Abstract
Nonribosomal nucleolar protein gar2 is required for 18S rRNA and 40S ribosomal subunit production in Schizosaccharomyces pombe. We have investigated the consequences of the absence of each structural domain of gar2 on cell growth, 18S rRNA production, and nucleolar structure. Deletion of gar2 RNA-binding domains (RBDs) causes stronger inhibition of growth and 18S rRNA accumulation than the absence of the whole protein, suggesting that other factors may be titrated by its remaining N-terminal basic/acidic serine-rich domain. These drastic functional defects correlate with striking nucleolar hypertrophy. Point mutations in the conserved RNP1 motifs of gar2 RBDs supposed to inhibit RNA–protein interactions are sufficient to induce severe nucleolar modifications but only in the presence of the N-terminal domain of the protein. Gar2 and its mutants also distribute differently in glycerol gradients: gar2 lacking its RBDs is found either free or assembled into significantly larger complexes than the wild-type protein. We propose that gar2 helps the assembly on rRNA of factors necessary for 40S subunit synthesis by providing a physical link between them. These factors may be recruited by the N-terminal domain of gar2 and may not be released if interaction of gar2 with rRNA is impaired.
INTRODUCTION
Ribosome biogenesis in eukaryotes requires the coordinated expression of >200 different genes that are dispersed throughout the genome. Still, most steps of ribosome synthesis take place in the nucleolus where rDNA repeats are located and transcribed. The structural organization of the nucleolus, already known in higher eukaryotes (reviewed by Hadjiolov, 1985; Mélèse and Xue, 1995; Shaw and Jordan, 1995) has recently been described also in the yeast Schizosaccharomyces pombe (Léger-Silvestre et al., 1997b). In both lower and higher eukaryotes, the nucleolus consists of lightly stained fibrillar centers that are surrounded by the dense fibrillar component. The fibrillar regions contain rDNA, RNA polymerase I (pol I),1 and factors required for the early steps of rRNA maturation. The fibrillar regions are embedded in the granular component that contains preribosomal particles undergoing the late stages of maturation. Structure and appearance of the nucleolus are the identification marks of active pre-rRNA transcription, processing, and efficient assembly of ribosomes (Warner, 1990; Hadjiolov, 1985; Shaw and Jordan, 1995). The insertion of a Drosophila ribosomal unit into non-nucleolar chromatin leads to the formation of a nucleolus-like structure around the new transcriptionally active rDNA (Karpen et al., 1988). Normally, a change at any stage of ribosome synthesis alters the structure of the nucleolus (reviewed in Hadjiolov, 1985). Microinjection of antibodies against pol I in mitotic cells prevents the postmitotic reformation of the nucleolus in higher eukaryotes (Benavente et al., 1987). Specific inhibition of rRNA synthesis with drugs interacting directly with pol I (d-galactosamin or cordycepin) leads to nucleolar fragmentation, whereas inhibition of rRNA transcription with drugs interacting with rDNA (actinomycin D or camptothecin) induces the segregation of nucleolar substructures. Similar effects are observed after drug-induced inhibition of topoisomerase II (Govoni et al., 1994). Finally, drugs specifically inhibiting rRNA processing (toyocamicyn) or the assembly of ribosomal particles (5-fluoropyrimidines) induce nucleolar hypertrophy by accumulation of granular structures (reviewed in Hadjiolov, 1985).
Among the large number of factors found to be required for the biogenesis of yeast ribosomes (reviewed in Venema and Tollervey, 1995), some are ribosomal proteins that are preserved in mature cytoplasmic ribosomes (Otaka and Osawa, 1981), and others present various enzymatic activities such as exonucleolytic (Stevens et al., 1991; Amberg et al., 1992; Mitchell et al., 1996, 1997), RNA helicase (Sachs and Davis, 1990; Ripmaster et al., 1993; Widner and Wickner, 1993; Eichler and Craig, 1994; Kressler et al., 1997; Liang et al., 1997; Venema et al., 1997; Weaver et al., 1997), and peptidyl isomerase (Benton et al., 1994; Shan et al., 1994) activities. Finally, many abundant nucleolar nonribosomal proteins lacking apparent enzymatic activities are also essential for ribosome biogenesis. The precise mode of action of most of these proteins is still unknown. In general, molecular phenotypes associated with the absence of any nucleolar protein are not of outstanding variety and often amount to the underaccumulation of the same precursor and/or mature rRNA species (reviewed in Venema and Tollervey, 1995). For example, Nop1p, Gar1p, Sof1p, Nsr1p, Mpp10p, Rrp5p, and Rrp7p (Lee et al., 1991; Tollervey et al., 1991; Girard et al., 1992; Kondo and Inouye, 1992; Jansen et al., 1993; Venema and Tollervey, 1996; Baudin-Baillieu et al., 1997; Dunbar et al., 1997) are all required for the production of the 18S rRNA, whereas Nop4p/Nop77p, Nop56p, Nop58p, Nop2p, and Nop1p (Tollervey et al., 1991; Bergès et al., 1994; Sun and Woolford, 1994; Gautier et al., 1997; Hong et al., 1997) are necessary for normal accumulation of the 25S rRNA. The similarity of the phenotypes resulting from the lack of either of these nucleolar proteins does not necessarily mean they have the same role in these processing pathways.
A possible clue to investigate the function of these nucleolar proteins is that they share various conserved sequence or structural domains (reviewed in Shaw and Jordan, 1995). The proteins Nsr1p, Drs1p, Nop4p/Nop77p, Npi46p/Fpr3p, Srp40p, Dbp3p, Nop56p, Nop58p, and Nop2p (Kondo and Inouye, 1992; Ripmaster et al., 1993; Benton et al., 1994; Bergès et al., 1994; De Beus et al., 1994; Shan et al., 1994; Sun and Woolford, 1994; Meier, 1996; Gautier et al., 1997; Weaver et al., 1997) have highly charged acidic, serine-containing domains that are sometimes interrupted by basic clusters and often contain numerous phosphorylated residues. These domains are believed to be involved in protein–protein interactions. Negatively charged clusters in some of them might interact with nuclear localization signal (NLS) peptides (Lee et al., 1991; Shan et al., 1994). Many nucleolar proteins share common, structurally highly related RNA-binding domains (RBDs) such as Nsr1p, Nop1p, Ssb1p, and Nop4p/Nop77p (Schimmang et al., 1989; Clark et al., 1990; Lee et al., 1991; Bergès et al., 1994; Sun and Woolford, 1994). Finally, the proteins Nsr1p, Ssb1p, Nop1p, and Gar1p (Schimmang et al., 1989; Clark et al., 1990; Lee et al., 1991; Girard et al., 1992) all contain glycine- and arginine-rich (GAR) domains that are implicated in nonspecific protein–RNA interactions (Burd and Dreyfuss, 1994) and sometimes in protein–protein interactions (Cartegni et al., 1996).
A nonribosomal nucleolar protein, the S. pombe gar2 protein (Gulli et al., 1995), and its functional homologue, Saccharomyces cerevisiae Nsr1p (Lee et al., 1992), possess a highly phosphorylated N terminus, two RBDs, and a GAR domain at their C termini. The Nsr1 protein has been identified as an NLS-binding protein (Lee et al., 1991). Gar2− and nsr1− strains have the same phenotype; they are cold sensitive and exhibit deficits in the production of both 18S rRNA and 40S ribosomal subunit (Kondo and Inouye, 1992; Lee et al., 1992; Gulli et al., 1995). Recently, our laboratory has demonstrated that the gar2 protein was phosphorylated during mitosis (Gulli et al., 1997). The Gar2 and Nsr1 proteins are expected to facilitate the assembly of ribosomal components (reviewed in Xue and Mélèse, 1994); however, the molecular mechanism underlying the nucleolar function of these proteins is still unknown. To investigate the role of gar2, we have constructed a series of deletion mutants of different structural domains of the S. pombe protein gar2. These mutations have different effects on cell growth and the production of 18S rRNA. Moreover, these phenotypes always correlate with strong alterations of nucleolar structure, illustrating the tight link between nucleolar structure and function.
