Abstract
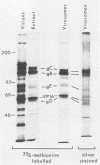
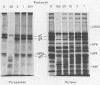
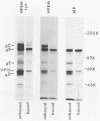
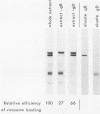
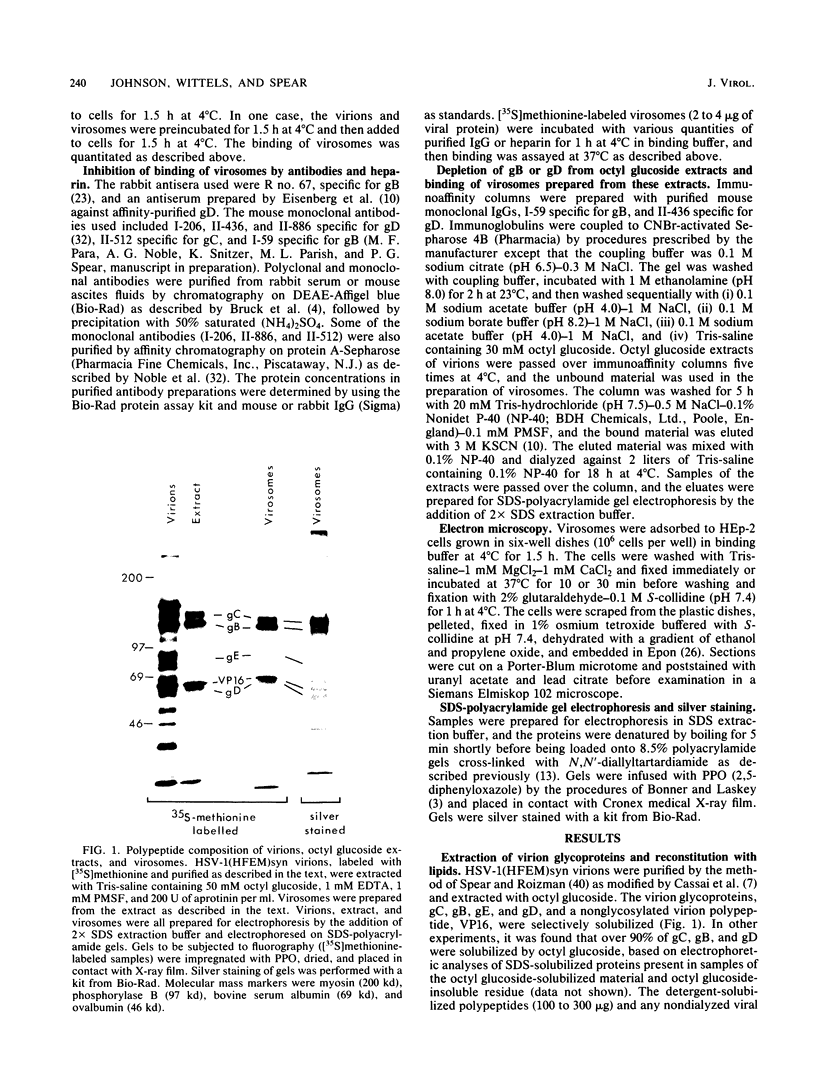
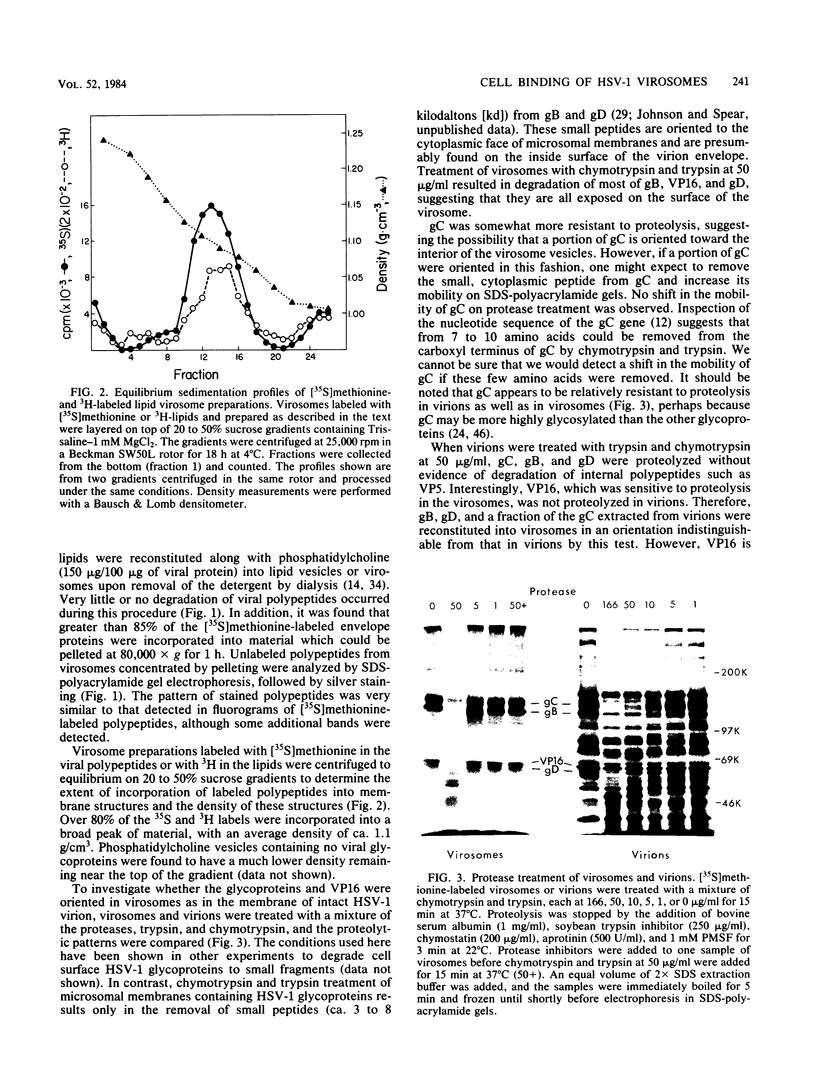
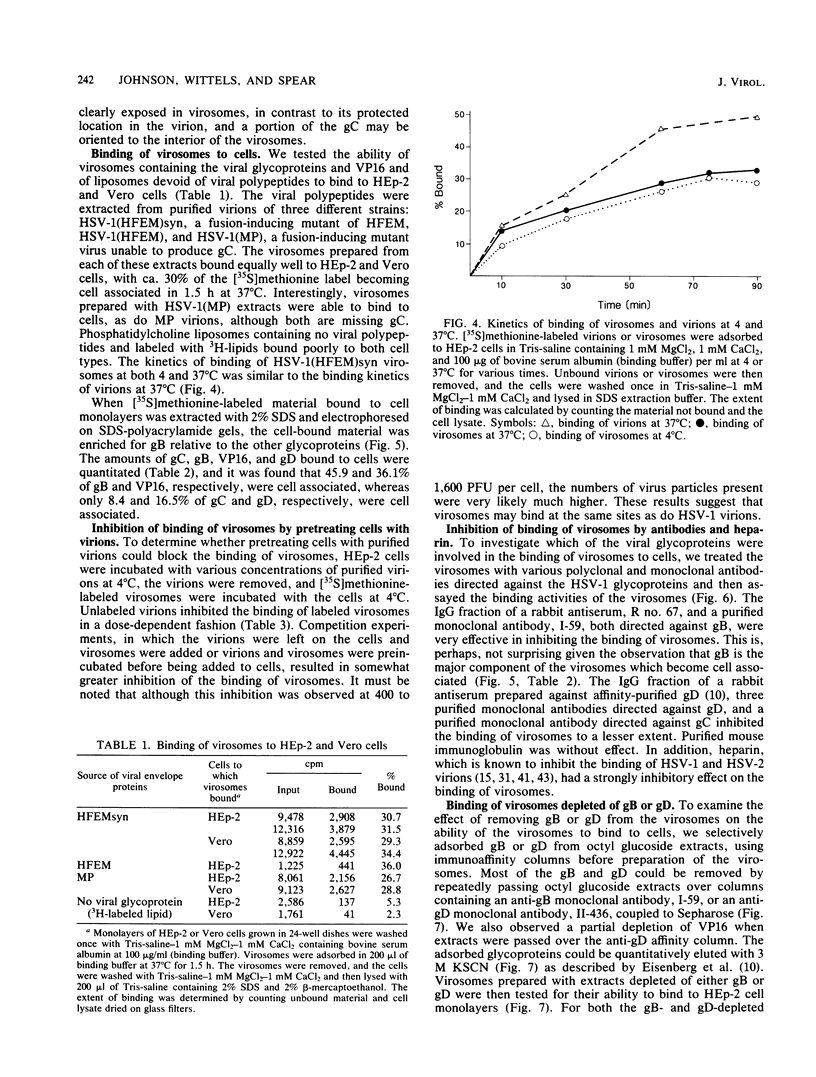
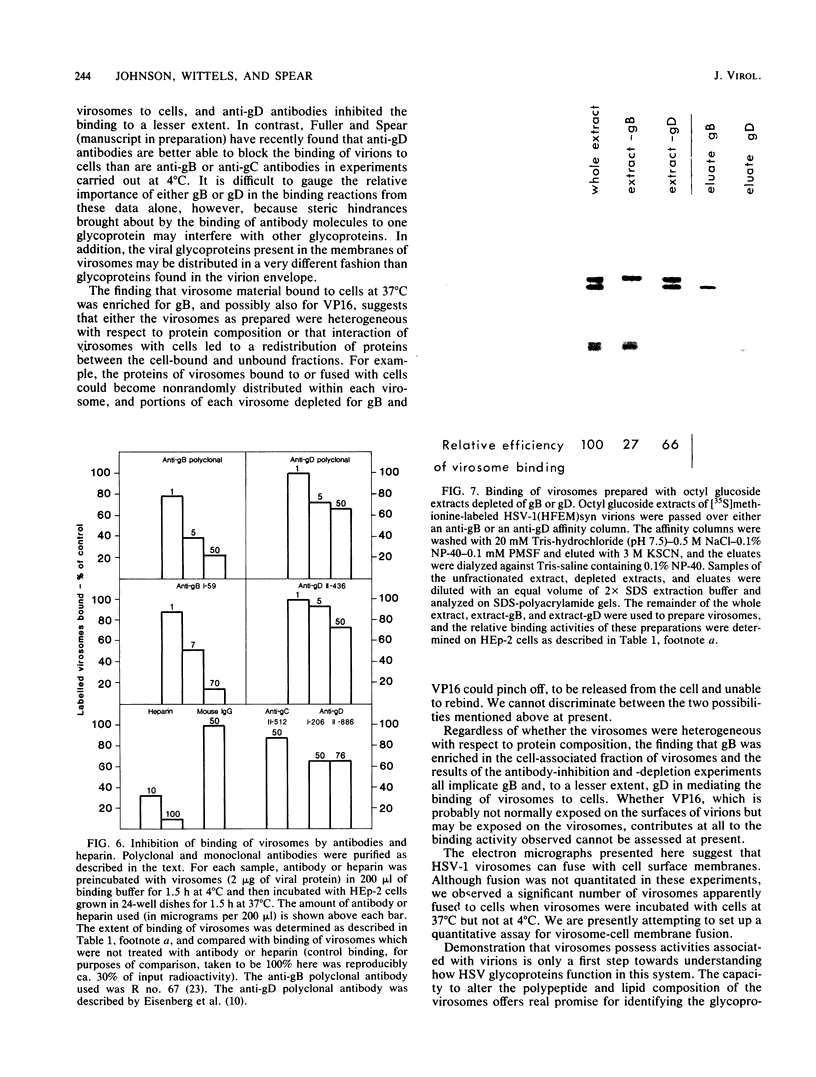
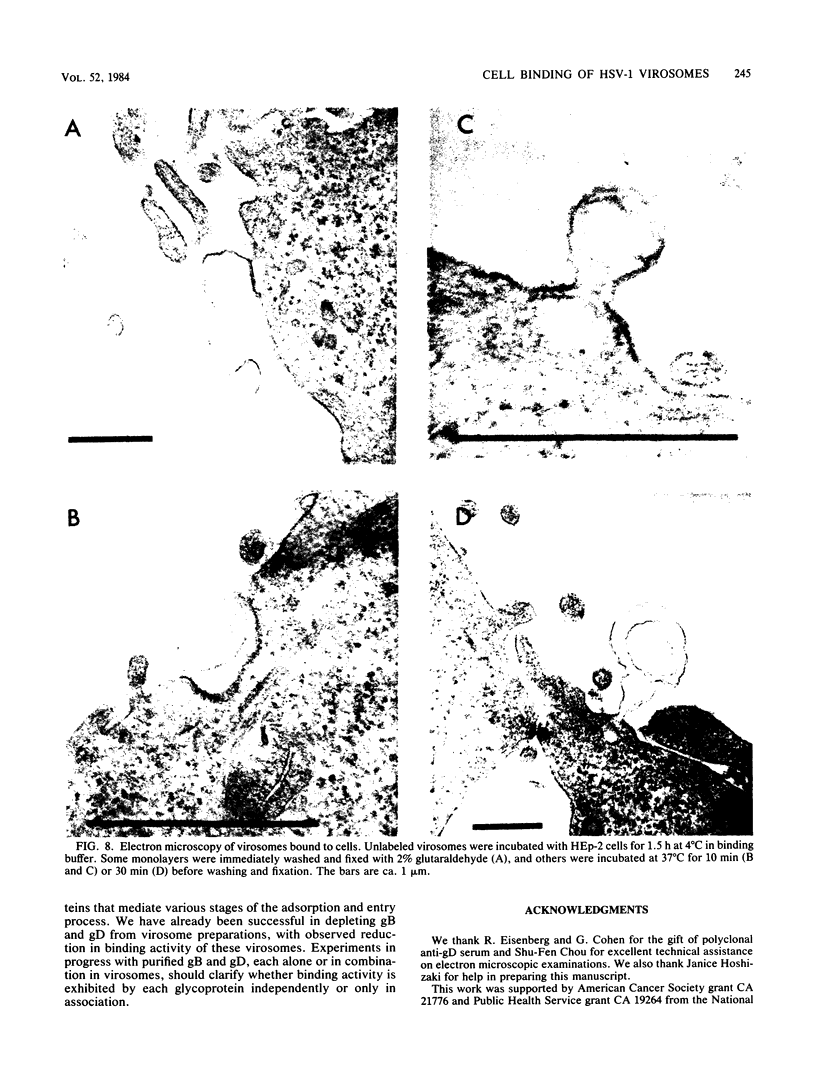
Envelope proteins and lipids were extracted from purified herpes simplex virus type 1 virions with octyl glucoside and mixed with phosphatidylcholine for preparation of virosomes by removal of the detergent. Greater than 85% of the extracted envelope proteins, including all the glycoproteins and the nonglycosylated protein designated VP16, were associated with virosomes, which ranged in density from ca. 1.07 to 1.13 g/cm3. All the glycoproteins except gC were as susceptible to degradation by added protease in virosomes as in virions, indicating similar orientations in both. Approximately 30 to 40% of radiolabel incorporated into virosomes bound to HEp-2 cells within 1.5 h at either 4 or 37 degrees C. The cell-bound