Abstract
In lysosomes isolated from rat liver and spleen, a percentage of the intracellular inhibitor of the nuclear factor κ B (IκB) can be detected in the lysosomal matrix where it is rapidly degraded. Levels of IκB are significantly higher in a lysosomal subpopulation that is active in the direct uptake of specific cytosolic proteins. IκB is directly transported into isolated lysosomes in a process that requires binding of IκB to the heat shock protein of 73 kDa (hsc73), the cytosolic molecular chaperone involved in this pathway, and to the lysosomal glycoprotein of 96 kDa (lgp96), the receptor protein in the lysosomal membrane. Other substrates for this degradation pathway competitively inhibit IκB uptake by lysosomes. Ubiquitination and phosphorylation of IκB are not required for its targeting to lysosomes. The lysosomal degradation of IκB is activated under conditions of nutrient deprivation. Thus, the half-life of a long-lived pool of IκB is 4.4 d in serum-supplemented Chinese hamster ovary cells but only 0.9 d in serum-deprived Chinese hamster ovary cells. This increase in IκB degradation can be completely blocked by lysosomal inhibitors. In Chinese hamster ovary cells exhibiting an increased activity of the hsc73-mediated lysosomal degradation pathway due to overexpression of lamp2, the human form of lgp96, the degradation of IκB is increased. There are both short- and long-lived pools of IκB, and it is the long-lived pool that is subjected to the selective lysosomal degradation pathway. In the presence of antioxidants, the half-life of the long-lived pool of IκB is significantly increased. Thus, the production of intracellular reactive oxygen species during serum starvation may be one of the mechanisms mediating IκB degradation in lysosomes. This selective pathway of lysosomal degradation of IκB is physiologically important since prolonged serum deprivation results in an increase in the nuclear activity of nuclear factor κ B. In addition, the response of nuclear factor κ B to several stimuli increases when this lysosomal pathway of proteolysis is activated.
INTRODUCTION
Transcription factors are the intermediates between receptor-mediated stimulation of the cell surface by hormones and growth factors and concomitant changes in cellular gene expression. One of the best-characterized transcription factors is the nuclear factor κ B (NF-κB),1 a heterodimeric protein with two subunits, p50 and p65. NF-κB is ubiquitously expressed and it regulates the expression of many genes (for review, see Baldwin, 1996). The best-characterized role of NF-κB is in the regulation of immune and inflammatory response genes (Verma et al., 1995). However, NF-κB also plays an important role in many other cellular processes such as cell proliferation and polarization (Weith et al., 1995), cell transformation and tumor growth (Higgins et al., 1993), programmed cell death (Beg et al., 1995), and synaptic plasticity, neurodegeneration, and neuronal development (O’Neill and Kaltschmidt, 1997). In response to various stimuli that include interleukin-1, tumor necrosis factors, bacterial and viral products, UV irradiation, and oxidative stress, NF-κB is released from the cytosol and translocates to the nucleus (reviewed in Baldwin, 1996). The cytosolic retention of NF-κB requires binding to inhibitor proteins known as inhibitor of the nuclear factor κB (IκB) (reviewed in Verma et al., 1995). Seven mammalian IκB molecules have been identified which differ in their inhibitory specificity for different members of the NF-κB family. The best-characterized IκB is IκBα that inhibits complexes containing p65/p50 (Scott et al., 1993).
Under most of the conditions analyzed, degradation of IκB requires phosphorylation and ubiquitination at its amino terminal region, followed by proteolysis by the 26S proteasome (Alkalay et al., 1995; Scherer et al., 1995). However, there are several circumstances in which participation of other protein degradation systems have been described. Thus, the calcium-activated calpain system is responsible for IκB degradation after silica-induced NF-κB activation (Chen et al., 1997) and for basal degradation of IκB in immature B cells (Miyamoto et al., 1998). Other proteolytic systems, the caspases, which are involved in the apoptotic process, can also be involved in IκB degradation in certain transformed cells (White and Gilmore, 1996), and an unphosphorylated form of IκBβ is degraded in the nucleus by an unknown proteinase which is not sensitive to proteasome inhibitors (Suyang et al., 1996). In addition, in pre-B cells degradation of IκB does not require phosphorylation and ubiquitination steps (Baeuerle and Baltimore, 1996). An IκB QL-rich region seems to be important in inducible degradation of IκB by the proteasome (Sun et al., 1996). Signal sequences for protein instability (PEST sequences) identified in the IκB carboxyl-terminal region have been proposed to be important for IκB degradation in unstimulated cells (van Antwerp and Verma, 1996) without participation of the 26S proteasome. These findings suggest that degradation of IκB can be by different pathways depending on the type of cell and the kind of stimulus applied.
In preliminary experiments in which a lysosomal membrane protein (Adra et al., 1996) was overexpressed in several cell lines, we found an activation and nuclear translocation of NF-κB (Hu and Lim, unpublished results). This led us to question whether lysosomes may be involved in IκB degradation. In the present study, we have investigated the possible participation of lysosomes in IκB degradation under different cellular conditions. In addition to the continuous lysosomal degradation of complete portions of cytosol, including organelles and soluble proteins, by a mechanism known as macroautophagy (Dunn, 1994), a selective degradation of cytosolic proteins in lysosomes has also been described (reviewed in Dice et al., 1990; Cuervo et al., 1997). Transport of specific cytosolic proteins into lysosomes requires the presence of a consensus peptide motif in the substrate protein biochemically related to the pentapeptide KFERQ (Chiang and Dice, 1988). A cytosolic chaperone of 73 kDa (hsc73) binds to the substrate proteins at the region containing the motif sequence, and this binding of hsc73 stimulates the direct transport of substrate proteins into lysosomes (Chiang et al., 1989; Terlecky et al., 1992). A second chaperone located within the lysosomal lumen (lysosomal hsc73) is also necessary for the selective uptake of substrate proteins (Agarraberes et al., 1997; Cuervo et al., 1997). An integral lysosomal membrane protein of 96 kDa (lgp96) acts as the receptor for the hsc73-mediated lysosomal degradation pathway (Cuervo and Dice, 1996). This selective lysosomal degradation is activated in conditions of serum deprivation in cultured cells (Neff et al., 1981) or long-term starvation in animals (Wing et al., 1991; Cuervo et al., 1995a), and shows a clear tissue-dependent activity (Wing et al., 1991). Approximately 30% of the total cytosolic proteins contain the KFERQ-related motif and are, therefore, putative substrates for this lysosomal pathway of degradation (Dice, 1990). The proteins experimentally identified as substrates include RNase A (Neff et al., 1981; McElligott et al., 1985), GAPDH (Aniento et al., 1993), aldolase (Aniento et al., 1993), components of other proteolytic systems such as some subunits of the 20S proteasome (Cuervo et al., 1995b), lipid-binding proteins such as annexins II, IV, and VI (Cuervo, Barnes, and Dice, unpublished results), a cytosolic form of α2-microglobulin (Cuervo, Hildebrand, Bomhard, and Dice, unpublished results), and the c-fos transcription factor (Aniento et al., 1996).
Here, we show that IκB is directly transported into lysosomes by the above-described hsc73-mediated pathway. This pathway of proteolysis can reduce intracellular levels of IκB and cause activation of NF-κB. Furthermore, when this selective lysosomal pathway of proteolysis is activated, the activity of NF-κB is more easily stimulated by diverse agents.
MATERIALS AND METHODS
Animals and Cells
Male Wistar rats of 200–250 g body weight were starved for 20 h before each experiment. Chinese hamster ovary (CHO) cells (American Type Culture Collection, Rockville, MD) were grown in 100-mm diameter round plates or 250 mm per side square plates until confluent in F-12 medium (Life Technologies, Gaithersburg, MD) containing 10% newborn calf serum (NCS) and antibiotics (penicillin/streptomycin) (Neff et al., 1981). WEHI231 cells lines stably transfected with and expressing a hemagglutinin (HA; amino acid 98–108 of the influenza protein) epitope-tagged SS32/36AA mutant IκBα were kindly provided by Dr. S. Miyamoto (Department of Human Oncology, University of Wisconsin, Madison, WI). These cells were maintained in RPMI 1640 (Mediatech, Asheville, NC) supplemented with 10% FBS, 5 × 10−5 M β-mercaptoethanol, and 500 μg/ml hygromycin. To deprive cells of serum, plates were extensively washed with HBSS (Life Technologies), and fresh medium not containing serum was added.
Chemicals and Antibodies
Sources of reagents and antibodies were as previously described (Terlecky and Dice, 1993; Cuervo et al., 1994; Adra et al., 1996; Cuervo et al., 1997). IL-1β (IL-1) was purchased from Oncogene (Cambridge, MA). Lipopolysaccharide (LPS), tumor necrosis factor α (TNF-α), phorbol 12-myristate 13-acetate (PMA), and pyrrolidine dithiocarbamate (PDTC) were purchased from Sigma (St. Louis, MO). The calf intestinal alkaline phosphatase was from Life Technologies. Polyclonal antibodies against IκBα and β, p65 and p50, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the mAb against conjugated ubiquitin was purchased from Zymed (South San Francisco, CA). The mAb (12CA5) against the amino acids 98–108 of the HA protein was from Dr. F. McKeon (Department of Cell Biology, Harvard Medical School, Boston, MA). The polyclonal antibody against the cytosolic region of lgp96 was prepared in our laboratory (Cuervo and Dice, 1996). The polyclonal antibody against the nuclear factor of activated T cells 1 was a gift from Dr. A. Rao (Department of Pathology, Harvard Medical School, Boston, MA). The plasmid containing the fusion protein GST-IκBα was kindly provided by Dr. S. Ghosh (Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT).