MATERIALS AND METHODS
Plasmids, Molecular Cloning, and Site-directed Mutagenesis
All the S. pombe expression plasmids carrying the deletions of the coding regions of the different domains of gar2 were obtained as follows; the gar2+ gene fused to the hemagglutinin (HA) tag was cloned into the BamHI site of the M13 mp18 phage, and appropriate restriction sites were created by directed mutagenesis (Kunkel et al., 1987) at both ends of each domain to be deleted. After digestions with the chosen restriction enzymes and ligation, the truncated gar2+–HA fusions were then cloned into the BamHI site of the pRWP expression vector, under the control of the strong thiamin-repressible nmt1 promoter (Basi et al., 1993). pHYNterm was obtained after deletion within gar2+ ORF of 636 bp corresponding to the coding sequence of the N-terminal basic and acidic regions and cloning into pRWP; ScaI restriction sites were created at the 5′ end and the 3′ end, respectively, with oligonucleotides G2P19 (5′-CTTAACGGAAGTACTATCCTTTTT-3′) and G2P28 (5′-CTCGTCTTCAGTACTGGATTCGGA-3′). pHYR was obtained after suppression of 534 bp corresponding to both RBD coding sequences; EcoRV restriction sites were created at the 5′ end and the 3′ end, respectively, with oligonucleotides G2P22 (5′-AAACAGTGCAGATATCGTTAGA-3′) and G2P27 (5′-GAGGAGTTGAGATATCCAAACGA-3′). pHYG was obtained after deletion of 114 bp corresponding to the coding region of the GAR domain; XhoI restriction sites were created at the 5′ end and the 3′ end, respectively, with oligonucleotides G2P25 (5′-ACCTCCACCAGCTCGAGGAGTTGA-3′) and G2P26 (5′-ACGGTTGGGGTCTCGAGAGCGAGC-3′). Mutations of the aromatic amino acids within the RNP1 motifs of each RBD were obtained by the same directed mutagenesis procedure, with oligonucleotide G2P31 (5′-CTCAAAATCAACAAGGCCAAGACCCTTTGAGCG-3′, changing Tyr306 and Tyr308 to Leu in RNP1 of the first RBD) and oligonucleotide G2P33 (5′-AGAGAAAGTAACGAGACCAAGACCCTTAAGACG-3′, changing Phe409 and Tyr411 to Leu in RNP1 of the second RBD). pHYRNP contains the sequence encoding gar2 with its four RNP1 aromatic residues changed to leucines. All mutations were verified by DNA sequencing (Sanger et al., 1977). All DNA manipulations and bacterial transformations were done according to described procedures (Sambrook et al., 1989).
Yeast Strains and Media
The wild-type gar2+ (Sp15) and the gar2-null (Sp91) strains were described previously (Gulli et al., 1997). The Sp91 strain was transformed with either pRWP, pMF938 (allowing the expression of the gar2–HA fusion; Gulli et al., 1997), pHYNterm, pHYR, pHYG, or pHYRNP, and the Sp15 was transformed with pRWP, pMF938, and pHYR by standard technique (Moreno et al., 1991). The S. pombe cells were grown in minimal medium, supplemented as required (Alfa et al., 1993). The genomic ΔRBD strain Sp108 was obtained by the replacement of the ura4+ gene inserted at the gar2+ locus in the Sp91 strain; two BamHI restriction sites were added by directed mutagenesis (oligonucleotides 5′-TTTTTGCCATTGGATCCACAACCTATA-3′ and 5′-ATGCCAAAAAGGATCCCAAAAACCCA-3′) on each side of the gar2+ ORF on a HindIII genomic fragment of 2034 bp. The intact gar2+ gene was then replaced by the sequence encoding gar2ΔRBDs-HA, taken from pHYR, after BamHI digestion and ligation. Sp91 (gar2::ura4+) was transformed with this linear construction, and ura transformants selected on 5′-FOA-supplemented rich medium. Candidate clones were verified by Western blotting with anti-HA antibodies for expression of the gar2ΔRBDs–HA fusion and then by Southern blotting to confirm the correct genomic replacement. Sp108 was then transformed with pRWP (to allow growth on minimal medium) and pMF938.
Expression of the Truncated Forms of gar2 in S. pombe
Minimal medium supplemented with thiamin was inoculated with an appropriate volume of a fresh repressed preculture of one of each strain and cells were grown to exponential phase (5 × 106–107 cells/ml) at 30°C. Cells were collected by centrifugation and washed three times with minimal medium (Alfa et al., 1993) to eliminate all traces of thiamin. Washed cells were then grown to midlog phase at 30°C in minimal medium without thiamin to allow the expression of the truncated proteins for 24 h. Cells were 1) analyzed immediately by electron microscopy (Léger-Silvestre et al., 1997b); 2) analyzed for rRNA accumulation; total RNAs were extracted by standard techniques (Tollervey and Mattaj, 1987), resolved on agarose gels under denaturing conditions, and observed after ethidium bromide staining; or 3) spotted onto minimal medium agar plates to compare their growth at nonpermissive temperature (19°C). For this latter experiment, 5 × 106-cells/ml 24-h derepressed cultures were serially diluted six times by 20%, and 5 μl of each dilution were spotted side by side onto minimal medium agar plates.
Embedding for Immunocytochemistry
The cells were placed in 1% low-melt agar. They were fixed with 4% formaldehyde in 0.1 M cacodylate buffer (pH 7.2) with 5 mM MgCl2 (45 min, room temperature). Cells were washed and incubated in 1% sodium metaperiodate (1 h) and treated with 500 mM ammonium chloride in cacodylate buffer (1 h). Samples were dehydrated in ethanol and infiltrated with medium grade LR White resin (Pelanne Instruments, Paris, France). The resin was polymerized (48 h, 50°C). Sections were cut on a Reichert (Vienna, Austria) Ultracut microtome, and ultrathin sections were mounted on 400-mesh nickel grids.
Immunoelectron Microscopy
The immunolocalization of the various antigens was performed according to Léger-Silvestre et al. (1997b). Grids were incubated with the primary antibodies diluted in PBS buffer with 2% BSA (2 h, room temperature) (anti-gar2, 1:20 [Gulli et al., 1995]; anti-HA, 1:500 [Field et al., 1988]; anti-gar1, 1:15 [Girard et al., 1993]). Sections were washed and then incubated with colloidal gold-conjugated antibodies diluted in PBS buffer with 1% BSA (1 h). The primary antibodies were omitted in the negative controls. Sections were contrasted with 5% aqueous uranyl acetate and imaged in a Jeol (Tokyo, Japan) 1200 EX electron microscope operating at 80 kV.
Glycerol Density Gradients
Sp91 cells from 300 ml of 2.107-cells/ml culture were collected after 24 h of expression of either gar2-HA or gar2ΔRBDs–HA, washed with sterile water, and resuspended in 2.5 ml of A200 buffer (200 mM K-acetate, 20 mM Tris-HCl, pH 8.0, 5 mM Mg-acetate, 0.2% Triton X-100, 1 mM dithiothreitol, and Protease Inhibitors Cocktail, Complete [Boehringer Mannheim, Indianapolis, IN]). Cells were lysed in a One Shot cell disrupter (Cell°D, Roquemaure, France), and the extract was clarified by centrifugation for 15 min at 10,000 × g. Total protein concentration was estimated in the supernatant with a Bio-Rad (Hercules, CA) assay. Four milligrams of total proteins were loaded in a 500-μl sample onto a 10–30% (vol/vol) glycerol gradient in 20 mM HEPES-KOH, pH 7.9, 60 mM KCl, 1 mM MgCl2. Gradients were centrifuged 10 h at 25,000 rpm in an SW41Ti rotor (Beckman, Palo Alto, CA), and 500-μl fractions were collected manually. Two hundred microliters from each fraction were trichloroacetic acid precipitated and migrated on SDS-PAGE. Samples were transferred onto nitrocellulose (Hybond C Super; Amersham, Arlington Heights, IL) and immunodetected with anti-HA antibodies.