virosomes were enriched for gB and deficient in other glycoproteins, in comparison with unbound or total virosomes. Binding of virosomes to HEp-2 cells could be inhibited by purified virus, heparin, and monospecific antiviral antibodies. Polyclonal and monoclonal anti-gB antibodies were more effective at inhibiting virosome binding than were anti-gD or anti-gC antibodies. Virosomes depleted of gB or gD did not bind to cells as efficiently as did virosomes containing all the extracted enveloped components; this loss of binding activity was especially pronounced on depletion of gB. The binding of herpes simplex virus type 1 virosomes to cells is discussed in relation to possible heterogeneity of the virosomes and comparisons with binding of virions to cells. We also present electron microscopic evidence that bound virosomes can fuse with the cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassai E., Manservigi R., Corallini A., Terni M. Plaque dissociation of herpes simplex viruses: biochemical and biological characters of the viral variants. Intervirology. 1975;6(4-5):212–223. doi: 10.1159/000149476. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D. J., Bond V. C., Person S., Snipes W. Nucleotide sequences of herpes simplex virus type 1 (HSV-1) affecting virus entry, cell fusion, and production of glycoprotein gb (VP7). Virology. 1982 Oct 30;122(2):411–423. doi: 10.1016/0042-6822(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Hogue-Angeletti R., Cohen G. H. Amino-terminal sequence of glycoprotein D of herpes simplex virus types 1 and 2. J Virol. 1984 Jan;49(1):265–268. doi: 10.1128/jvi.49.1.265-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fries E., Helenius A. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur J Biochem. 1979 Jun;97(1):213–220. doi: 10.1111/j.1432-1033.1979.tb13105.x. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). I. Assembly of hemolytic and fusion factors from envelopes solubilized by Nonidet P40. Virology. 1972 Sep;49(3):627–639. doi: 10.1016/0042-6822(72)90519-3. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Association of the envelope glycoproteins of influenza virus with liposomes--a model study on viral envelope assembly. Virology. 