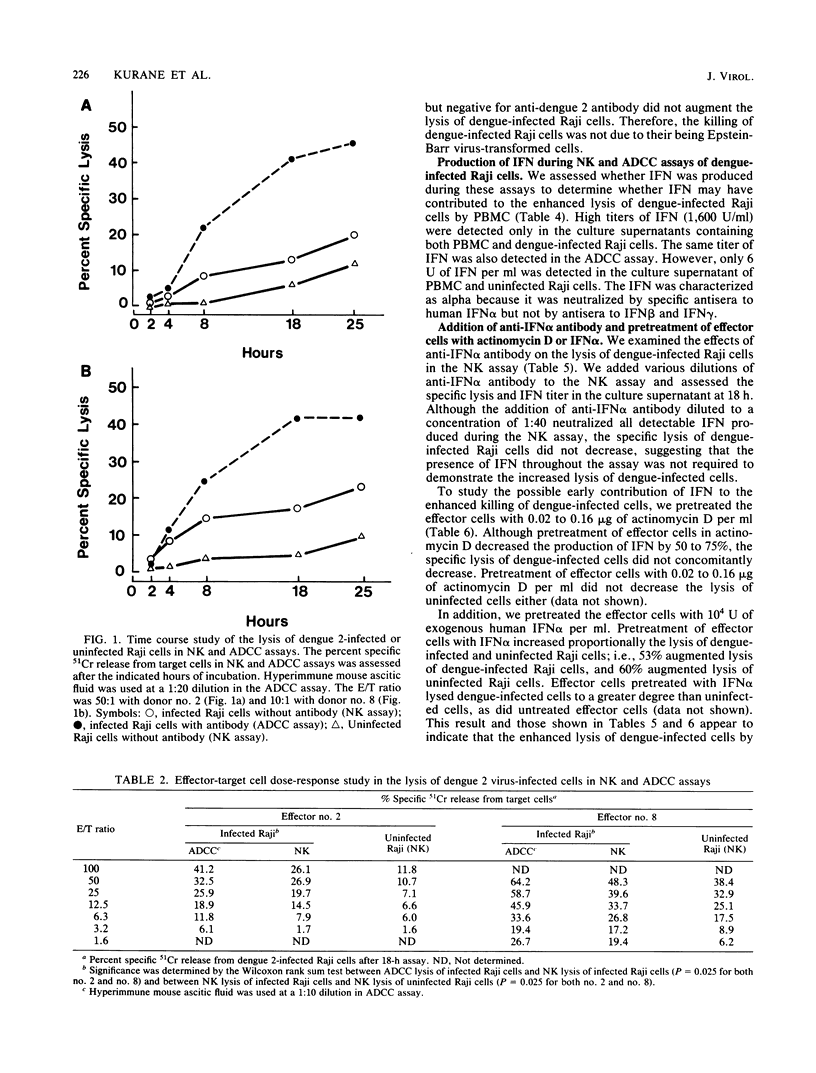
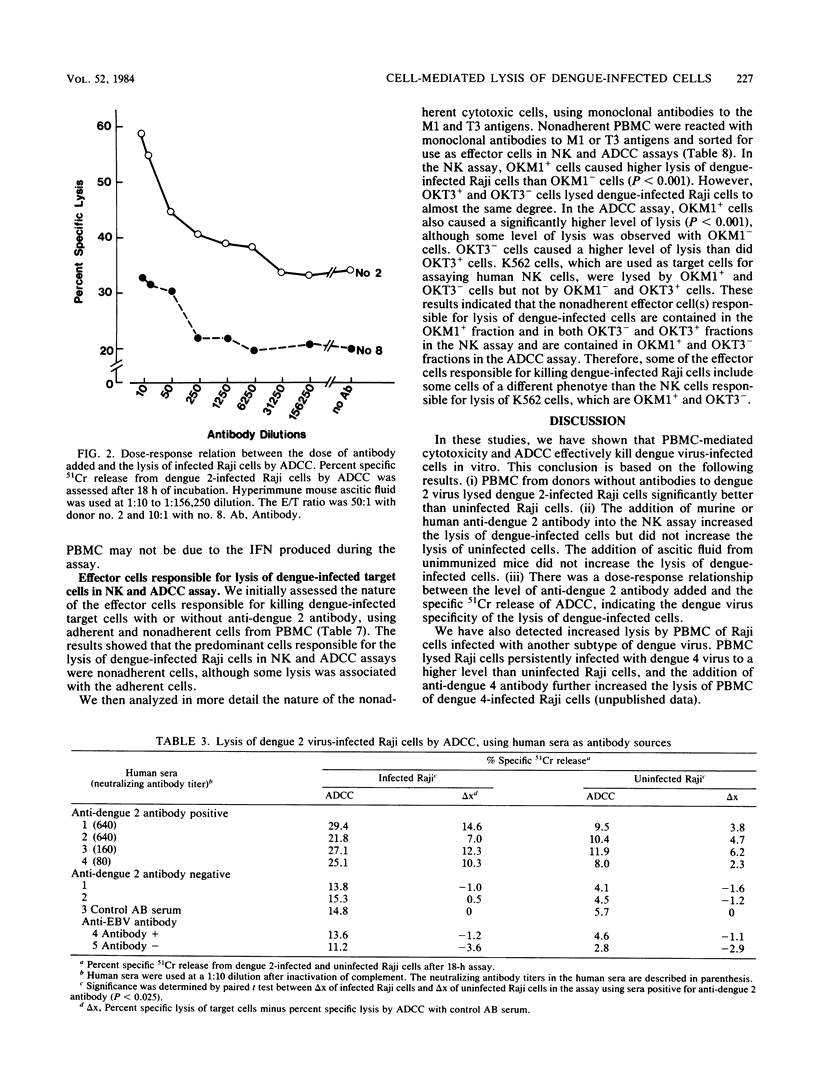
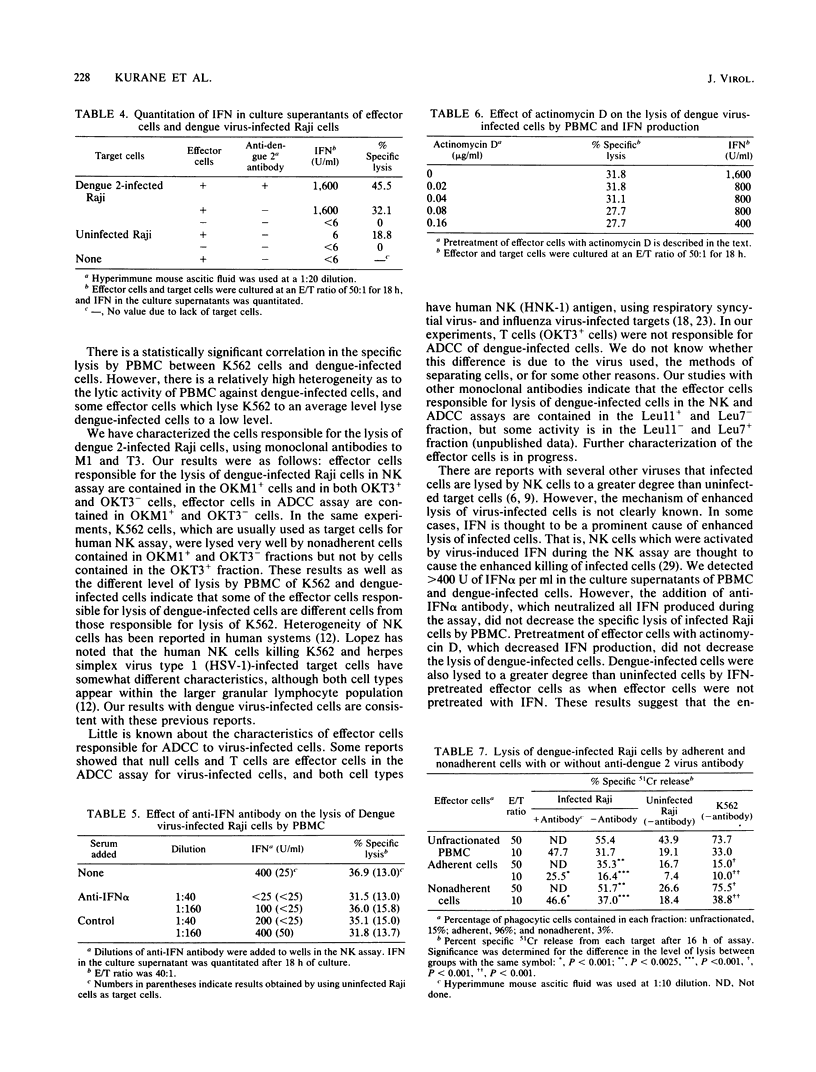
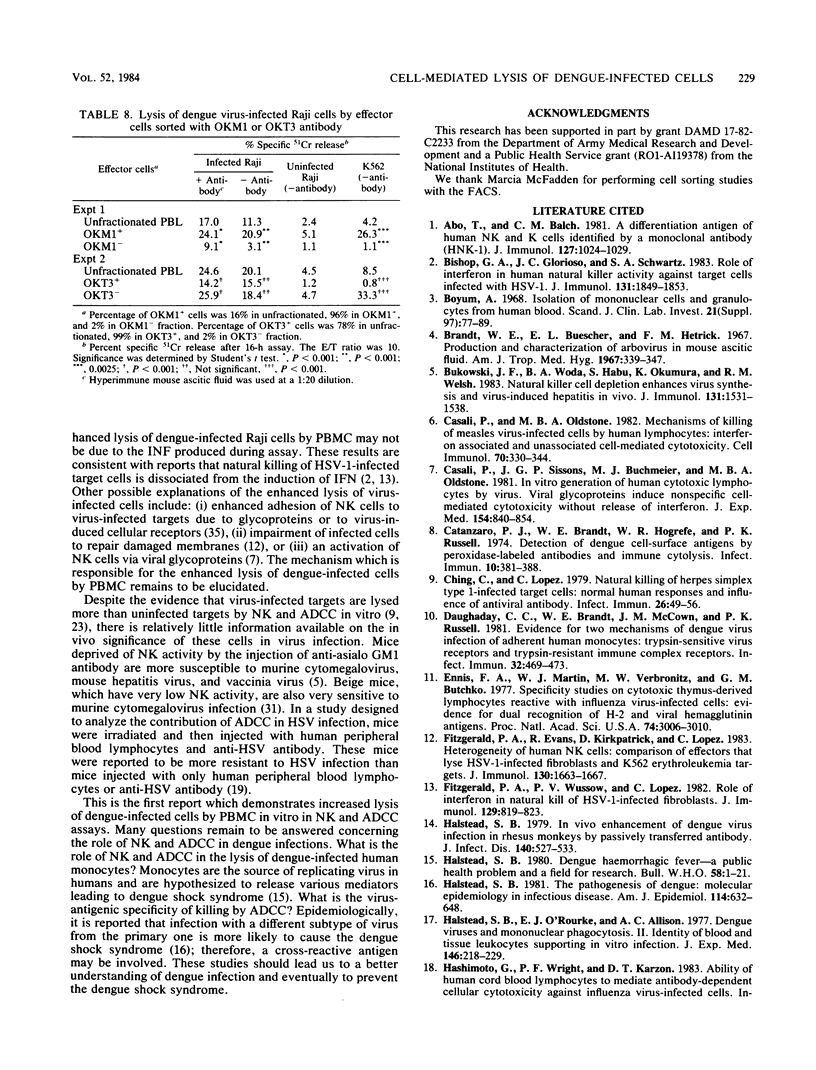
Peripheral blood mononuclear cells (PBMC) from humans without antibodies to dengue 2 virus lysed dengue 2 virus-infected Raji cells to a significantly greater degree than uninfected Raji cells. The addition of mouse anti-dengue antibody increased the lysis of dengue-infected Raji cells by PBMC. Dengue 2 immune human sera also increased lysis of dengue-infected Raji cells by PBMC. These results indicate that both PBMC-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) can cause significant lysis of dengue-infected Raji cells. The lysis of infected Raji cells in the ADCC assay correlated with the dilution of dengue-specific antibody which was added, indicating the dengue virus specificity of the lysis of dengue virus-infected Raji cells. Alpha interferon (IFN alpha) was detected in the culture supernatant of PBMC and dengue-infected Raji cells. However, enhanced lysis of dengue-infected Raji cells by PBMC may not be due to the IFN produced, because neutralization of all IFN activity with anti-IFN alpha antibody did not decrease the lysis of dengue-infected cells, and effector cells pretreated with exogenous IFN alpha also lysed dengue-infected cells to a greater degree than uninfected cells. The effector cells responsible for lysis of dengue virus-infected Raji