Expression and Purification of gar2 Recombinant Protein and Its Truncated Forms
The BamHI fragments containing the coding sequences of gar2, gar2ΔRBDs, gar2ΔNterm, and gar2RNP1* were inserted into the bacterial expression vector pAR3040 under the control of the T7 promoter (Studier et al., 1990) and used to transform Escherichia coli BL21 (DE3) Lys S. Expression and purification were performed essentially as described by Gulli et al. (1997), except that BL21 cells were grown in M9 minimal medium for induction of expression with 0.4 mM isopropyl-1-thio-β-d-galactopyranoside.
RESULTS
Several Domains of gar2 Are Dispensable for Production of 18S rRNA and Function of the Nucleolus
To study the nucleolar function of the S. pombe gar2 protein, we have investigated the consequences of the absence of the structural domains of gar2 on cell growth, 18S rRNA production and nucleolar structure. Each deletion mutant of the structural domains of gar2 (Figure 1A) was placed under the control of the strong, thiamin-repressible nmt1 promoter (Basi et al., 1993) and expressed in the gar2-null strain (Gulli et al., 1997). Comparison of growth rates was performed as follows. After exponential growth under repressive conditions (with thiamin) at 30°C, expression of each mutant protein was achieved by shifting the cells into medium devoid of thiamin. After 24 h of growth, the cultures were then spotted onto agar plates that were incubated at 19°C to enhance growth rate differences. To compare 18S accumulation, total RNA was extracted from each strain grown for 24 h at 30°C. The RNAs were size fractionated by denaturing agarose gel electrophoresis and visualized by ethidium bromide staining. The ratio of 25 to 18S rRNA was measured and used to evaluate the deficits in the production of 18S rRNA. Observations at the electron microscopic levels were done on exponential phase cells, after 24 h of expression of each mutant protein at 30°C. A wild-type gar2+ strain and a gar2-null strain both transformed with an empty vector, as well as a gar2-null strain expressing intact gar2, were used as references. To facilitate detection, the C terminus of every mutant protein was fused with a triple HA epitope (Field et al., 1988). It was verified that the HA tag had no effect on the nucleolar accumulation and function of the gar2 protein.
Figure 1.
(A) Schematic representation of gar2 protein and its derivatives used in this study. Hatched box, N-terminal basic/acidic serine-rich domain; light gray boxes, RBDs containing conserved RNP1 motifs (checkered boxes); vertical-hatched box, GAR domain; black box, HA epitope; *, Y306, Y308, F409, and Y411 residues have been replaced with leucines. (B) Effects of gar2 mutations on cell growth. Growth comparisons were done by spotting each strain onto solid minimal medium after growing for 24 h in liquid medium at 30°C under derepressive conditions. The plates were incubated for 8 d at 19°C. From left to right, 25000, 5000, 1000, 200, and 40 cells were spotted.
Compared with gar2+ cells, gar2-null cells grow more slowly at low temperature (Figure 1B, lanes 1 and 2) and exhibit a 2 to 2.5 times higher 25-to-18S ratio (Figure 2, columns 1 and 2). The nucleolus of the gar2-null cells shows slight ultrastructural changes (Figure 3, A and B). It is wider, less dense, and less round but still has the characteristic organization in three regions observed in S. pombe cells (Léger-Silvestre et al., 1997b). Expression of the gar2 protein restores the normal accumulation of 18S rRNA (Figure 2, column 3) and wild-type nucleolar structure (Figure 4A). Immunodetection with anti-HA antibodies reveals that the protein is localized to the nucleolus (Figure 4A). Surprisingly, growth comparison indicates that expression of gar2 in the gar2-null strain induces a cell growth rate significantly higher than that of the wild-type gar2+ strain (Figure 1B, lane 3). In the gar2-null strain, expression of gar2 lacking either its N-terminal charged domain (gar2ΔNterm) or its GAR domain (gar2ΔGAR) restores the normal ratio of 25 and 18S rRNAs (Figure 2, columns 4 and 5) and supports formation of normal nucleolar structures. Growth induction after expression of gar2ΔNterm is, however, less efficient than with gar2 or gar2ΔGAR (Figure 1B, lanes 3–5).
Figure 2.
Effects of gar2 mutations on 18S rRNA accumulation. 25-to-18S ratios were measured on three samples of RNAs from each strain, after extraction, resolution on agarose gel under denaturing conditions, ethidium bromide staining, and observation under UV light to evaluate the deficit in 18S rRNA. Overexpression of gar2 either without its N-terminal or GAR domain restores normal 18S rRNA accumulation in the Sp91 (gar2-null) strain. Overexpression of gar2 lacking its RBDs increases the deficit in 18S rRNA, and point mutations in the conserved RNP1 motifs of the RBDs prevent total restoration of 18S level. The Sp108 strain expressing the gar2ΔRBDs protein under the control of the gar2 promoter also exhibits stronger deficit in 18S rRNA than the gar2-null strain.
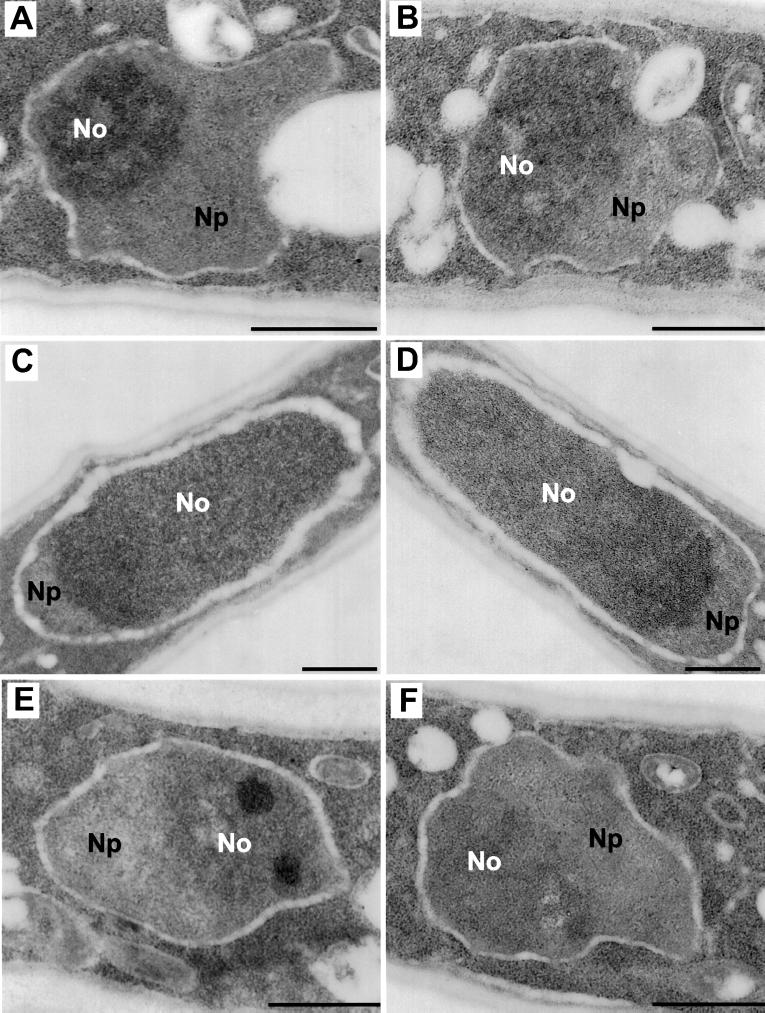
Figure 3.