Stable Cellular Transfection
Cells were transfected using the calcium phosphate method (Maniatis et al., 1982). The cDNA for human lamp2 (Fukuda et al., 1988) was subcloned in the pCR3 mammalian expression vector (Invitrogen, San Diego, CA). Selection of transfected cells was performed by resistance to Geneticin (400 μg/ml; Life Technologies) for at least 15 d. Individual colonies were grown in 48-well plates in the presence of the antibiotic for another 7 d. Expression of the protein was verified by immunofluorescence of fixed cells and immunoblot of cellular lysates after separation by SDS-PAGE.
Isolation of Subcellular Fractions
Spleen and liver from previously starved rats were homogenized in 0.25 M sucrose. Subcellular fractions were prepared by differential centrifugation and further centrifugation in a density gradient of metrizamide as described previously (Cuervo et al., 1995; Adra et al., 1996). Lysosomal matrix and membranes were obtained as described previously (Ohsumi et al., 1983). Briefly, lysosomes were pelleted and resuspended in a hypotonic solution (0.025 M sucrose) for 30 min at 0°C. Lysosomal membranes were sedimented by centrifugation at 105,000 × g for 30 min, and the supernatant corresponding to the lysosomal matrix was also separately recovered. In some experiments, two different groups of lysosomes with different contents of hsc73 were isolated as previously described (Cuervo et al., 1997). Lysosomes from cultured cells were isolated as described by Storrie and Madden (1990). In all of the experiments, lysosomal integrity was verified after isolation and at the end of the incubation period by measuring the activity of β-hexosaminidase, a lysosomal enzyme, in the incubation medium (Storrie and Madden, 1990). Experiments with more than 10% broken lysosomes were discarded.
Proteolysis Measurements
Degradation of the IκB already located in the lysosomal matrix was measured by incubation of intact lysosomes (50 μg of protein) in 10 mM MOPS/0.25 M sucrose/5 mM DTT (pH 7.2) at 37°C. At selected times aliquots were removed, separated by SDS-PAGE, and the remaining IκB inside lysosomes was detected by immunoblot. Lysosomal degradation of exogenously added proteins was assayed by incubation of intact lysosomes in the above medium with GAPDH radiolabeled by reductive methylation (Jentoft and Dearborn, 1983) ([14C]GAPDH; 1.2 × 106 dpm/nmol), a pool of cytosolic proteins from human fibroblasts, or GST-IκB metabolically labeled with [3H]leucine or [35S]methionine/cysteine, respectively ([3H]proteins, 2.0 × 106 dpm/μg; [35S]GST-IκB, 7.3 × 106 dpm/nmol). Reactions were stopped with a final concentration of 10% trichloroacetic acid and, after filtration in the Millipore Multiscreen Assay System (Millipore, Bedford, MA) with a 0.45-μm pore membrane, radioactivity in the flow-through was measured in a 2100TR Packard liquid scintillation analyzer (Packard Instruments, Meriden, CT). Proteolysis was expressed as the percentage of the initial acid-precipitable radioactivity transformed to acid-soluble radioactivity during the incubation time.
Uptake of Proteins by Lysosomes
Transport of proteins into isolated lysosomes was analyzed using a previously described in vitro system (Terlecky and Dice, 1993; Cuervo et al., 1994). Briefly, lysosomal proteolytic activity was inhibited by treatment of freshly isolated lysosomes with 100 μM chymostatin for 10 min at 0°C. After dilution in two volumes of 10 mM MOPS/0.25 M sucrose (pH 7.2) buffer, lysosomes were incubated with GST-IκB (5 μg) for 20 min at 37°C. At the end of the incubation, the protein remaining outside lysosomes was removed by treatment with proteinase K (3 μg) at 0°C for 10 min. Lysosomes were recovered by centrifugation and subjected to SDS-PAGE. GST-IκB transported into lysosomes was detected by immunoblot after SDS-PAGE. Where indicated GST-IκB was previously incubated with calf intestinal alkaline phosphatase (40 U) in 50 mM Tris-HCl/0.1 mM EDTA/0.3 M NaCl/0.1 mM MgCl2/10 μM ZnCl2 (pH 8.5) for 1 h at 37°C.
Determination of IκB Half-Life
CHO cells at 60–70% confluency were radiolabeled with an [35S]methionine/cysteine mixture (0.2 mCi/ml) for 48 h in medium supplemented with 10% NCS. In some experiments the incubation lasted only 2 h to preferentially label short-lived proteins. After extensive washing, cells were maintained in the presence of 10% NCS (serum+) or in medium without serum (serum−). Where indicated, NH4Cl was added to a final concentration of 15 mM. In some experiments PDTC or H2O2 was added at the indicated concentrations in the fresh medium immediately after the labeling. At increasing times cells were lysed in lysis buffer [50 mM Tris-HCl (pH 8)/150 mM NaCl/1% Nonidet-P40/0.5% sodium deoxycholate/0.1% SDS]. Lysates were cleared by centrifugation, and supernatants were incubated with specific antibodies against IκB or p65 previously conjugated to protein A-Sepharose beads. After extensive washing with lysis buffer, the immunoprecipitate was subjected to SDS-PAGE. Gels were exposed to a PhosphorImager screen and the immunoprecipitated IκB or p65 was quantified with a PhosphorImager system (Molecular Dynamics, Sunnyvale, CA). The half-life of the protein was calculated from the formula t1/2 = ln2/degradation rate.
Electromobility Gel Shift Assay
Nuclear extracts from CHO cells were prepared as follows. Cells (1.5 × 107) were grown until confluent in medium supplemented with 10% NCS and then, after extensive washing, the medium was replaced with fresh medium with or without serum as indicated. After 16 h (except where indicated) cells were harvested, washed, and resuspended in hypotonic buffer [10 mM Tris-HCl (pH 7.4)/10 mM NaCl/30 mM MgCl2/0.02% sodium azide] with a broad range of proteinase inhibitors (0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 100 μM leupeptin, 1 μM pepstatin A, 1 mM EDTA, and 0.01% sodium azide). After 10 min at 0°C, 0.05% of Nonidet P-40 was added, and nuclei were pelleted by centrifugation at 2500 × g for 5 min. Pellets were washed with the same buffer and resuspended in 20 mM HEPES (pH 7.4)/420 mM NaCl/1.5 mM MgCl2/0.2 mM EDTA/25% glycerol/0.01% sodium azide with proteinase inhibitors for 30 min. After centrifugation at 12,000 × g for 10 min, nuclear proteins were recovered in the supernatant and stored at −70°C. A double-stranded oligonucleotide containing the immunoglobulin κ enhancer kB site of NF-κB (CAGAGGGGACTTTCCGAGA) was end labeled with T4 polynucleotide kinase in the presence of 20 μCi of [γ-32P]ATP. Binding assays were performed by incubation of nuclear proteins (5 μg) with the radiolabeled probe (10,000 dpm) and 0.5 μg of poly(dI-dT) in 8.5 mM HEPES (pH 7)/ 104 mM NaCl/0.2 mM DTT/8.5% glycerol for 20 min at 25°C. Samples were subjected to electrophoresis in a 4% nondenaturing polyacrylamide gel. After drying, the gel was exposed to a PhosphorImager screen. In some experiments, nuclear transport of NF-κB was induced by addition of PMA, LPS, IL-1, TNF-α, or H2O2 at the indicated concentrations in the culture medium 4 h prior to cell harvesting. The specificity of the binding to NF-κB in the nuclear extracts was determined by competition in the presence of a 200-fold excess of unlabeled oligonucleotide probe.
General Methods
SDS-PAGE (Laemmli, 1970), immunoblotting (Towbin et al., 1979), and fluorography (Bonner and Laskey, 1974) were performed by standard procedures. Protein concentration in samples was measured according to the Lowry et al. (1951) method using BSA as a standard. Hsc73 was purified from rat liver by affinity chromatography using an ATP-agarose matrix (Welch and Feramisco, 1985). GST-IκB was isolated from Escherichia coli previously transformed with the above-mentioned vector and after induction with 1 mM isopropyl-β-d-thiogalactopyranoside for 4 h (Maniatis et al., 1982). The GST-IκB was purified with a glutathione-agarose column. Densitometric analyses were performed with an Image Analyzer System (Inotech S-100, Sunnyvale, CA).