cells in the natural killer and ADCC assays were analyzed. Nonadherent PBMC caused more lysis than did adherent cells. Characterization of nonadherent cells with monoclonal antibodies showed that the predominant responsible effector cells were contained in OKM1+ and OKT3- fraction in the natural killer and ADCC assays.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bishop G. A., Glorioso J. C., Schwartz S. A. Role of interferon in human natural killer activity against target cells infected with HSV-1. J Immunol. 1983 Oct;131(4):1849–1853. [PubMed] [Google Scholar]
- Brandt W. E., Buescher E. L., Hetrick F. M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967 May;16(3):339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Casali P., Oldstone M. B. Mechanisms of killing of measles virus-infected cells by human lymphocytes: interferon associated and unassociated cell-mediated cytotoxicity. Cell Immunol. 1982 Jul 1;70(2):330–344. doi: 10.1016/0008-8749(82)90334-3. [DOI] [PubMed] [Google Scholar]
- Casali P., Sissons J. G., Buchmeier M. J., Oldstone M. B. In vitro generation of human cytotoxic lymphocytes by virus. Viral glycoproteins induce nonspecific cell-mediated cytotoxicity without release of interferon. J Exp Med. 1981 Sep 1;154(3):840–855. doi: 10.1084/jem.154.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Brandt W. E., Hogrefe W. R., Russell P. K. Detection of dengue cell-surface antigens by peroxidase-labeled antibodies and immune cytolysis. Infect Immun. 