Abstract
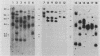
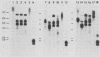
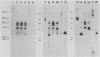
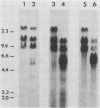
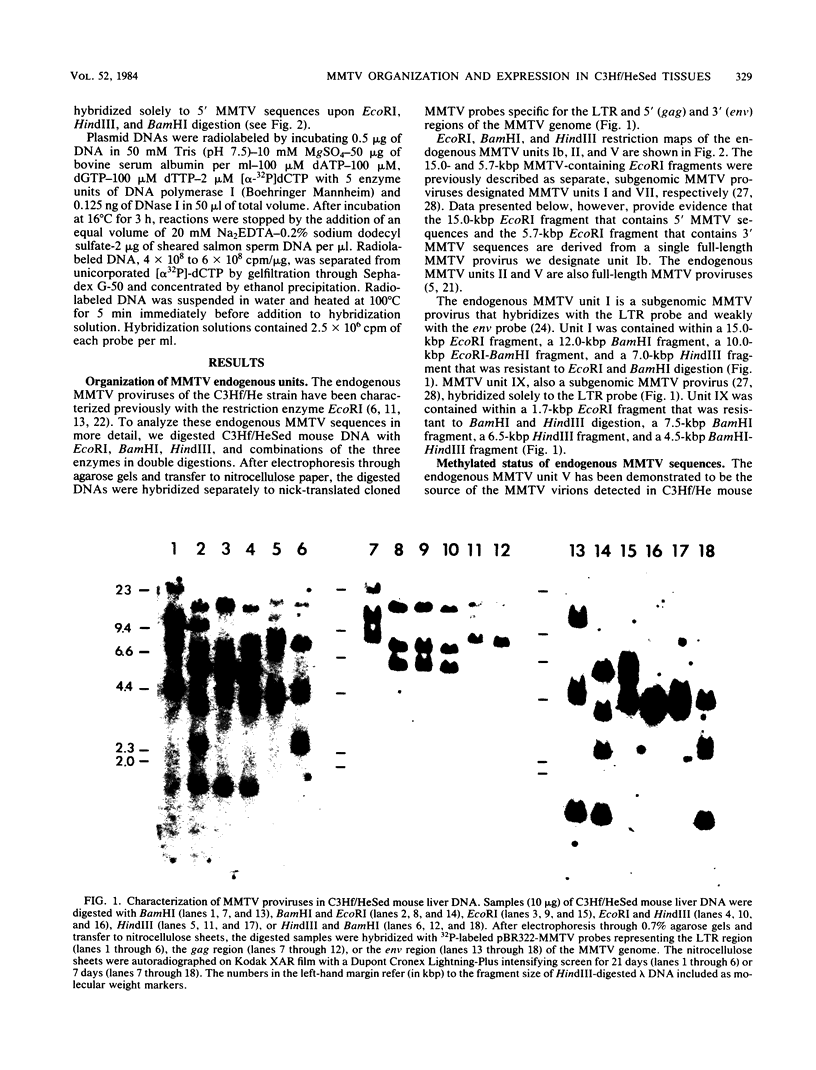
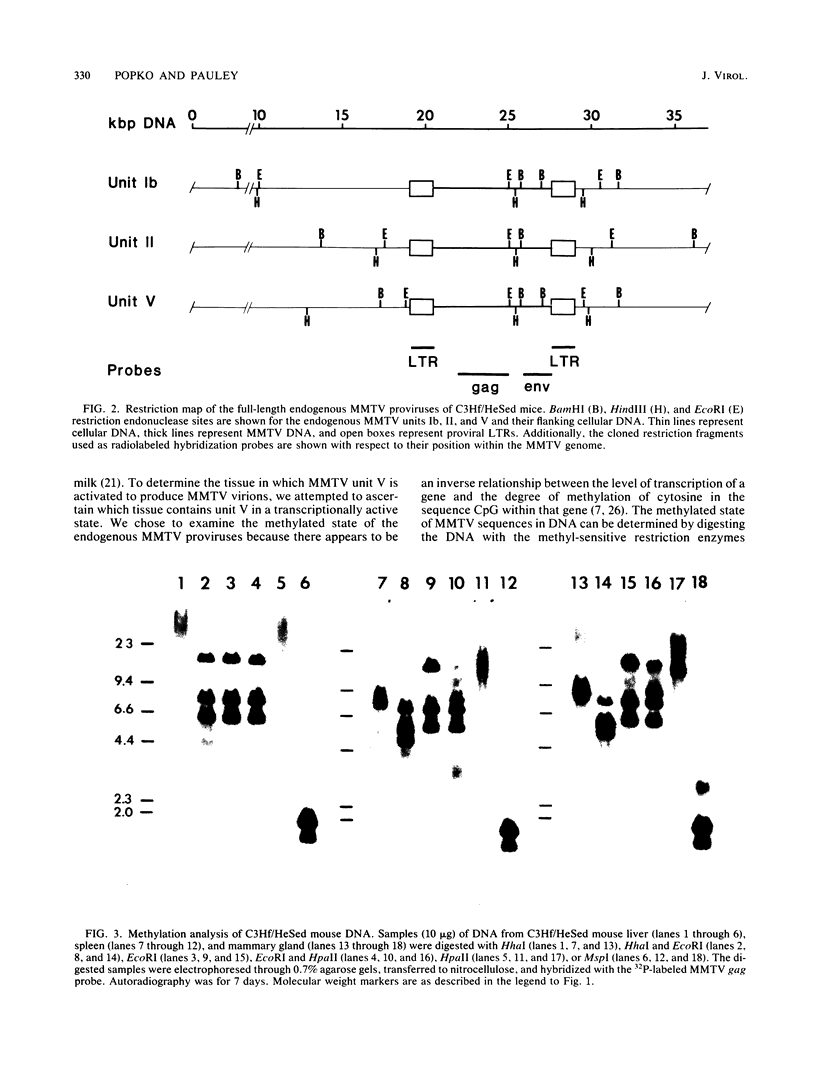
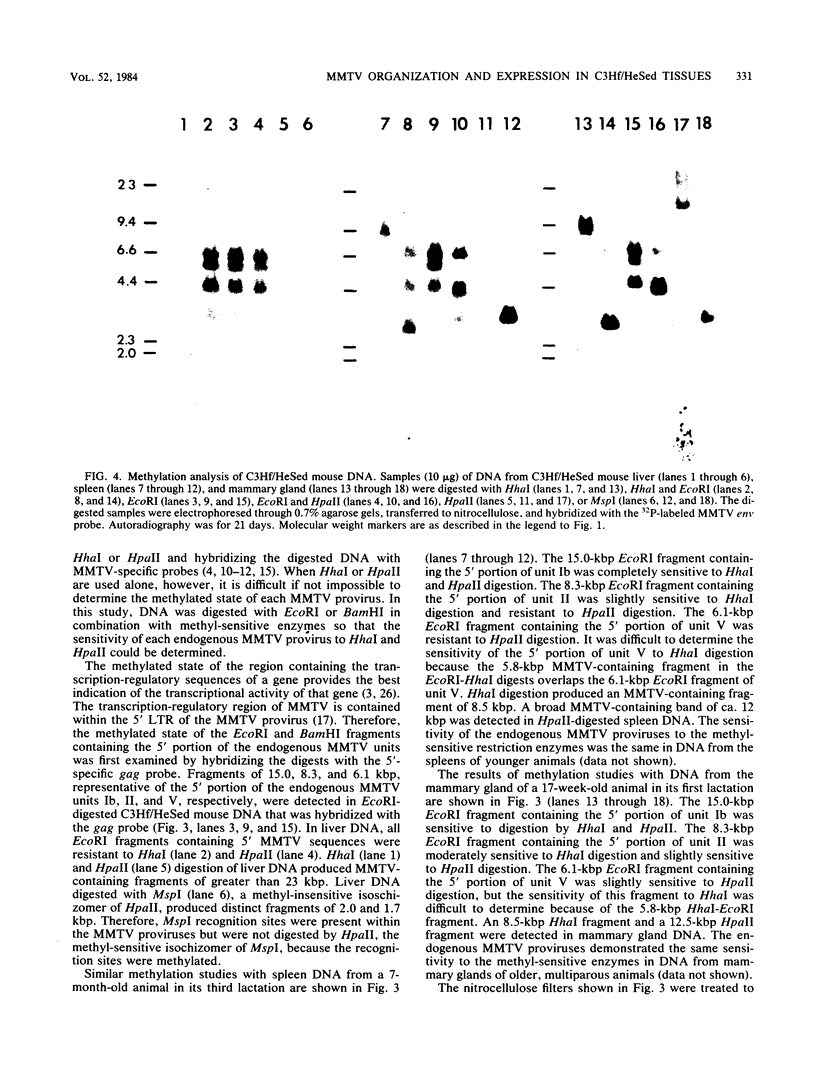
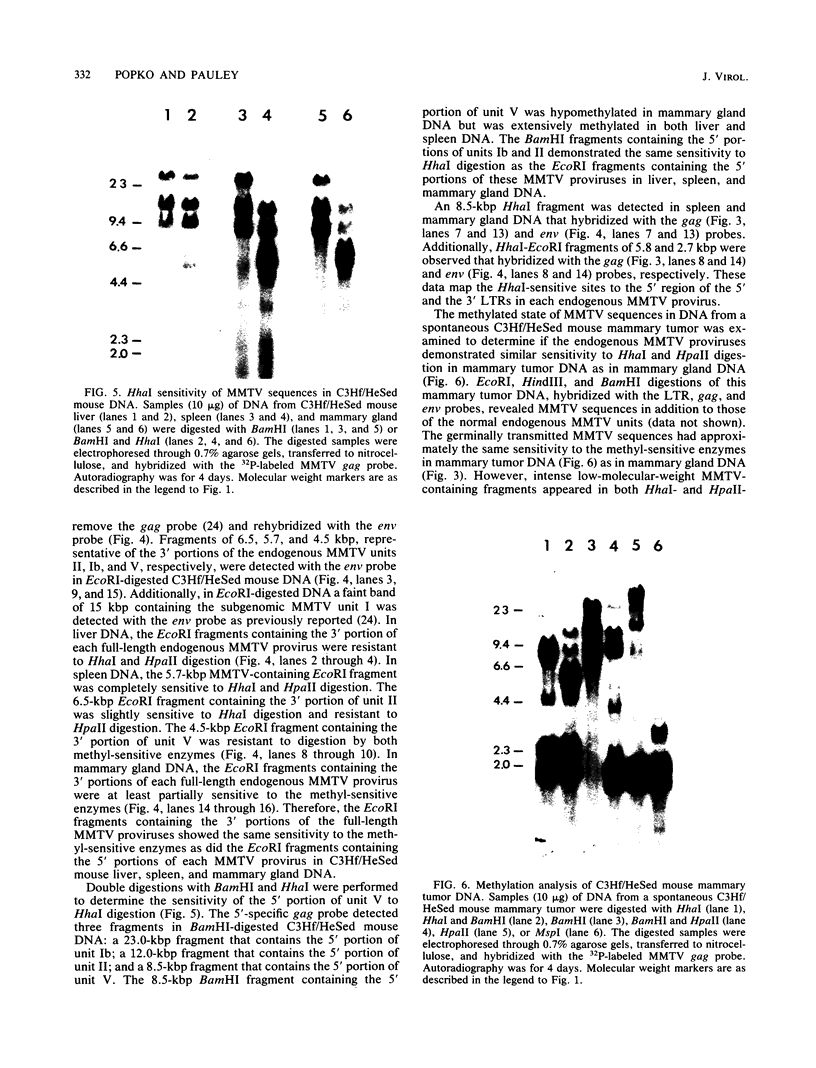
The organization and expression of germinally transmitted mouse mammary tumor virus (MMTV) proviruses in C3Hf/HeSed mouse tissues were examined. Digestion with the restriction enzymes EcoRI, BamHI, and HindIII and hybridization with cloned probes specific for the long terminal repeat and the 5' and 3' regions of the MMTV genome revealed three full-length (units Ib, II, and V) and two subgenomic (units I and IX) MMTV proviruses in C3Hf/HeSed mouse germ line DNA. The EcoRI fragments (15.0 and 5.7 kilobase pairs [kbp]) that contained unit Ib were previously described as separate, subgenomic MMTV proviruses. The methylated state of each full-length MMTV provirus was examined in DNA from C3Hf/HeSed mouse livers, spleens, mammary glands, and mammary tumors by digestion with EcoRI or BamHI in combination with the methyl-sensitive restriction enzymes HhaI or HpaII. Unit Ib contained HhaI- and