RESULTS
Immunolocalization of IκB in Lysosomes
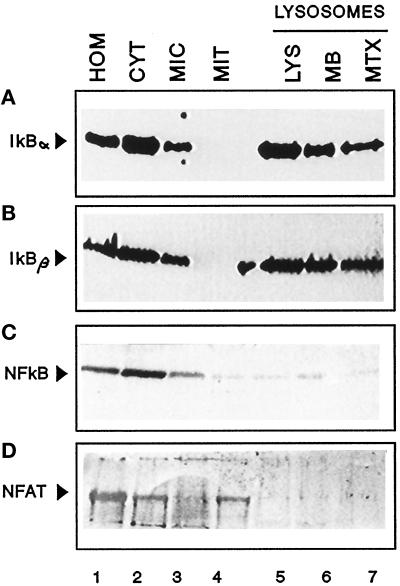
As a first step toward determining whether lysosomes were involved in the intracellular degradation of IκB, we analyzed whether or not IκB could be detected in the lysosomal fraction. As shown in Figure 1, A and B, most of the intracellular IκB α and β is located in the cytosol. Both proteins also associate to some extent with microsomes but are not detected in mitochondria. In lysosomes it is possible to detect IκB α and β which account for 1.5% and 1.0% of the IκB in spleen homogenate, respectively, when corrected for lysosomal recovery. A portion of the lysosome-associated IκB (30–42%) is located at the lysosomal membrane, probably bound to its cytosolic surface, but the remaining IκB is within the lysosomal matrix. Only slight differences were found in the lysosomal content of IκB α and β. The presence of IκB in lysosomes is not the result of a cytosolic contamination in the process of isolation since the mitochondrial fraction, obtained by similar methods, does not show detectable levels of IκB. In addition, no detectable levels of two other transcriptional factors (p65 of NF-κB and NFAT-1) were evident in the lysosomes (Figure 1, C and D).
Figure 1.
A portion of intracellular IκB is located in lysosomes. Rat spleens were fractionated after homogenization as described in MATERIALS AND METHODS. After the isolation, part of the lysosomal fraction was subjected to hypotonic shock, and lysosomal membranes and matrix were separated by centrifugation. The same amount of protein (100 μg) of the indicated fractions (and the membrane and matrix derived from 100 μg of lysosomal protein) was then subjected to SDS-PAGE and immunoblotted with specific antibodies against IκB α (A), IκB β (B), p65 (C), or nuclear factor of activated T cells 1 (NFAT, D). HOM, homogenate; CYT, cytosol; MIC, microsomes; MIT, mitochondria; LYS, lysosomes; MB, membrane; MTX, matrix.
Lysosomal Degradation of IκB
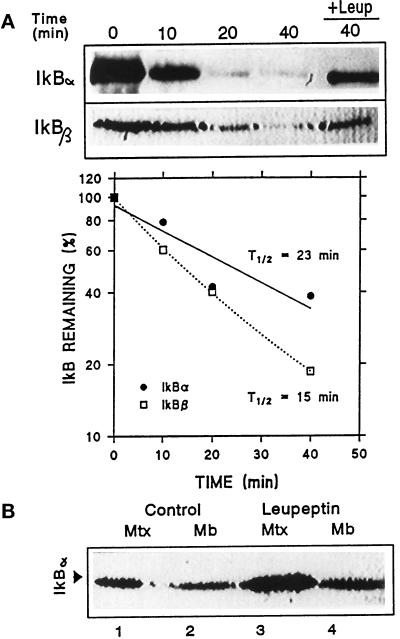
The lysosome-associated IκB can be readily degraded once inside the lysosomal matrix. Thus, when liver lysosomes were incubated in an isotonic medium to maintain lysosomal integrity and levels of IκB associated with lysosomes were analyzed by immunoblot (Figure 2A), the lysosomal content of IκB decreased with the incubation time, and this decrease was slowed in the presence of leupeptin, an inhibitor of cathepsin B, H, and L (Salvelsen and Nagase, 1989). Quantification of four immunoblots similar to the ones shown revealed that the half-life of IκB α and β once inside lysosomes was approximately 20 min. Since IκBα and β behaved similarly in these initial studies, we chose to analyze further only IκBα.
Figure 2.
Degradation of IκB inside lysosomes. (A) Rat liver lysosomes were incubated in an isotonic medium at 37°C in the absence or presence (+Leup) of leupeptin. At the indicated times, reactions were stopped by addition of Laemmli sample buffer and levels of IκB α and β present in the lysosomal fraction were detected by immunoblot following SDS-PAGE. The graph shows the quantification of four different experiments similar to the one shown and the calculated half-life for both proteins. (B) Lysosomes isolated from liver of nontreated or leupeptin-treated rats (see MATERIALS AND METHODS) were subjected to hypotonic shock and matrix and membranes were separated. Levels of IκB in each lysosomal fraction were detected by immunoblot with a specific antibody following SDS-PAGE.
To estimate degradation rates of IκB that could be attributed to lysosomes in vivo, we compared lysosomal levels of this protein in control rats and in rats previously treated with leupeptin for 1 h to partially inhibit the lysosomal proteolytic activity. As shown in Figure 2B, after leupeptin treatment, there is a significant increase in lysosome-associated IκB (52% more than in untreated animals), and this increase is mainly detected in the lysosomal matrix. On the basis of these results and the above-mentioned half-life of IκB once inside the lysosomal matrix, we calculated that in starved rats approximately 0.4% of the total intracellular content of IκB is transported into lysosomes per hour. Notice that the final value has been corrected for the percentage of lysosomal recovery in the isolation.
Pathway for Lysosomal Uptake of IκB
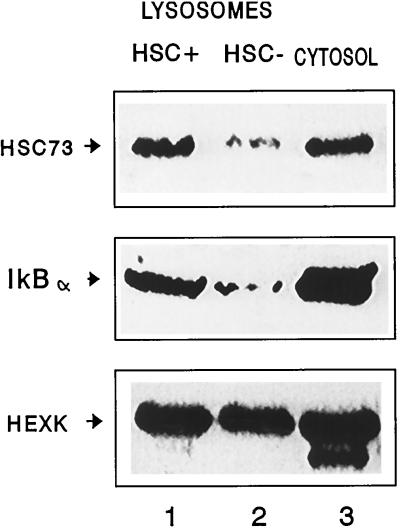
The significant differences between the lysosomal amount of IκB and that of other transcription factors could be related to their different proteolytic susceptibility once inside lysosomes. However, experiments with broken lysosomes revealed that half-lives of IκB and NF-κB were similar once in contact with the lysosomal proteinases (approximately 15 min; our unpublished results). These results suggest that the transport of IκB into lysosomes is selective and preferred over other cytosolic proteins. To determine whether IκB could be entering lysosomes through the hsc73-mediated pathway, we isolated two lysosomal populations with very different activities for the hsc73-mediated transport (Cuervo et al., 1997a). The major difference between these groups of lysosomes is their different content of hsc73, a necessary factor for substrate protein uptake (Agarraberes et al., 1997; Cuervo et al., 1997a; Figure 3, top). As shown in Figure 3 (middle), levels of IκB in the lysosomal population active in the direct transport pathway were significantly higher than in the less active population. As a control, levels of hexokinase, a cytosolic protein that has been reported to not follow the selective pathway for its transport to lysosomes (Cuervo et al., 1997a), were similar in both groups (Figure 3, bottom). These results suggest that IκB might be a substrate for the selective lysosomal pathway of protein degradation.
Figure 3.
Distribution of hsc73, IκB, and hexokinase in different lysosomal populations. Two different lysosomal populations were isolated from rat liver as described in MATERIALS AND METHODS. The same amounts of protein (100 μg) of each lysosomal group (HSC+ and HSC−) and the cytosolic fraction were subjected to SDS-PAGE and immunoblotted with specific antibodies for hsc73 (top), IκBα (middle), and hexokinase (HEXK, bottom).
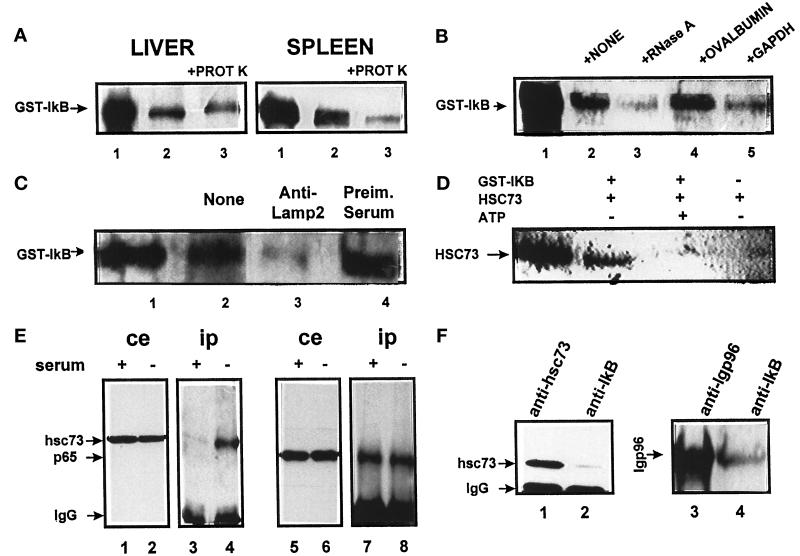
We used isolated lysosomes to analyze a possible direct uptake of IκB (Terlecky and Dice, 1993; Cuervo et al., 1994). Lysosomes from rat spleen (Figure 4A, right) or liver (Figure 4A, left) were incubated with the radiolabeled fusion protein GST-IκB to differentiate it by size from the IκB already present in the lysosomal matrix. When lysosomes were sedimented after the incubation, part of GST-IκB was associated with lysosomes. Some of this protein was resistant to proteinase treatment and therefore was located in the lysosomal matrix. These results suggest that GST-IκB can be transported in vitro into lysosomes.