Electron microscopic transversal sections of actively growing S. pombe cells at 30°C. (No, nucleolus; Np, nucleoplasm). (A) A wild-type (Sp15) strain. (B) A gar2-null strain (Sp91) with a slightly larger but structurally well-organized nucleolus. (C) Sp91 expressing gar2ΔRBDs protein presents an unusual hypertrophied nucleolus. (D) Sp15 expressing gar2ΔRBDs protein exhibits the same nucleolar hypertrophy. (E) Sp91 overexpressing gar2 mutated in the RNP1 motifs (gar2RNP1*) presents local dense fibrillar structures. (F) The peculiar fibrillar structures are absent from the nucleolus of Sp91 expressing gar2RNP1* lacking the N-terminal end of the protein, indicating that these modifications are dependent on the presence of the N-terminal domain of gar2 when binding to RNA is impaired. Bar, 500 nm.
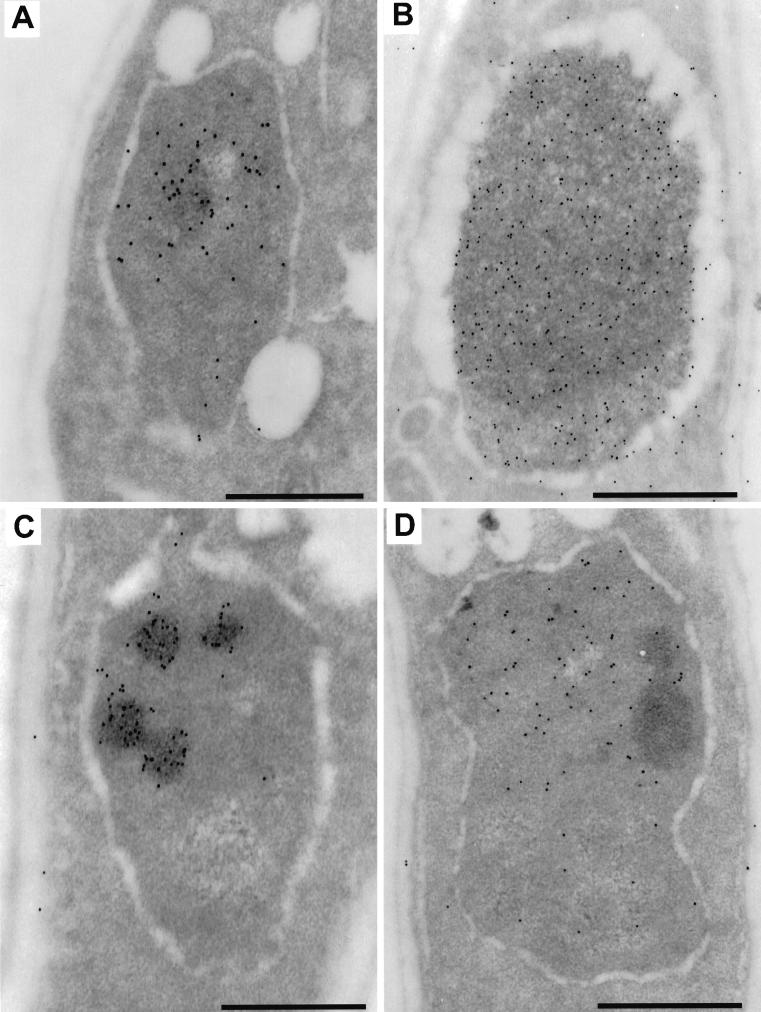
Figure 4.
Immunostaining of various nucleolar antigens on S. pombe cells. (A) Immunodetection with anti-HA antibodies on Sp91 (gar2-null) overexpressing gar2–HA. The nucleolus is normal, and the protein is exclusively nucleolar. (B) Immunodetection with anti-HA antibodies of gar2ΔRBDs overexpressed in Sp91. The truncated protein is both in the large fibrillar nucleolus and in the nucleoplasm. (C and D) Anti-HA (C) and anti-Spgar1 (D) antibodies on Sp91 overexpressing gar2RNP1*. gar2 precisely colocalizes with the dense structures in the nucleolus, which appears larger than these dense zones according to gar1 localization. Bar, 500 nm.
Expression of gar2 Lacking Its RBDs Has Severe Inhibitory Effects
The presence of two RBDs in the gar2 protein indicates it is most probably an RNA-binding protein. To test the consequences of the abolition of gar2-RNA interaction in vivo, the same tests as described above were performed on gar2-null cells expressing gar2 deleted of its two RBDs (gar2ΔRBDs). The growth rate of the resulting gar2ΔRBD strain (Figure 1B, lane 6) is significantly lower than that of the gar2-null strain in which gar2 is simply missing (Figure 1B, lane 2). These cells also exhibit a very strong decrease in 18S rRNA accumulation (Figure 2, column 6), as indicated by the 25-to-18S ratio that is more than two or four times higher than in the gar2-null or in the wild-type cells, respectively. Expression of gar2ΔRBDs protein in the gar2-null strain also leads to the appearance of a very large and dense nucleolus (Figure 3C) that loses its ultrastructural characteristics and possesses a uniformly fibrillar-like structure. After DAPI staining, the nucleoplasm appears as a thin crescent around the enormous, faintly stained nucleolus (Léger-Silvestre, unpublished results). The enlargement of the nucleolus induced by the expression of this truncated form of the gar2 protein strongly resembles an aberrant nucleolar accumulation of cellular components, as the nucleus of this mutant becomes at least twice as wide as a normal one on electron microscopy sections. Immunodetection using anti-HA antibodies indicates that gar2ΔRBDs is dispersed throughout the nucleus (Figure 4B) and not confined to the nucleolus anymore, probably because it has lost its binding capacity for nucleolar RNAs. In situ hybridization with a 35S rRNA probe reveals no obvious accumulation of pre-rRNA in the nucleolus (Léger-Silvestre, unpublished result), rendering unlikely a potential deficit in export of preribosomal particles. We have found that expression of gar2ΔRBDs in wild-type cells that contain the endogenous wild-type gar2 protein also impairs cell growth, although to a lesser extent (Sicard, unpublished observation), and induces the same type of nucleolar hypertrophy (Figure 3D).
To verify that the strong phenotypes of the gar2-null strain expressing gar2ΔRBDs are not due to the overexpression of this mutant protein but indeed to the absence of the RBDs of gar2, the coding region of the chromosomal gar2+ gene was replaced with that of the gar2ΔRBDs construct (Figure 1A). The correct integration was verified by Southern blot and by Western immunoblot with anti-HA antibodies. Expression of the gar2ΔRBDs protein-coding gene is directed by the gar2 promoter under more physiological conditions. The cold sensitivity and 18S rRNA deficit of this new strain were found to be stronger than those of the gar2-null strain (Figures 1B, lane 8, and 2, column 8), confirming that these drastic effects on nucleolar function are not dependent on the level of expression of gar2ΔRBDs. Clearly, the absence of gar2 is less inhibitory for the cell than the presence of gar2 lacking its RBDs.