1979 Aug;97(1):212–217. doi: 10.1016/0042-6822(79)90390-8. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. Virology. 1980 Jul 30;104(2):294–302. doi: 10.1016/0042-6822(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousoulas K. G., Pellett P. E., Pereira L., Roizman B. Mutations affecting conformation or sequence of neutralizing epitopes identified by reactivity of viable plaques segregate from syn and ts domains of HSV-1(F) gB gene. Virology. 1984 Jun;135(2):379–394. doi: 10.1016/0042-6822(84)90194-6. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., White J., Helenius A. Interactions of Semliki Forest virus spike glycoprotein rosettes and vesicles with cultured cells. J Cell Biol. 1983 Feb;96(2):455–461. doi: 10.1083/jcb.96.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Cohen G. H., Eisenberg R. J. Synthesis and processing of glycoprotein D of herpes simplex virus types 1 and 2 in an in vitro system. J Virol. 1983 Nov;48(2):521–533. doi: 10.1128/jvi.48.2.521-533.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Feuer B. I., Vanderoef R., Lenard J. Reconstituted G protein-lipid vesicles from vesicular stomatitis virus and their inhibition of VSV infection. J Cell Biol. 1980 Feb;84(2):421–429. doi: 10.1083/jcb.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- Uchida T., Kim J., Yamaizumi M., Miyake Y., Okada Y. Reconstitution of lipid vesicles associated with HVJ (Sendai virus) sikes. Purification and some properties of vesicles containing nontoxic fragment A of diphtheria toxin. J Cell Biol. 1979 Jan;80(1):10–20. doi: 10.1083/jcb.80.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAHERI A., CANTELL K. THE EFFECT OF HEPARIN ON HERPES SIMPLEX VIRUS. Virology. 1963 Dec;21:661–662. doi: 10.1016/0042-6822(63)90242-3. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Mapping of the structural gene for the herpes simplex virus type 2 counterpart of herpes simplex virus type 1 glycoprotein C and identification of a type 2 mutant which does not express this glycoprotein. J Virol. 1984 Mar;49(3):741–747. doi: 10.1128/jvi.49.3.741-747.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]