Figure 4.
IκB and the hsc73-mediated pathway for protein degradation. (A) Direct uptake of IκB by lysosomes. The fusion protein GST-IκB (5 μg) was incubated with intact rat liver and spleen lysosomes (100 μg of protein) under standard conditions. At the end of the incubation, proteinase K (5 μg) was added to part of the samples, as indicated, and samples were subjected to SDS-PAGE and immunoblotted for IκB. (B) Effect of different proteins on IκB uptake by lysosomes. IκB was incubated under standard conditions with rat liver lysosomes without additions or in the presence of equimolar amounts of the indicated proteins. (C) Effect of blockage of lgp96 on IκB lysosomal uptake. Rat liver lysosomes were preincubated with a specific antibody against lgp96 (lane 3) or with preimmune serum (lane 4). In B and C uptake of IκB was assayed as described in A by adding proteinase K to all of the samples. (D) Binding of hsc73 to IκB. Hsc73 from rat liver was incubated with GST-IκB with the indicated additions for 1 h at 37°C. At the end of the incubation, the sample was subjected to affinity chromatography with glutathione immobilized on agarose beads. Levels of hsc73 associated with GST-IκB or to the beads alone were detected after SDS-PAGE by immunoblot with a specific antibody against hsc73. GST-IκB (1 μg) is in lane 1 in A–C. Hsc73 (0.5 μg) is in lane 1 in D. (E) Coimmunoprecipitation of IκB and hsc73 in CHO cells. CHO cells maintained in the presence or absence of serum for 12 h (as labeled) were subjected to immunoprecipitation with a specific antibody against IκB. Total cellular extracts (ce) and immunoprecipitates (ip) were analyzed by SDS-PAGE and immunoblot for the presence of hsc73 (lanes 1–4) and p65 (lanes 5–8). (F) Association of IκB proteins at the lysosomal membrane. Lysosomes isolated from rat liver were subjected to immunoprecipitation with specific antibodies against hsc73 (lane 1), IκB (lanes 2 and 4), or lgp96 (lane 3). Levels of hsc73 (lanes 1 and 2) or lgp96 (lanes 3 and 4) in the immunoprecipitates were analyzed as in E.
These transport experiments were performed without addition of exogenous hsc73, because we have previously demonstrated that the levels of hsc73 associated with the membrane of rat liver lysosomes are sufficient to mediate the transport of substrate proteins into the lysosomal matrix (Cuervo et al., 1994). In addition, when 35S-labeled IκB was incubated with intact lysosomes from CHO cells with low levels of membrane-associated hsc73, a clear dependence on hsc73 and ATP for the uptake and degradation of IκB was observed (see below).
To determine whether the transport system used by IκB was the hsc73-mediated pathway previously described, or at least whether IκB shared some of the components of this system for its transport into lysosomes, we analyzed the effect of different substrates (RNase A, GAPDH) and nonsubstrate proteins (ovalbumin) on the IκB lysosomal uptake. After incubation, all samples were treated with proteinase K to detect the amount of protein transported into the lysosomal matrix. As shown in Figure 4B, in the presence of two of the substrates for the hsc73-mediated transport, the amount of IκB transported into lysosomes significantly decreased. However, addition of ovalbumin in the incubation medium did not modify IκB uptake. In addition, when lgp96, the receptor protein at the surface of the lysosomes for the specific substrates was previously blocked with a specific antibody, a decrease in the uptake of IκB was also observed. Incubation of lysosomes with a preimmune serum did not significantly modify levels of IκB uptake (Figure 4C).
Finally, another characteristic of the cytosolic proteins that can be directly transported into lysosomes is their ability to bind to hsc73, a molecular chaperone. This binding is nucleotide dependent because ADP is required for substrate binding and ATP is necessary for substrate release. A consensus motif for binding to hsc73 has been previously identified in substrates for this pathway (Dice, 1990). Analysis of the IκB sequence revealed the presence of a region biochemically related with the proposed pentapeptide (VKELQ amino acids 46–50). When GST-IκB was incubated with hsc73 and the fusion protein was recovered by affinity binding to GST-agarose beads, part of the hsc73 was also recovered (Figure 4D). Levels of hsc73 recovered in the presence of ATP were significantly lower. Most hsc73 bound directly to the GST-IκB, since in the absence of GST-IκB hsc73 was hardly detected (Figure 4D, lane 4). No binding of hsc73 occurred to GST itself (our unpublished results). This interaction of IκB with hsc73 was also detected in vivo. Using a specific antibody against IκB, we were able to coimmunoprecipitate hsc73 from cytosolic extracts of CHO cells (Figure 4E, lane 3). Interestingly, after serum removal cytosolic levels of hsc73 remain constant (Figure 4E, lane 2) but the amount of hsc73 coprecipitated with IκB was significantly higher (12-fold increase; Figure 4E, lane 4). As shown in Figure 4E, lanes 7 and 8, the amount of NF-κB coprecipitated with IκB remained unchanged at this early point of serum deprivation. In isolated rat liver lysosomes not only hsc73 (Figure 4F, lane 2) but also lgp96 (Figure 4F, lane 4) was coimmunoprecipitated with IκB. These results together suggest that, at least in vitro, the direct transport of IκB through the lysosomal membrane requires most of the components of the hsc73-mediated system.
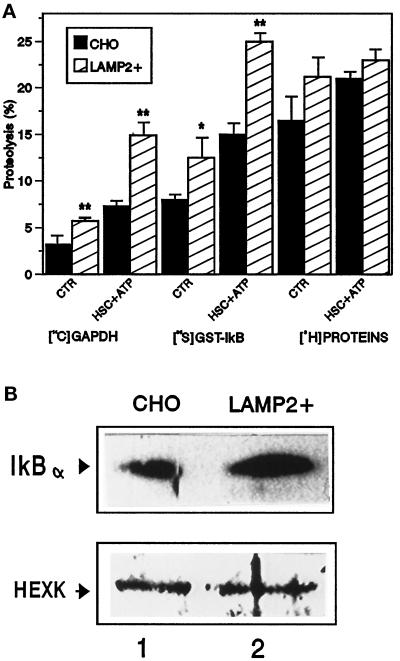
To determine whether the lysosomal degradation of IκB in living cells is mediated by the selective lysosomal pathway, we analyzed the effect that an increase in the activity of this pathway has on IκB uptake and degradation. We have previously demonstrated that an increase in the lysosomal levels of lgp96 results in an increased activity of the pathway (Cuervo and Dice, 1996). As shown in Figure 5A, lysosomes from CHO cells stably overexpressing lgp96 (twofold increase) show a significant increase in the uptake and degradation of GAPDH but not of a pool of cytosolic proteins, most of which are not substrates for this lysosomal pathway of proteolysis. Uptake and degradation of IκB by those lysosomes was also increased (Figure 5A). Differences in degradation rates for IκB among those groups of lysosomes were mainly due to differences in the protein transported, since endogenous levels of IκB in lysosomes from transfected cells were significantly higher than in lysosomes from untransfected cells (Figure 5B). Lysosomal levels of hexokinase, a nonsubstrate for the hsc73-mediated pathway, were similar in the two groups analyzed.
Figure 5.
Effect of overexpression of lamp2 in CHO cells on the uptake and degradation of IκB. (A) Proteolytic activity of lysosomes from transfected cells. Intact lysosomes isolated from nontransfected CHO cells or cells transfected with the cDNA for human lamp2 (LAMP2+) were incubated with radiolabeled GAPDH, GST-IκB, or a pool of cytosolic proteins without additions or in the presence of hsc73 (10 μg/ml) and ATP (5 mM) (HSC + ATP) as labeled. Proteolysis rates were measured as described in MATERIALS AND METHODS. Values are the means ± SD of three different experiments. (B) Lysosomal levels of IκB and hexokinase. Intact lysosomes (100 μg of protein) from the cells described in A were subjected to SDS-PAGE and immunoblot with a specific antibody against IκB (top panel) or hexokinase (HEXK, bottom panel).