HpaII-sensitive sites in spleen, mammary gland, and mammary tumor DNA but was completely methylated in liver DNA. Units II and V contained HhaI- and HpaII-sensitive sites in mammary gland and mammary tumor DNA, but the sites were extensively methylated in spleen and liver DNA. The HhaI-sensitive sites were mapped to the 5' end of the 5' and 3' long terminal repeats of each full-length MMTV provirus. C3Hf/HeSed mouse tissue RNA was examined for MMTV transcripts. Mammary glands contained MMTV RNA species of 9.0, 3.8, and 1.7 kb. Mammary tumors contained high levels of the 9.0- and 3.8-kb transcripts but lacked the 1.7-kb species. A very low level of the 3.8-kb MMTV transcript was present in spleens. Livers lacked detectable MMTV RNA. These results implicate mammary tissue as the site of unit V activation in the formation of MMTV virions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P. Interaction between host and viral genomes in mouse mammary tumors. Annu Rev Genet. 1982;16:273–295. doi: 10.1146/annurev.ge.16.120182.001421. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W. N., Benade L. E., Graham D. E., Smith G. H. Mouse mammary tumor virus proviral sequences congenital to C3H/Sm mice are differentially hypomethylated in chemically induced, virus-induced, and spontaneous mammary tumors. J Virol. 