Point Mutations in gar2 RBDs Induce Structural Modifications of the Nucleolus
It has been shown that alterations of the aromatic residues at positions 3 and 5 within the conserved RNP1 motif of the RBD are sufficient to reduce the RNA binding affinity of the tested RNA-binding protein in vitro (Caceres and Krainer, 1993; Zuo and Manley, 1993; Mayeda et al., 1994). To check whether the inhibition of gar2-RNA interaction has the same drastic effects on nucleolar organization as the absence of the whole RBDs does, we changed both aromatic amino acids in the RNP1 motifs of both RBDs to leucines and overexpressed this new mutant form of the gar2 protein (gar2RNP1*; Figure 1A) in the gar2-null strain. The growth rate of the transformed cells is comparable with that of the wild-type cells (Figure 1B, lane 7). Therefore, these point mutations prevent the growth induction observed when intact gar2 is overexpressed in the gar2-null strain (Figure 1B, lane 3). Production of 18S rRNA is only partially restored by the gar2RNP1* protein (Figure 2, column 7). Moreover, expression of this mutant protein results in structural alterations in the nucleolus, where several dense zones appear (Figure 3E). These structural changes are reminiscent in their fibrillar aspect of the huge nucleolus of the gar2ΔRBDs-expressing strain, although the changes in the gar2RNP1* strain are less drastic. By use of antibodies directed against the S. pombe gar1 nucleolar protein (Girard et al., 1993), it became apparent that the nucleolus is not confined to the dense fibrillar areas (Figure 4D). Interestingly, although gar1 is excluded from these dense structures (Figure 4D), gar2RNP1* is almost exclusively located in them (Figure 4C). It is noteworthy that these nucleolar modifications are highly reminiscent of those previously described for the gar2-interrupted strain (Gulli et al., 1995): we have recently found that the N-terminal part of gar2 that remains is still expressed and colocalizes with the spherical “dense bodies” of the nucleolus that are characteristic of this strain (Léger-Silvestre et al., 1997a). These dense structures in the nucleolus look like an accumulation of nucleolar components around or linked to the N-terminal end of gar2 when its RBDs are missing or altered.
A gar2-null strain overexpressing a double mutant bearing both point mutations in the RNP1 motifs of the two RBDs as described above and a deletion of the N-terminal highly charged region presents no obvious modification in its nucleolar structure (Figure 3F). This result supports the hypothesis that the N-terminal domain of the gar2 protein is responsible for the abnormal accumulation of nucleolar molecules within the dense fibrillar regions in strains in which gar2–RNA interaction is inhibited. This double mutant has no additional inhibitory effect, probably because it has lost its capacity to titrate other factors.
Gar2 and gar2ΔRBDs Are Not Assembled in the Same Complexes
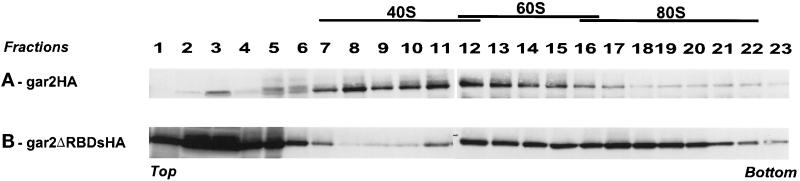
To study the ribonucleoprotein complexes with which the wild-type gar2 or the mutant gar2ΔRBDs proteins are associated in vivo, cellular extracts obtained from a gar2-null strain expressing either the gar2 or the gar2ΔRBDs protein were fractionated by sedimentation on 10–30% glycerol gradients. Immunodetection revealed that wild-type gar2 is sedimenting mainly in a region that exhibits 40–60S sedimentation coefficient (Figure 5A), indicating that it is associated with higher-order structures in the nucleolus. It has been verified that the endogenous gar2 protein from a wild-type strain behaves similarly. In contrast, gar2 that lacks its RBDs is present in two size fractions (Figure 5B). The majority of this protein, because it is at the top of the gradient, likely is in a free form. We assume that gar2ΔRBDs is predominantly detached from nucleolar RNAs. However, the other fraction of gar2ΔRBDs sediments with 80S structures, indicating that it is associated with more complex nucleolar structures than the wild-type protein. The existence of these abnormally large complexes suggests that the N-terminal domain of gar2 has different affinity for nucleolar components in vivo in the absence of its RBDs.
Figure 5.
gar2 and gar2ΔRBDs migrations in glycerol velocity gradients. Equivalent amounts of total extracts from Sp91 (gar2-null) overexpressing gar2–HA or gar2ΔRBDs–HA were loaded onto 10–30% glycerol gradients and subjected to centrifugation. Both proteins were detected with anti-HA antibodies. Whereas gar2 belongs to 40–60S sedimentation coefficient complexes, gar2ΔRBDs protein is found either free at the top of the gradient or associated with structures larger than those with which the wild-type protein is associated.
Gar2 Specifically Interacts with Ribosomal Proteins In Vitro
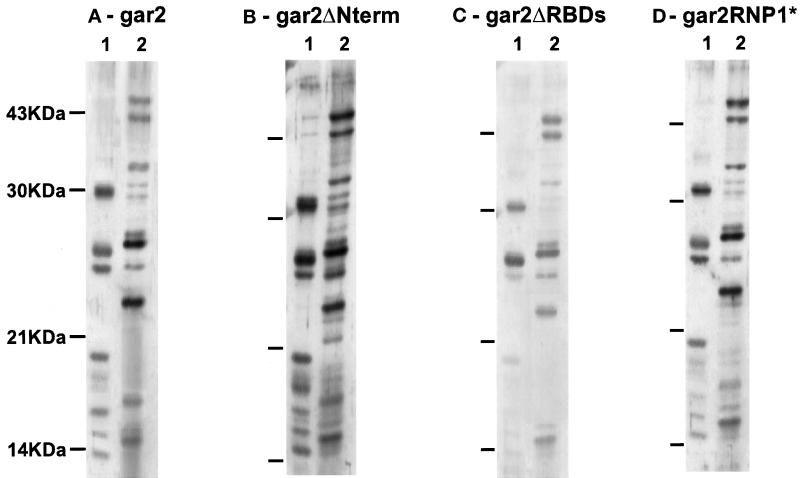
It has been previously proposed that nucleolar proteins bearing highly charged domains may interact with ribosomal proteins and help their assembly into ribosomal subunits (Xue and Mélèse, 1994). To check whether ribosomal proteins could bind to the N-terminal end of gar2, we have tested the ability of recombinant gar2 to interact with S. pombe ribosomal proteins in vitro in far-Western assays. Equal amounts of ribosomal proteins from either the small or the large subunit were resolved by SDS-PAGE, transferred onto a nitrocellulose membrane, and incubated with purified recombinant gar2 protein tagged with the T7 epitope. After immunodetection with anti-T7 and anti-rabbit HRP conjugate antibodies, the gar2–ribosomal protein interactions were revealed by autoradiography. These experiments indicate that gar2 is able to bind directly some ribosomal proteins from both subunits (Figure 6A). The absence of cross-reaction with some abundant ribosomal proteins, as judged by comparison with the Ponceau staining of the membrane, shows that the observed interactions are indeed specific. As a negative control, the membranes were also incubated with a 17-kDa T7-tagged peptide containing the first ∼70 N-terminal amino acids of gar2 fused to ∼50 unrelated amino acids (see Methods in Léger-Silvestre et al., 1997a for detailed description of this peptide). This gar2-related T7-tagged peptide fails to interact with any ribosomal protein on an equivalent blot (Sicard, unpublished result). A far-Western experiment on an analogous ribosomal protein blot was performed using the same concentration of purified recombinant T7-tagged gar2ΔNterm or gar2ΔRBDs proteins (Figure 6, B and C). Unexpectedly, gar2 without its N-terminal domain displays the same pattern of interaction with ribosomal proteins in vitro as intact gar2 (Figure 6B). This renders unlikely the in vivo titration of ribosomal proteins by the N-terminal part alone. The usual hypothesis of an interaction between ribosomal proteins and the highly charged domains of nucleolar proteins is unlikely. In the absence of the RBDs of gar2, interactions with some ribosomal proteins are lost, and generally, the affinity of gar2 for ribosomal proteins is weaker (Figure 6C). It is likely that the deletion of the RBDs induces strong alterations in the structure of the gar2 protein in vitro, and this may explain the reproducible weak pattern of interaction between gar2ΔRBDs and ribosomal proteins. We cannot exclude that the appearance of a hypertrophied nucleolus in the gar2ΔRBDs-expressing strain is not solely due to the loss of interaction with nucleolar RNAs in vivo but also to important structural changes in this mutant protein. In the same far-Western experiment, we have shown that the RNP1 point mutations neither quantitatively nor qualitatively prevent the gar2 protein from interacting with ribosomal proteins in vitro (Figure 6D). Thus, the accumulations of nucleolar material observed in the strain expressing gar2RNP1* are most likely solely a consequence of an inhibition of the interaction of gar2 with its target RNA.