Role of Ubiquitination and Phosphorylation of IκB in Its hsc73-mediated Transport into Lysosomes
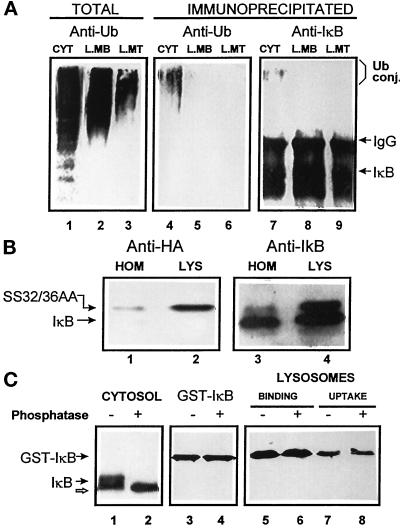
As described above, IκB undergoes phosphorylation and polyubiquitination under specific conditions, and polyubiquitination targets the proteins for degradation by the 26S proteasome complex. The presence of polyubiquitinated proteins bound to the lysosomal membrane and inside the lysosomal matrix has been reported previously (Lenk et al., 1992). To determine whether covalent binding of ubiquitin to IκB was also necessary for its lysosomal uptake, we separately immunoprecipitated the IκB in the lysosomal matrix and membrane. Using an antiubiquitin antibody, we further analyzed by immunoblot for the presence of ubiquitin in the IκB immunoprecipitates. As shown in Figure 6A, this antibody recognized high molecular weight ubiquitinated complexes in cytosol and in lysosomal membranes and matrix not subjected to immunoprecipitation (lanes 1–3), but none of those high molecular weight complexes were detected in the IκB immunoprecipitated from lysosomal membrane and matrix (lanes 5 and 6). Covalent binding of a single molecule of ubiquitin to IκB is also not necessary for its lysosomal uptake because the immunoprecipitated IκB was not recognized by the specific antibody against ubiquitin (lanes 5 and 6). Thus, ubiquitination seems to be unnecessary for the selective transport of IκB to lysosomes because the IκB located in the lysosomal matrix or associated with the lysosomal membrane is not ubiquitinated.
Figure 6.
Lysosome-associated IκB is not ubiquitinated or phosphorylated. (A) Cytosol (100 μg of protein), lysosomal membranes, and lysosomal matrix (derived from 100 μg of lysosomal protein) were isolated as described in MATERIALS AND METHODS and then directly subjected to SDS-PAGE (lanes 1–3) or immunoprecipitated with a specific antibody against IκB (lanes 4–9). Filters were immunoblotted with a specific antibody against ubiquitin (lanes 1–6) or against IκB (lanes 7–9). (B) Homogenate and lysosomes isolated from WEHI231 cells stably transformed with a HA epitope-tagged SS32/36AA IκB. Samples (50 μg of protein) were subjected to SDS-PAGE and immunoblot with a specific antibody against HA (lanes 1 and 2) or against IκB (lanes 3 and 4). (C) Rat liver cytosol (isolated in the presence of phosphatase inhibitors, 100 μg of protein, lanes 1 and 2) or purified GST-IκB (lanes 3–8) were treated with phosphatase as described in MATERIALS AND METHODS and then subjected to SDS-PAGE and immunoblot with a specific antibody against IκB. Binding and uptake of phosphatase-treated and untreated GST-IκB by isolated rat liver lysosomes (lanes 5–8) was analyzed as described in Figure 4A.
Phosphorylation of IκB at serines 32 and 36 is required for its signal-induced degradation by the 26S proteasome (Alkalay et al., 1995; Scherer et al., 1995). To determine whether phosphorylation of those residues is also required for the lysosomal degradation of IκB, we analyzed the lysosomal content of IκB in WEHI231 cells stably transfected with a HA epitope-tagged S32/36A IκB mutant. As shown in Figure 6B, the mutated IκB can be detected inside lysosomes using a specific antibody against the HA tag (Figure 6B, lane 2). Levels of endogenous and mutated IκB inside lysosomes correlate with their levels in the total homogenate, suggesting that both forms of IκB are equally transported inside lysosomes (Figure 6B, lanes 3 and 4). Phosphorylation of IκB on other residues also appeared not to be necessary for its lysosomal transport. When the fusion protein GST-IκB used to analyze direct transport of IκB into isolated lysosomes was pretreated with a phosphatase, we found no significant differences in the rates of IκB binding or uptake by lysosomes (Figure 6C, compare lanes 5 and 6 and lanes 7 and 8).
Physiological Relevance of IκB Degradation by the hsc73-mediated Lysosomal Pathway
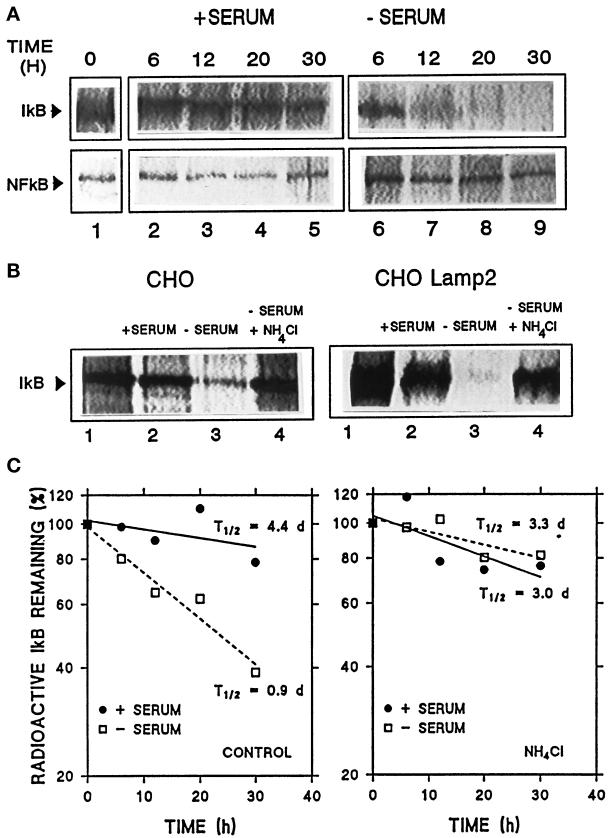
The hsc73-mediated lysosomal pathway is activated during serum deprivation in cultured cells or prolonged starvation in animals. To analyze the participation of lysosomes in IκB degradation, we compared the IκB half-life in CHO cells in the presence or absence of serum and the effect of NH4Cl, a lysosomal inhibitor (Figure 7). Rates of degradation of IκB were measured by radioactive labeling of CHO cells followed by immunoprecipitation of IκB at different times. As shown in Figure 7A (top), we found a significant increase in IκB degradation when serum was removed from the culture medium. No change in NF-κB degradation under conditions of serum deprivation were found (Figure 7A, bottom). In addition, when lysosomal degradation was partially inhibited by NH4Cl, a marked decrease in IκB degradation was observed in the absence of serum (Figure 7B, left). Changes in IκB degradation during serum removal are even more dramatic in cells overexpressing lamp2 when compared with normal cells (approximately 1.8 times faster) (Figure 7B, right). After determining IκB degradation rates in three independent experiments similar to the ones described above (Figure 7C), we calculated that the half-life of intracellular IκB in the presence of serum is 4.4 d and in the absence of serum is 0.9 d. When NH4Cl was added, the half-lives obtained were 3.0 and 3.3 d in the presence and absence of serum, respectively. No changes were detected in the degradation rates of NF-κB in the absence of serum when NH4Cl was added (our unpublished results). These results show that IκB is more rapidly degraded during serum deprivation, a condition known to activate the hsc73-mediated selective lysosomal pathway. Furthermore, during serum deprivation, lysosomes are the major site for this accelerated degradation because treatment with NH4Cl results in more than 90% inhibition of the serum-regulated IκB degradation.
Figure 7.
Intracellular degradation of IκB and p65 under different conditions. Nontransfected CHO cells (A) or cells transfected with lamp2 as labeled (B) were radiolabeled with [35S]methionine/cysteine for 48 h as described in MATERIALS AND METHODS. After extensive washing, cells were kept in medium with serum (+serum) or without serum (−serum). At indicated times (A) or after 18 h (B), cells were subjected to immunoprecipitation with a specific antibody against IκB (A, top panel, and B) or p65 (A, bottom panel). Immunoprecipitates were resolved by SDS-PAGE and gels were exposed in a PhosphorImager screen. In B (lanes 4), 15 mM NH4Cl was added to the medium during the chase period. (C) Average value of IκB degradation rates of three different experiments similar to the one shown in A (left panel) and similar experiments performed in the presence of 15 mM NH4Cl (right panel). t1/2 were calculated from the formula t1/2 = ln2/degradation rate.
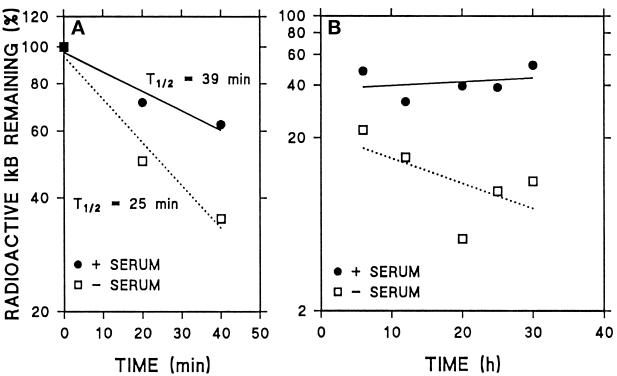
We also analyzed the degradation of IκB after 2 h of radiolabeling to determine whether IκB was degraded with single exponential kinetics or whether two different degradation patterns could be detected in CHO cells. After the short labeling, most of the radiolabeled IκB was degraded in the first 40 min both in the absence and in presence of serum (Figure 8A). However, after long exposure of the fluorographies containing the immunoprecipitates, it was possible to detect a portion of IκB that was slowly degraded in cells supplemented with serum (Figure 8B). That portion of IκB was also detected in cells deprived of serum but its degradation was markedly faster (Figure 8B). When IκB proteolytic rates were analyzed in three separate experiments, we observed a pool of IκB with a very short-half life (39 and 25 min in the presence and in absence of serum, respectively) along with a second pool of IκB with a half-life of 3 d or more. As shown in Figure 8A, during the first hour of chase the degradation of IκB was slightly faster in the absence of serum than in the presence of serum, but differences in degradation were markedly higher during 6–30 h of chase (Figure 8B). The detectable serum-dependent increase in IκB degradation in the first hour of chase was not altered by NH4Cl (our unpublished results), suggesting that this increase is not due to lysosomal proteolysis. Thus, only the degradation rates of the long-lived pool significantly increased in response to serum deprivation and only under those conditions was IκB degradation inhibited by NH4Cl (Figure 7C).