1974 Aug;10(2):381–388. doi: 10.1128/iai.10.2.381-388.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday C. C., Brandt W. E., McCown J. M., Russell P. K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981 May;32(2):469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W., Butchko G. M. Specificity studies on cytotoxic thymus-derived lymphocytes reactive with influenza virus-infected cells: evidence for dual recognition of H-2 and viral hemagglutinin antigens. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3006–3010. doi: 10.1073/pnas.74.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Fitzgerald P. A., von Wussow P., Lopez C. Role of interferon in natural kill of HSV-1-infected fibroblasts. J Immunol. 1982 Aug;129(2):819–823. [PubMed] [Google Scholar]
- Halstead S. B. Dengue haemorrhagic fever--a public health problem and a field for research. Bull World Health Organ. 1980;58(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol. 1981 Nov;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Kohl S., Loo L. S. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J Immunol. 1982 Jul;129(1):370–376. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Kunkel L. A., Welsh R. M. Metabolic inhibitors render "resistant" target cells sensitive to natural killer cell-mediated lysis. Int J Cancer. 1981 Jan 15;27(1):73–79. doi: 10.1002/ijc.2910270112. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Okabe N., Hashimoto G., Abo T., Wright P. F., Karzon D. T. Characterization of the human peripheral blood effector cells mediating antibody-dependent cell-mediated cytotoxicity against respiratory syncytial virus. Clin Immunol Immunopathol. 1983 May;27(2):200–209. doi: 10.1016/0090-1229(83)90070-3. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Bloom B. R. Natural killer cells in resistance to virus-infected cells. Springer Semin Immunopathol. 1982;4(4):397–414. doi: 10.1007/BF02053741. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Udomsakdi S., Halstead S. B. Antibody response in dengue and dengue hemorrhagic fever. Jpn J Med Sci Biol. 1967 Dec;20 (Suppl):103–108. [PubMed] [Google Scholar]
- SABIN A. B. Research on dengue during World War II. Am J Trop Med Hyg. 1952 Jan;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978 Aug;121(2):532–538. [PubMed] [Google Scholar]
- Scott R. M., McCown J. M., Russell P. K. Human immunoglobulin specificity after group B arbovirus infections. Infect Immun. 1972 Sep;6(3):277–281. doi: 10.1128/iai.6.3.277-281.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Allan J. E., Papadimitriou J. M., Bancroft G. J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Hallenbeck L. A. Effect of virus infections on target cell susceptibility to natural killer cell-mediated lysis. J Immunol. 1980 May;124(5):2491–2497. [PubMed] [Google Scholar]