Figure 6.
Far-Western blots on S. pombe ribosomal proteins from the small (1) or the large (2) subunit. Blots were incubated with 5 μg/ml recombinant gar2–T7 (A), gar2ΔNterm–T7 (B), gar2ΔRBDs–T7 (C), or gar2RNP1*–T7 (D) proteins. Protein–protein interactions were revealed by immunostaining with anti-T7 antibodies and ECL. The full-length gar2 protein interacts in vitro with ribosomal proteins from both subunits (A), and affinity of gar2ΔNterm for ribosomal proteins is identical to that of wild-type gar2 (B). The affinity of gar2ΔRBDs for ribosomal proteins in vitro is generally low, suggesting strong alteration of gar2 structure after the deletion of the RBDs. Only a few interactions are significantly lost (C). gar2RNP1* has the same affinity for ribosomal proteins in vitro as wild-type gar2 (D).
DISCUSSION
In this study, we have investigated the role of the S. pombe nonribosomal nucleolar protein gar2 by expressing several deletion mutants of the functional domains of the protein in a gar2-null strain. We found that the presence of a truncated form of the gar2 protein has very strong inhibitory effects on cell growth as well as on nucleolar structure and function. Expression of a gar2 protein that lacks its RBDs induces slow growth, nucleolar hypertrophy and 18S rRNA deficit. These phenotypes are significantly more drastic than those observed in the gar2-null strain. Point mutations in the conserved RNP1 motifs of the RBDs of the gar2 protein are also inhibitory to nucleolar structure and function as they induce aggregations of nucleolar material. The amino acids we have changed in the RNP1 motifs of gar2 RBDs have been extensively studied for other RBD-containing proteins; they are implicated in direct contacts with nucleotides (Jessen et al., 1991; Mayeda et al., 1994), are required for RNA–protein interactions in vitro (reviewed in Burd and Dreyfuss, 1994), and are often crucial in vivo for the function of the proteins they belong to (Watanabe and Yamamoto, 1994; Sun and Woolford, 1997). Therefore, we assume that these point mutations prevent the interaction of gar2 with RNA in vivo. In the case of the gar2 protein, a double mutant with both mutated RNP1 motifs and deletion of the N-terminal domain is unable to induce such nucleolar modifications. This result indicates that the inhibition of interaction of gar2 with RNA is deleterious for the cells only if the highly charged domain is present. In vivo, gar2ΔRBDs protein is found in higher-order nucleolar structures that sediment significantly faster than structures containing the wild-type protein (Figure 5). This result suggests that the N-terminal domain of gar2 has different or increased affinity for other factors in the absence of the RBDs. Because it had been suggested previously that nucleolar proteins could help ribosomal proteins associate with pre-rRNA by interacting with them through highly charged domains (Xue and Mélèse, 1994), we have tested whether the factors associated with the N-terminal domain of gar2 are ribosomal proteins. Using far-Western assays, we have compared the interaction of S. pombe ribosomal proteins with gar2 and its various deletion mutants. Indeed, we found that the gar2 protein interacts with some ribosomal proteins in vitro but that this interaction depends on the presence of its C-terminal domain. It is thus unlikely that ribosomal proteins bind in vivo to the highly charged domain of gar2. Very recently, a similar observation has been made by Sun and Woolford (1997), who showed that the acidic motifs located between the RBDs of nucleolar protein Nop4p were unlikely to bind to ribosomal proteins in vivo. The factors linked to the N-terminal domain of gar2 remain to be characterized.
Several data suggest that the gar2 protein binds S. pombe rRNA in vivo. We can assume that a nucleolar RNA-binding protein interacts with some nucleolar RNAs, most likely with small nucleolar RNAs and/or pre-rRNA. Consistent with this, a SELEX experiment (Klug and Famulok, 1994) with a recombinant gar2 protein has revealed a consensus sequence that is present in S. pombe 18S rRNA (Sicard, unpublished results). Furthermore, gar2 exhibits a structural organization very similar to vertebrate nucleolin (Gulli et al., 1995) that is thought to be implicated in the synthesis (Bouche et al., 1984) and packaging of pre-rRNA (Herrera et Olson, 1986). Supporting this hypothesis, nucleolin has been shown to interact with several sites on pre-rRNA in vivo (Ghisolfi-Nieto et al., 1996; Serin et al., 1996).
We propose that the S. pombe nucleolar gar2 protein provides a link between pre-rRNA and factors necessary for ribosome synthesis. The gar2 protein binds these nucleolar factors, which are probably not ribosomal proteins, with its N-terminal domain and interacts with pre-rRNA through its RBDs. In the absence of gar2, this assembly is still possible, but less efficient, especially at low temperatures, at which protein–protein interactions are thermodynamically impaired. This could explain why the gar2-null strain shows a cold-sensitive phenotype. When the gar2 protein lacks its RBDs, it still can interact with its natural target factors, but it is unable to tether the resulting complexes to the pre-rRNA. Therefore, they accumulate in the nucleolus, making it much larger, as seen on Figure 3C. Supporting this model, inhibition of the ability of gar2 to bind RNA by point mutations in the RNP1 motifs is sufficient to induce profound nucleolar modifications resembling accumulation of nucleolar factors, and this happens only in the presence of the N-terminal domain of gar2. We envisage that in the wild-type strain, association among gar2, rRNA, and factors required for ribosome production is only transient. Anchoring of gar2 to rRNA is necessary and sufficient to induce the release of the associated factors from the N-terminal highly charged domain. If this anchoring is prevented because of the absence of the RBDs, factors remain linked to the N-terminus of gar2, producing the observed large complexes sedimenting rapidly in glycerol gradients.
This model also explains the strong growth induction after overexpression of gar2 in a gar2-null strain. This positive influence on growth cannot be observed when gar2 is overexpressed in wild-type cells (Sicard, unpublished observation), indicating that gar2 is not in limiting amounts in normal cells. In the unbalanced context attributable to the absence of gar2, artificial overexpression of this protein can bring together molecules that had been produced normally but that were being recruited and assembled more slowly. As a consequence, more ribosomes could be formed, and growth rate increases. An evidence that some ribosomal material strongly accumulates, whereas others are produced in limiting quantities, can be found by observing free ribosomal subunit profiles after separation on sucrose gradients. The deficit in 40S subunit in the gar2-null strain is accompanied by an overaccumulation of free 60S (Sicard, unpublished results). After 24 and even 48 h of overexpression of gar2 (which is largely sufficient to restore the 40S subunit steady-state level), there is still an excess of 60S subunit, suggesting that a large amount of 60S had indeed accumulated while the 40S subunit was still missing (Sicard, unpublished results). On the molecular level, it is tempting to imagine that factors that are normally recruited by gar2 are also “accumulating” in the nucleolus of the gar2-null cells because they are not efficiently assembled, and that a sudden overexpression of gar2 helps recruit them more rapidly. This idea is also supported by the observation that the overexpression of gar2 in the strain in which gar2ΔRBDs is expressed from the genome does not make the cells grow faster but only restores wild-type growth rate (Sicard, unpublished observation). This could mean that in this case, factors titrated by the N-terminal domain of gar2 cannot be recruited. Only those factors that are expressed during the expression of gar2 can be recruited and used for ribosome biogenesis.