Figure 8.
Two different pools of IκB can be detected in CHO cells. Cells were radiolabeled and subjected to immunoprecipitation with anti-IκB antibody as described in Figure 7, with the exception that radiolabeling was shortened to 2 h. (A) Quantification of levels of IκB immunoprecipitated during 40 min of chase in three different experiments. (B) Quantification of levels of IκB immunoprecipitated during 6–30 h of chase in three different experiments.
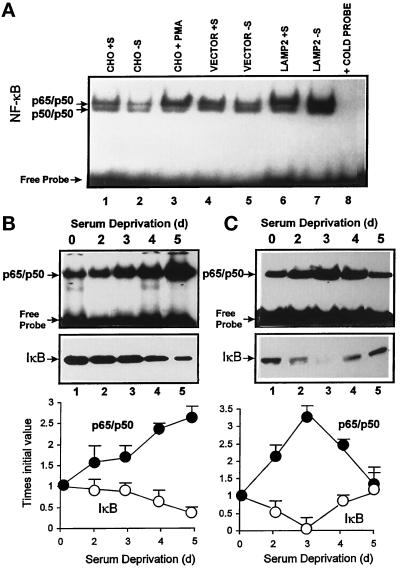
Finally, we analyzed the effect of the increase in lysosomal IκB degradation on NF-κB activity using electrophoretic mobility shift assays with a radiolabeled probe containing the immunoglobulin κ enhancer NF-κB-binding site. In nuclear extracts from CHO cells, this probe bound to a high molecular weight complex identified with specific antibodies (our unpublished results) as the p65/p50 heterodimer of the NF-κB family (as labeled in Figure 9). In many of the nuclear preparations, the probe also specifically bound to a lower molecular weight complex identified as the p50/p50 homodimer of the NF-κB family. However, since this complex was not always detected, all of the quantification of data refer to the p65/p50 heterodimer. First of all, we observed a constitutive activation of NF-κB in CHO cells. When serum was removed for 16 h, there were no detectable changes in NF-κB activation. If anything, the levels of nuclear NF-κB were slightly decreased. However, nuclear levels of NF-κB in serum-supplemented cells overexpressing lamp2 were 3.5 times the levels in nontransfected cells (Figure 9A). Moreover, when serum was removed for 16 h, the difference between transfected and nontransfected cells was even higher (nuclear NF-κB was 5.6 times more in cells overexpressing lamp2). Transfection of cells with an empty expression vector did not modify nuclear levels of NF-κB. No significant differences in NF-κB activation in the presence or the absence of serum under those conditions were found for nontransfected cells. However, in nontransfected cells, when serum deprivation was prolonged to more than 2 d (Figure 9B, top panel), we detected a significant increase in NF-κB activation. This increase in NF-κB nuclear activity correlated well with a decrease in the cytosolic levels of IκB in serum-deprived cells (Figure 9B, bottom panel). Interestingly, the pattern of NF-κB activation by prolonged serum deprivation showed certain cell-type specificity. For example, in human fibroblasts there was a rise and fall in nuclear NF-κB corresponding with a decrease and increase in levels of IκB (Figure 9C, bottom panel). The serum-induced increase in nuclear NF-κB activity also started earlier but it lasted for a shorter time than in CHO cells (Figure 9C, top panel).
Figure 9.
Effect of overexpression of lamp2 and serum deprivation on NF-κB nuclear translocation. (A) Nuclear extracts were prepared from nontransfected CHO cells or cells transfected with lamp2 (LAMP2) or with an empty vector (vector) maintained in the presence (+S) or absence (−S) of serum for 16 h prior to harvesting. Analysis of NF-κB-binding activity was performed as described in MATERIALS AND METHODS. In lane 3, cells were stimulated with PMA for 6 h. Lane 8 shows a binding assay performed as in lane 7 but in the presence of an excess of unlabeled probe. Results similar to those in lane 8 were obtained when unlabeled probe was added in each of the conditions analyzed (our unpublished results). (B and C) CHO cells (B) or human fibroblasts (C) were maintained in the absence of serum for the indicated times, and after harvesting nuclear extracts and cytosolic fractions were prepared as described in MATERIALS AND METHODS. NF-κB activity in the nuclear extracts (top panel) was analyzed as above. Content of IκB in the cytosolic fractions (middle panel) was detected by immunoblot with a specific antibody for IκB. Bottom panel shows the densitometric quantification of three experiments similar to the ones shown the in top and middle panels.
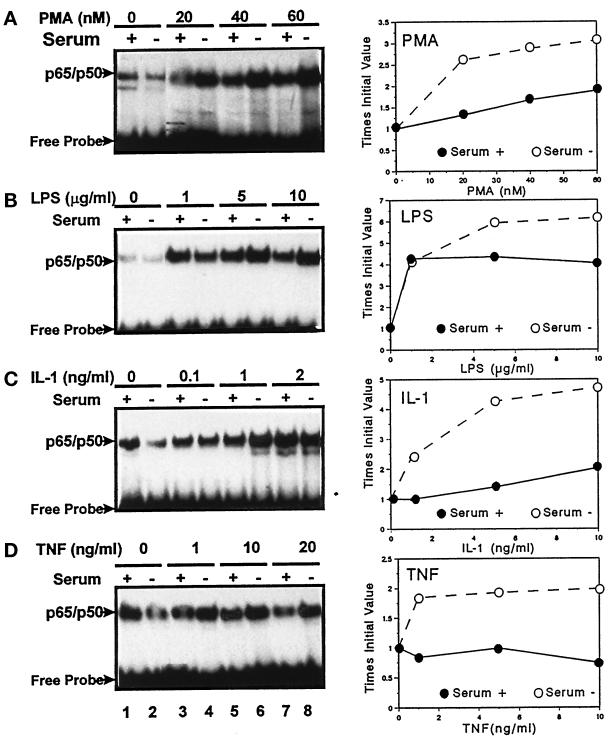
We have previously described that the hsc73-mediated lysosomal pathway of protein degradation is already active in CHO cells after 16 h of serum deprivation, and its activity progressively increases with the starvation time (Cuervo and Dice, 1996). As we have shown before (Figure 7), it is possible to detect a significant increase in the degradation of intracellular IκB after 12 h of serum deprivation, but we found increased NF-κB activity only after 2 d or more of starvation (Figure 9, B and C). However, the initial increased degradation of IκB after serum removal clearly has an effect on the sensitivity of the NF-κB activation in response to several activating agents. Thus, in CHO cells deprived of serum for only 12 h, we were able to detect a consistent increase in the NF-κB response to PMA, LPS, IL-1, and TNF-α when compared with serum-supplemented cells (Figure 10).
Figure 10.
Effect of serum deprivation on the NF-κB response to different stimuli. CHO cells were maintained in the presence (serum+) or absence (serum−) of serum for 12 h. After that incubation, PMA (A), LPS (B), IL-1 (C), or TNF-α (D) was added to the culture medium at the indicated concentrations for 4 h. At the end of the incubation, cells were harvested and NF-κB activity was assayed in the nuclear extracts as described in MATERIALS AND METHODS. The right side of the figure corresponds to the densitometric quantification of nuclear levels of NF-κB in three or four different experiments similar to the ones shown here.
Targeting of IκB to Lysosomes for Its Degradation
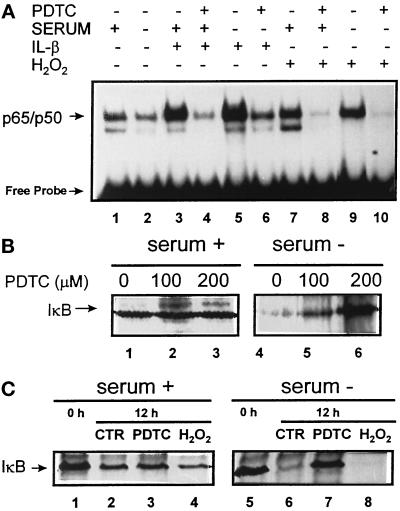
The exact mechanism that triggers IκB and other substrates of the hsc73-mediated pathway for its degradation in lysosomes remains unclear. It is known that the NF-κB response can be inhibited by antioxidants (Baeuerle and Henker, 1994) that somehow suppress a reaction required for release of IκB from NF-κB (Schreck et al., 1992). In addition, certain oxidized proteins are better substrates for the hsc73-mediated pathway (Cuervo and Knecht, unpublished results). To determine a possible role of reactive oxygen species in the lysosomal targeting of IκB, we further analyzed the effect of PDTC, a known antioxidant, in the degradation of the long-lived pool of IκB. As shown in Figure 11A, PDTC was able to inhibit the NF-κB response to agents such as IL-1 and H2O2 not only in the presence but also in the absence of serum in CHO cells. In the presence of PDTC, there is a decrease in the degradation rates of IκB after serum removal, and this inhibitory effect in the IκB degradation is proportional to the amount of PDTC added (Figure 11B, lanes 4–6). The fact that the addition of H2O2 to the incubation medium under conditions of serum deprivation markedly increase IκB degradation (Figure 11C) also suggests the participation of reactive oxygen species in the targeting of IκB to lysosomes in response to serum withdrawal.
Figure 11.
Reactive oxygen species and lysosomal targeting of IκB. (A) CHO cells were maintained in the presence or absence of serum and 200 μM PDTC (as indicated) during 12 h. Four hours before harvesting, IL-1 (1 ng/ml) or H2O2 (250 μM) were added to some of the samples. NF-κB activity was measured in the nuclear extracts as described in MATERIALS AND METHODS. (B and C) CHO cells were radiolabeled and subjected to immunoprecipitation 10 h after labeling with an anti-IκB antibody as described in Figure 7. Where indicated, PDTC (100 or 200 μM) or H2O2 (125 μM) was added to the incubation medium after the washing postlabeling. The percentage of living cells after the treatment, monitored by trypan blue staining, was comparable to untreated cells. Lanes 1 and 5 in C show initial levels of IκB after labeling.
DISCUSSION
The inducible degradation of IκB bound to NF-κB by the 26S proteasome is a well-characterized process that results in activation of NF-κB (van Antwerp and Verma, 1996). Although other proteolytic systems have been implicated in IκB degradation under specific circumstances (see INTRODUCTION), there have been no published studies implicating lysosomes in IκB degradation. In this work, we have identified in cultured cells a long-lived pool of IκB that is more rapidly degraded in response to serum deprivation (Figure 7). This finding may explain why serum deprivation can activate NF-κB in several cell types in culture (Grimm et al., 1996; Figures 9 and 10).
The long-lived pool of IκB might allow cells to increase NF-κB activity more slowly than by the proteasome pathway. In fact, NF-κB activity increases only after 2 d of serum deprivation (Figure 9). The long-lived pool of IκB may consist of conformational variants or heteromeric assemblies that have buried signals for ubiquitination. Under conditions of serum deprivation, this long-lived IκB might be modified to expose the putative KFERQ motif, leading to binding by hsc73 and to transport into lysosomes. Ubiquitination or phosphorylation of IκB is not required for this lysosomal uptake (Figure 6).
The stimuli that determine IκB interaction with hsc73 and its subsequent degradation by this selective lysosomal pathway remain unclear. It has been previously described that the heat shock response inhibits IκB degradation (Wong et al., 1997); however, the hsc73 involved in the lysosomal transport is not the heat shock inducible form but its constitutively expressed family member. The trigger for IκB recognition by hsc73 might be located in the IκB molecule itself. Our studies with the antioxidant agent PDTC (Figure 11) indicate that oxidation of IκB might play an important role in its lysosomal targeting under conditions of serum deprivation.
The lysosomal population isolated from rat liver has been previously well characterized and it mainly corresponds to primary lysosomes derived from hepatocytes with undetectable levels of autophagic vacuoles or multivesicular bodies (Aniento et al., 1993; Cuervo et al., 1994). Compared with liver, spleen has a very heterogenous cellular composition. However, the method that we used to isolate lysosomes allows separation of lysosomes that are mainly derived from lymphocytes (Bowers, 1974). In addition, lysosomes isolated from a lymphocyte cell line in culture (Jurkat cells) also contain IκB (our unpublished results). The recovery of lysosomes from rat spleen was approximately 4%, a slightly lower value than from rat liver (5.5%; Cuervo et al., 1995a). The purity of both lysosomal preparations, determined by assaying activity of mitochondrial and cytosolic marker enzymes as possible contaminants, was very high (>99%).
The percentage of total intracellular IκB detected in lysosomes from spleen (Figure 1) is only 1.0–1.5% of the total in homogenates. However, this value corresponds to a steady-state condition of import and degradation inside the lysosomal matrix. As shown in Figure 2, using rat liver lysosomes, once IκB reaches the lysosomal matrix, it is rapidly degraded. This conclusion is also supported by the effect of leupeptin, a lysosomal inhibitor, on levels of lysosomal IκB (Figure 2B). In those experiments, we found that approximately 0.4% of the total IκB in liver was degraded in lysosomes per hour (see RESULTS, Lysosomal Degradation of IκB). Based on that degradation rate and by applying the formula t1/2= ln2/degradation rate, we calculated that the half-life of IκB due to lysosomal degradation is approximately 7 d. However, only 40% inhibition of lysosomal degradation has been reported after leupeptin injection into rats (discussed in Cuervo et al., 1995b). Therefore, the half-life of the IκB that can be attributed to lysosomal proteolysis would be closer to the value of 4 d experimentally determined in CHO cells (Figure 7).
Several of the results presented here strongly implicate IκB as a substrate for the hsc73-mediated pathway of lysosomal proteolysis: 1) the higher IκB content in lysosomes active in the direct uptake of proteins (Figure 3); 2) the direct interaction of IκB with the cytosolic hsc73 (Figure 4D) and with lgp96, the receptor at the lysosomal membrane (Figure 4C); 3) the direct transport in vitro of IκB into isolated rat liver and CHO cell lysosomes (Figures 4A and 5A); 4) the ATP/hsc73 dependence of this transport (Figure 5A); 5) the competitive effect of other substrates of the hsc73-mediated pathway on IκB lysosomal uptake (Figure 4B); 6) the serum-dependent activation of IκB lysosomal degradation in cultured cells (Figure 7); and 7) the increase in IκB lysosomal degradation in cells with an increased activity for the hsc73-mediated lysosomal pathway (Figures 5 and 7B).
It is likely that only free forms of IκB are directly transported into lysosomes, since no NF-κB was detected inside lysosomes. Whether or not the free IκB has been previously bound to NF-κB or whether it corresponds to a pool of newly synthesized free IκB remains unclear. Previous studies reported a half-life for free IκB of approximately 30 min (van Antwerp and Verman, 1996). However, degradation of overexpressed IκB in cultured cells was analyzed instead of the endogenous IκB, and the radioactive labeling was performed for only 1 h. When we performed a short labeling of CHO cells for 2 h, instead of our initial 2 d, we also found a very fast NH4Cl-insensitive degradation of IκB during the first hour of the chase period (Figure 8). This short labeling period strongly favors labeling of rapidly turning over proteins. Thus, only by prolonging the labeling time can the slowly turning over IκB be labeled and therefore clearly detected after immunoprecipitation.
The inhibitory effect of NH4Cl on the degradation of IκB in the absence of serum revealed that, under those conditions, >90% of the intracellular IκB is degraded by lysosomes. In contrast with chloroquine, another common lysosomal inhibitor, NH4Cl, at the concentrations used in this study, does not interfere with IκB synthesis (Chen et al., 1997). The absence of an effect of lysosomal inhibition on IκB half-life in other studies (Verma et al., 1995) can be explained by analysis of degradation being performed only in the presence of serum. Under such conditions we also found little effect of NH4Cl (Figure 7C).
There is a constitutive activation of NF-κB in CHO cells (Figure 9A). We have also observed constitutive NF-κB activation in other cell lines such as HL-60 hematopoietic cells and human fibroblasts. We have found a consistent slight decrease in the NF-κB activity during the first 12 h of serum deprivation. The decrease in many regulatory factors present in serum could in some manner cause that initial decrease in NF-κB activity. Then the NF-κB activity clearly increases with increases in the activity of the lysosomal pathway, such as in CHO cells overexpressing lamp 2 (Figure 9A), or after prolonged serum removal (Figure 9B). The differences in the kinetics of activation of NF-κB between CHO cells and human fibroblasts (Figure 9) might be explained by the difference in the activity of the hsc73-mediated pathway in the two cell types. We have previously described that the activity of this pathway is significantly higher in human fibroblasts than in CHO cells (Cuervo and Dice, 1996). The faster decrease in the cytosolic levels of IκB in human fibroblasts by a higher rate of lysosomal degradation can explain the more rapid increase in NF-κB activity in these cells. This increase in NF-κB activity can also more quickly induce the expression of IκB, an NF-κB responsive gene, and thereby limiting the duration of the NF-κB response (Sun et al., 1993). Among normal hematopoietic lineages, B cells are the only cell type in which there is a constitutive activation of NF-κB. In these cells, an enhanced degradation of the pool of rapidly turning over IκB was found to be the main reason for the activation of NF-κB without external stimuli (Miyamoto et al., 1994; Verma et al., 1995). The response of NF-κB to serum deprivation has been previously reported in cultured cells (Grimm et al., 1996), and we have demonstrated that this serum-regulated activation of NF-κB correlates with the activation of the selective lysosomal degradation of IκB (Figure 9, B and C).