The striking phenotypes observed with our mutants confirm that there exists a deep relationship between nucleolar structure and correct ribosome production (Hadjiolov, 1985). But our model only partially explains why the nucleolar modifications are so different after overexpression of either gar2ΔRBDs or gar2 mutated in the conserved RNP1 motifs. The altered amino acids are known to be crucial for protein–RNA interactions in vitro (Caceres and Krainer, 1993; Zuo and Manley, 1993; Mayeda et al., 1994; Serin et al., 1997) but are not essential in vitro for the binding of gar2 to ribosomal proteins (this study). The function of the whole RBDs appears more complex. Protein–protein interactions mediated by consensus RBDs have already been described (Scherly et al., 1990; Kessler and Sachs, 1998). The concomitant loss of binding to rRNA and other factors, as well as the probable structural changes induced by the deletion of the RBDs, might lead to a more drastic nucleolar disorganization than the sole impairment of the interaction between gar2 and RNA that is observed with the gar2RNP1* mutant.
The tight correlation between alteration of nucleolar structure and function in our mutants is in favor of a functional role of gar2 in ribosome assembly rather than simply a structural one. Other nonribosomal nucleolar proteins have also been shown to influence nucleolar structure at different levels. For example, yeast nucleolar protein Nop2p, which is required for 60S subunit production (Hong et al., 1997), has an N-terminal domain made of clusters of acidic and basic residues (De Beus et al., 1994), but its precise function is still largely unknown. Overexpression of Nop2p induces nucleolar structure modifications but, surprisingly, does not alter cell growth and ribosome formation (De Beus et al., 1994). A role for Nop2p in the maintenance of nucleolar ultrastructure has been therefore proposed. Because nucleolar changes after gar2 mutations are linked to drastic effects on growth and small ribosomal subunit accumulation, we can envisage a more direct functional interaction between this nucleolar protein and factors required for ribosome biogenesis. Most probably, these factors functionally linked to gar2 are more important than gar2 itself. We are currently trying to identify these factors.
ACKNOWLEDGMENTS
We are grateful to T. Kiss, Y. Henry, P. Bouvet, and J.-P. Girard for critical reading of the manuscript and to J.-P. Gélugne and C. Desplats for fruitful discussions. We thank Y. de Préval for oligonucleotide synthesis and D. Villa for the photographs. This work was supported by the Association pour la Recherche sur le Cancer, Région Midi-Pyrénées, the Ligue Nationale contre le Cancer, the Centre National de la Recherche Scientifique, and the Université P. Sabatier. H.S. has been supported by fellowships from the Centre National de la Recherche Scientifique and the Ligue Nationale contre le Cancer.
Abbreviations used:
- GAR
glycine- and arginine-rich
- HA
hemagglutinin
- NLS
nuclear localization signal
- pol I
RNA polymerase I
- RBD
RNA-binding domain
REFERENCES
- Alfa CE, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor NY: Cold Spring Harbor Laboratory; 1993. [Google Scholar]
- Amberg DC, Goldstein AL, Cole CN. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamin repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Baudin-Baillieu A, Tollervey D, Cullin C, Lacroute F. Functional analysis of Rrp7p, an essential yeast protein involved in pre-rRNA processing and ribosome assembly. Mol Cell Biol. 1997;17:5023–5032. doi: 10.1128/mcb.17.9.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R, Rose KM, Reimer G, Hügle-Dörr B, Scheer U. Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol. 1987;105:1483–1491. doi: 10.1083/jcb.105.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton BM, Zang JH, Thorner J. A novel FK506- and rapamycin-binding protein (FPR3 gene product) in the yeast Saccharomyces cerevisiae is a proline rotamase localised to the nucleolus. J Cell Biol. 1994;127:623–639. doi: 10.1083/jcb.127.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergès T, Petfalski E, Tollervey D, Hurt EC. Synthetic lethality with fibrillarin identifies NOP77p, a nucleolar protein required for pre-rRNA processing and modification. EMBO J. 1994;13:3136–3148. doi: 10.1002/j.1460-2075.1994.tb06612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G, Caizergues-Ferrer M, Bugler B, Amalric F. Interrelations between the maturation of a 100KDa nucleolar protein and pre-rRNA synthesis in CHO cells. Nucleic Acids Res. 1984;12:3025–3035. doi: 10.1093/nar/12.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Caceres J, Krainer AR. Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 1993;12:4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartegni L, Maconi M, Morandi E, Cobianchi F, Riva S, Biamonti G. hnRNP A1 selectively interacts through its Gly-rich domain with different RNA-binding proteins. J Mol Biol. 1996;259:337–348. doi: 10.1006/jmbi.1996.0324. [DOI] [PubMed] [Google Scholar]
- Clark MW, Yip ML, Campbell J, Abelson J. SSB-1 of the yeast Saccharomyces cerevisiae is a nucleolar-specific, silver-binding protein that is associated with the snR10 and snR11 small nuclear RNAs. J Cell Biol. 1990;11:1741–1751. doi: 10.1083/jcb.111.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beus E, Brockenbrough JS, Hong B, Aris JP. Yeast NOP2 encodes an essential nucleolar protein with homology to a human proliferation marker. J Cell Biol. 1994;127:1799–1813. doi: 10.1083/jcb.127.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DA, Wormsley S, Agentis TM, Baserga SJ. M Pp 10p, a U3 small nucleolar ribonucleoprotein component required for pre-18S rRNA processing in yeast. Mol Cell Biol. 1997;17:5803–5816. doi: 10.1128/mcb.17.10.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler DC, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- Field J, Nikawa JI, Broek D, MacDonald B, Rogers L, Wilson IA, Lerner RA, Wigler M. Purification of a RAS-responsive adenyl cyclase complex from S. cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier T, Bergès T, Tollervey D, Hurt E. Nucleolar KKE/D repeat proteins Nop56p and Nop58p interact with Nop1p and are required for ribosome biogenesis. Mol Cell Biol. 1997;17:7088–7098. doi: 10.1128/mcb.17.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisolfi-Nieto L, Joseph G, Puvion-Dutilleul F, Amalric F, Bouvet P. Nucleolin is a sequence-specific RNA-binding protein: characterization of targets on pre-ribosomal RNA. J Mol Biol. 1996;260:34–53. doi: 10.1006/jmbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Girard J-P, Caizergues-Ferrer M, Lapeyre B. The SpGAR1 gene of Schizosaccharomyces pombe encodes the functional homologue of the snoRNP protein GAR1 of Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:2149–2155. doi: 10.1093/nar/21.9.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J-P, Lehtonen H, Caizergues-Ferrer M, Amalric F, Tollervey D, Lapeyre B. Gar1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992;11:673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni M, Farabegoli F, Pession A, Novello F. Inhibition of topoisomerase II activity and its effect on nucleolar structure and function. Exp Cell Res. 1994;211:36–41. doi: 10.1006/excr.1994.1055. [DOI] [PubMed] [Google Scholar]
- Gulli M-P, Faubladier M, Sicard H, Caizergues-Ferrer M. Mitosis-specific phosphorylation of gar2, a fission yeast nucleolar protein structurally related to nucleolin. Chromosoma. 1997;105:532–541. doi: 10.1007/BF02510490. [DOI] [PubMed] [Google Scholar]
- Gulli M-P, Girard J-P, Zabetakis D, Lapeyre B, Mélèse T, Caizergues-Ferrer M. gar2 is a nucleolar protein from Schizosaccharomyces pombe required for 18S rRNA and 40S ribosomal subunit accumulation. Nucleic Acids Res. 1995;23:1912–1918. doi: 10.1093/nar/23.11.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiolov AA. The nucleolus and ribosome biogenesis. New York: Springer-Verlag; 1985. [Google Scholar]
- Herrera AH, Olson MOJ. Association of protein C23 with rapidly labeled nucleolar RNA. Biochemistry. 1986;25:6258–6264. doi: 10.1021/bi00368a063. [DOI] [PubMed] [Google Scholar]
- Hong B, Brockenbrough JS, Wu P, Aris JP. Nop2p is required for pre-rRNA processing and 60S ribosome subunit synthesis in yeast. Mol Cell Biol. 1997;17:378–388. doi: 10.1128/mcb.17.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Tollervey D, Hurt EC. U3 snoRNP protein with homology to splicing factor PRP4 and GB domains is required for ribosomal RNA processing. EMBO J. 1993;12:2549–2558. doi: 10.1002/j.1460-2075.1993.tb05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen TH, Oubridge C, Teo CH, Pritchard C, Nagai K. Identification of molecular contacts between the U1 A small nuclear ribonucleoprotein and U1 RNA. EMBO J. 1991;10:3447–3456. doi: 10.1002/j.1460-2075.1991.tb04909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen GH, Schaefer JE, Laird CD. A drosophila rRNA gene located in euchromatin is active in transcription and nucleolus formation. Genes Dev. 1988;2:1745–1763. doi: 10.1101/gad.2.12b.1745. [DOI] [PubMed] [Google Scholar]
- Kessler SH, Sachs AB. RNA recognition motif 2 of yeast Pab1p is required for its functional interaction with eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998;18:51–57. doi: 10.1128/mcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug SJ, Famulok M. All you wanted to know about SELEX. Mol Biol Rep. 1994;20:97–107. doi: 10.1007/BF00996358. [DOI] [PubMed] [Google Scholar]
- Kondo K, Inouye M. Yeast NSR1 protein that has structural similarity to mammalian nucleolin is involved in pre-rRNA processing. J Biol Chem. 1992;267:16252–16258. [PubMed] [Google Scholar]
- Kressler D, de la Cruz J, Rojo M, Linder P. Fal1p is an essential DEAD-box protein involved in 40S-ribosomal-subunit biogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:7283–7294. doi: 10.1128/mcb.17.12.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee WC, Xue Z, Mélèse T. The NSR1 gene encodes a protein that specifically binds nuclear localisation sequences and has two RNA recognition motifs. J Cell Biol. 1991;113:1–12. doi: 10.1083/jcb.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Zabetakis D, Mélèse T. NSR1 is required for pre-rRNA processing and for the proper maintenance of steady-state levels of ribosomal subunits. Mol Cell Biol. 1992;12:3865–3871. doi: 10.1128/mcb.12.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger-Silvestre I, Gulli M-P, Noaillac-Depeyre J, Faubladier M, Sicard H, Caizergues-Ferrer M, Gas N. Ultrastructural changes in the Schizosaccharomyces pombe nucleolus following the disruption of the gar2+ gene, which encodes a nucleolar protein structurally related to nucleolin. Chromosoma. 1997a;105:542–552. doi: 10.1007/BF02510491. [DOI] [PubMed] [Google Scholar]
- Léger-Silvestre I, Noaillac-Depeyre J, Faubladier M, Gas N. Structural and functional analysis of the nucleolus of the fission yeast Schizosaccharomyces pombe. Eur J Cell Biol. 1997b;72:13–23. [PubMed] [Google Scholar]
- Liang WQ, Clark JA, Fournier MJ. The rRNA-processing function of the yeast U14 small nucleolar RNA can be rescued by a conserved RNA helicase-like protein. Mol Cell Biol. 1997;17:4124–4132. doi: 10.1128/mcb.17.7.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda A, Munroe SH, Caceres JF, Krainer AR. Function of the conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 1994;13:5483–5495. doi: 10.1002/j.1460-2075.1994.tb06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier UT. Comparison of the rat nucleolar protein No Pp 140 with its yeast homologue SRP40. J Biol Chem. 1996;271:19376–19384. [PubMed] [Google Scholar]
- Mélèse T, Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′->5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Petfalski E, Tollervey D. The 3′-end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev. 1996;10:502–513. doi: 10.1101/gad.10.4.502. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetics analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Otaka E, Osawa S. Yeast ribosomal proteins: V. correlation of several nomenclatures and proposal of a standard nomenclature. Mol Gen Genet. 1981;181:176–182. [Google Scholar]
- Ripmaster TL, Vaughn GP, Woolford JL. DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:7901–7912. doi: 10.1128/mcb.13.12.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs AB, Davis RW. Translation initiation and ribosomal biogenesis: involvement of a putative rRNA helicase and RPL46. Science. 1990;247:1077–1079. doi: 10.1126/science.2408148. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D, Dathan NA, Boelens W, van Venrooij WJ, Mattaj I. The U2B" RNP motif as a site of protein-protein interaction. EMBO J. 1990;9:3675–3681. doi: 10.1002/j.1460-2075.1990.tb07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Tollervey D, Kern H, Frank R, Hurt EC. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and essential for viability. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serin G, Joseph G, Faucher C, Ghisolfi L, Bouche G, Amalric F, Bouvet P. Localization of nucleolin binding sites on human and mouse pre-ribosomal RNA. Biochimie. 1996;78:530–538. doi: 10.1016/0300-9084(96)84759-6. [DOI] [PubMed] [Google Scholar]
- Serin G, Joseph G, Ghisolfi L, Bauzan M, Erard M, Amalric F, Bouvet P. Two RNA-binding domains determine the RNA-binding specificity of nucleolin. J Biol Chem. 1997;272:13109–13116. doi: 10.1074/jbc.272.20.13109. [DOI] [PubMed] [Google Scholar]
- Shan X, Xue Z, Mélèse T. Yeast NPI46 encodes a novel prolyl cis-trans isomerase that is located in the nucleolus. J Cell Biol. 1994;126:853–862. doi: 10.1083/jcb.126.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Stevens A, Hsu CL, Isham KR, Larimer FW. Fragments of the internal transcribed spacer 1 of pre-rRNA accumulate in Saccharomyces cerevisiae lacking 5′->3′ exoribonuclease 1. J Bacteriol. 1991;173:7024–7028. doi: 10.1128/jb.173.21.7024-7028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sun C, Woolford JL. The yeast NOP4 gene product is an essential nucleolar protein required for pre-rRNA processing and accumulation of 60S ribosomal subunits. EMBO J. 1994;13:3127–3135. doi: 10.1002/j.1460-2075.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Woolford JL. The yeast nucleolar protein Nop4p contains four RNA recognition motifs necessary for ribosome biogenesis. J Biol Chem. 1997;272:25345–25352. doi: 10.1074/jbc.272.40.25345. [DOI] [PubMed] [Google Scholar]
- Tollervey D, Lehtonen H, Carmo-Fonseca M, Hurt EC. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991;10:573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D, Mattaj I. Fungal small nuclear ribonucleoproteins share properties with plant and vertebrate U-sn RNPs. EMBO J. 1987;6:469–476. doi: 10.1002/j.1460-2075.1987.tb04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, Bousquet-Antonelli C, Gélugne J-P, Caizergues-Ferrer M, Tollervey D. Rok1p is a putative RNA helicase required for rRNA processing. Mol Cell Biol. 1997;17:3398–3407. doi: 10.1128/mcb.17.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- Venema J, Tollervey D. RRP5 is required for formation of both 18S and 5.8S rRNA in yeast. EMBO J. 1996;15:5701–5714. [PMC free article] [PubMed] [Google Scholar]
- Warner JR. The nucleolus and ribosome formation. Curr Opin Cell Biol. 1990;2:521–527. doi: 10.1016/0955-0674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yamamoto M. S. pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell. 1994;78:487–498. doi: 10.1016/0092-8674(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Weaver PL, Sun C, Chang TH. Dbp3p, a putative RNA helicase in Saccharomyces cerevisiae, is required for efficient pre-rRNA processing predominantly at site A3. Mol Cell Biol. 1997;17:1354–1365. doi: 10.1128/mcb.17.3.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner WR, Wickner RB. Evidence that the SKI antiviral system of Saccharomyces cerevisiae acts by blocking expression of viral mRNA. Mol Cell Biol. 1993;13:4331–4341. doi: 10.1128/mcb.13.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z, Mélèse T. Nucleolar proteins that bind NLSs: a role in nuclear import and ribosome biogenesis? Trends Cell Biol. 1994;4:414–417. doi: 10.1016/0962-8924(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Zuo P, Manley JL. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993;12:4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]