In addition to the regulation of the constitutive activity of NF-κB after prolonged starvation, the selective lysosomal degradation of IκB also contributes to the modulation of stimuli-mediated activation of NF-κB. Although there is no significant difference in the levels of total IκB between normal CHO cells versus those deprived of serum for 12 h (our unpublished results), it is possible that the serum-starved cells contain a reduced level of the long-lived pool of IκB. This would explain the increased NF-κB activation in response to the different stimulatory agents in those cells (Figure 10). The amount of long-lived IκB compared with short-lived IκB seems to depend on the cell type. In unstimulated lymphoid cells the long-lived pool of IκB can also be detected, but it constitutes a lower percentage (18%) of the total IκB than in CHO cells (our unpublished results). It has been suggested that an excess of IκBα in unstimulated cells would prevent rapid inducibility and reduce the sensitivity of the NF-κB system (Baeuerle and Henker, 1994). In contrast, a reduction in levels of IκB would increase the sensitivity of the NF-κB system. The selective degradation of IκB in lysosomes, for example, under conditions of stress and starvation, appears to be one of the operative mechanisms.
Together, our data show that the long-lived pool of IκB can influence the sensitivity of the NF-κB system and that this may be modulated by a selective lysosomal proteolytic pathway. The sensitivity of the selective lysosomal degradation of IκB to stress such as serum starvation indicates that this regulatory pathway has a physiological role. Thus, our findings support the proposal that the IκB protein has evolved multiple mechanisms of degradation (van Antwerp and Verma, 1996). The lysosomal degradation of IκB described in this article offers a new mechanism of control of the NF-κB function through modifications of the activity of the hsc73-mediated lysosomal pathway.
ACKNOWLEDGMENTS
We thank Dr. Fernando Macian for his expert advice and technical assistance in the electromobility gel shift assays of NF-κB. We also thank Elizabeth Frutiger for the critical reading of the manuscript. Special thanks to Dr. S. Ghosh for the GST-IκBα plasmid and Dr. S. Miyamoto for the WEHI231 cell lines expressing SS32/36AA IκBα mutant protein. This work was supported by National Institutes of Health grant DK-07542 (to A.M.C.), National Institutes of Health grant AG-06116 (to J.F.D.), National Institutes of Health grant DK-47636, and Council for Tobacco Research grant 4488 (to B.L.). B.L. is also a Scholar of the Leukemia Society of America.
Abbreviations used:
- CHO
Chinese hamster ovary
- HA
hemagglutinin
- IκB
inhibitor of the nuclear factor κ B
- LPS
lipopolysaccharide
- NCS
newborn calf serum
- NF-κB
nuclear factor κ B
- PDTC
pyrrolidine dithiocarbamate
- PMA
phorbol 12-myristate 13-acetate
- TNF-α
tumor necrosis factor α
REFERENCES
- Adra CN, Zhu S, Ko J-L, et al. LAPTM5: a novel lysosomal-associated multispanning membrane protein preferentially expressed in hematopoietic cells. Genomics. 1996;35:328–337. doi: 10.1006/geno.1996.0364. [DOI] [PubMed] [Google Scholar]
- Agarraberes F, Terlecky SR, Dice JF. Requirement for an intralysosomal hsc73 for a selective pathway of lysosomal proteolysis. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–1070. [PubMed] [Google Scholar]
- Aniento F, Papavassiliou AG, Knecht E, Roche E. Selective uptake and degradation of c-fos and v-fos by rat liver lysosomes. FEBS Lett. 1996;390:47–52. doi: 10.1016/0014-5793(96)00625-4. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henker T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bonner WN, Laskey RA. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974;46:83–85. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowers WE. Isolation of lysosomes from lymphoid tissues. Methods Enzymol. 1974;31:353–356. doi: 10.1016/0076-6879(74)31038-5. [DOI] [PubMed] [Google Scholar]
- Chen F, Lu Y, Kuhn DC, Maki M, Shi X, Sun S-C, Demers LM. Calpain contributes to silica-induced IκB-alpha degradation and nuclear factor-κB activation. Arch Biochem Biophys. 1997;342:383–388. doi: 10.1006/abbi.1997.0132. [DOI] [PubMed] [Google Scholar]
- Chiang H-L, Dice JF. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem. 1988;263:6797–6805. [PubMed] [Google Scholar]
- Chiang H-L, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997a;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Hayes SA, Dice JF. Structure and Function of Molecular Chaperones: The Role of Chaperones in the Life Cycle of Proteins. A.L. Fink and J. Goto, New York: Marcel Dekker; 1997b. Molecular chaperones and intracellular protein degradation with emphasis on a selective lysosomal pathway of proteolysis; pp. 491–509. [Google Scholar]
- Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995a;269:C1200–C1208. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Palmer A, Rivett AJ, Knecht E. Degradation of proteasomes by lysosomes in rat liver. Eur J Biochem. 1995b;227:792–800. doi: 10.1111/j.1432-1033.1995.tb20203.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Terlecky SR, Dice JF, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269:26374–26380. [PubMed] [Google Scholar]
- Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- Dice JF, Terlecky SR, Chiang H-L, Olson TS, Isenman LD, Short-Russell SR, Freundlieb S, Terlecky LJ. A selective pathway for degradation of cytosolic proteins by lysosomes. Semin Cell Biol. 1990;1:449–455. [PubMed] [Google Scholar]
- Dunn WA. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Viitala J, Matteson J, Carlsson SR. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988;263:18920–18928. [PubMed] [Google Scholar]
- Grimm S, Bauer MKA, Baeuerle PA, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KA, Perez JR, Coleman TA, Dorshkind K, McComas WA, Sarmiento UM, Rosen CA, Narayanan R. Antisense inhibition of the p65 subunit of NF-κB blocks tumorigenicity and causes tumor regression. Proc Natl Acad Sci USA. 1993;90:9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N, Dearborn DG. Protein labeling by reductive alkylation. Methods Enzymol. 1983;91:570–579. doi: 10.1016/s0076-6879(83)91052-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenk SE, Dunn WA, Trausch JS, Ciechanover A, Schwartz AL. Ubiquitin-activating enzyme, E1, is associated with maturation of autophagic vacuoles. J Cell Biol. 1992;118:301–308. doi: 10.1083/jcb.118.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maniatis T, Fritsch WF, Sambrook J. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Laboratory Press; 1982. [Google Scholar]
- McElligott MA, Miao P, Dice JF. Lysosomal degradation of ribonuclease A and ribonuclease S-protein microinjected into the cytosol of human fibroblasts. J Biol Chem. 1985;260:11986–11993. [PubMed] [Google Scholar]
- Miyamoto S, Chiao PJ, Verma IM. Enhanced I kappa B alpha degradation is responsible for constitutive NF-kappa B activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Seufzer BJ, Shumway SD. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff NT, Bourret L, Miao P, Dice JF. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981;91:184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Kaltschmidt C. NF-kB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–256. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y, Ishikawa T, Kato K. A rapid and simplified method for the preparation of lysosomal membranes from rat liver. J Biochem. 1983;93:547–556. [PubMed] [Google Scholar]
- Salvelsen G, Nagase H. Inhibition of proteolytic enzymes. In: Beynon RJ, Bond JS, editors. Proteolytic Enzymes. Oxford: IRL Press; 1989. pp. 83–104. [Google Scholar]
- Scherer DC, Brockman HA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of IκBa requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Meier B, Manner DN, Droge W, Baeurle P. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ML, Fujita T, Liou HC, Noalan GP, Baltimore D. The p65 subunit of NF-κB regulates IκB by two distinct mechanisms. Genes Dev. 1993;7:1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- Storrie B, Madden EA. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Elwood H, Greene WC. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-C, Ganchi PA, Ballard DW, Greene WC. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlecky SR, Chiang H-L, Olson TS, Dice JF. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem. 1992;267:9202–9209. [PubMed] [Google Scholar]
- Terlecky SR, Dice JF. Polypeptide import and degradation by isolated lysosomes. J Biol Chem. 1993;268:23490–23495. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4353. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Antwerp DJ, Verma IM. Signal-induced degradation of IκBα: association with NF-κB and the PEST sequence in IκBα are not required. Mol Cell Biol. 1996;16:6037–6045. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Weith F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Feramisco JR. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985;5:1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Gilmore TD. Bcl-2 and crmA have different effects on transformation, apoptosis and the stability of I kappa B-alpha in chicken spleen cells transformed by temperature-sensitive v-Rel oncoproteins. Oncogene. 1996;13:891–899. [PubMed] [Google Scholar]
- Wing SS, Chiang HL, Goldberg AL, Dice JF. Proteins containing peptide sequences related to KFERQ are selectively depleted in liver and heart, but not skeletal muscle, of fasted rats. Biochem J. 1991;275:165–169. doi: 10.1042/bj2750165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Ryan M, Wispe JR. The heat shock response inhibits inducible nitric oxide synthase gene expression by blocking I kappa-B degradation and NF-kappa B nuclear translocation. Biochem Biophys Res Commun. 1997;231:257–263. doi: 10.1006/bbrc.1997.6076. [DOI] [PubMed] [Google Scholar]