1982 Sep;43(3):876–884. doi: 10.1128/jvi.43.3.876-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Sarkar N. H. Integration of new endogenous mouse mammary tumor virus proviral DNA at common sites in the DNA of mammary tumors of C3Hf mice and hypomethylation of the endogenous mouse mammary tumor virus proviral DNA in C3Hf mammary tumors and spleens. J Virol. 1983 Jan;45(1):114–123. doi: 10.1128/jvi.45.1.114-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Vassos A. B., Cardiff R. D. Methylation and amplification of mouse mammary tumor virus DNA in normal, premalignant, and malignant cells of GR/A mice. J Virol. 1982 Mar;41(3):1007–1013. doi: 10.1128/jvi.41.3.1007-1013.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Buetti E., Diggelmann H., Hynes N. E. Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with site-specific molecular clones. J Virol. 1980 Dec;36(3):734–745. doi: 10.1128/jvi.36.3.734-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESTON W. E. Mammary tumors in agent-free mice. Ann N Y Acad Sci. 1958 Sep 30;71(6):931–942. doi: 10.1111/j.1749-6632.1958.tb46815.x. [DOI] [PubMed] [Google Scholar]
- Hu W. S., Fanning T. G., Cardiff R. D. Mouse mammary tumor virus: specific methylation patterns of proviral DNA in normal mouse tissues. J Virol. 1984 Jan;49(1):66–71. doi: 10.1128/jvi.49.1.66-71.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Morris V. L., Flintoff W. F., Kozak C. A. Characterization and chromosomal location of endogenous mouse mammary tumor virus loci in GR, NFS, and DBA mice. Virology. 1984 Jan 15;132(1):12–25. doi: 10.1016/0042-6822(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Groner B., Hynes N. E. Mammary tumor virus proviral DNA in normal murine tissue and non-virally induced mammary tumors. J Virol. 1981 Aug;39(2):367–376. doi: 10.1128/jvi.39.2.367-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- PULLINGER B. D., IVERSEN S. Mammary tumour incidence in relation to age and number of litters in C3Hf and RIIIf mice. Br J Cancer. 1960 Jun;14:267–278. doi: 10.1038/bjc.1960.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Hubbell E. S., Goldberg R. J., O'Neill F. J., Scolnick E. M. High frequency variation in mammary tumor virus expression in cell culture. Cell. 1976 May;8(1):87–93. doi: 10.1016/0092-8674(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Pauley R. J., Parks W. P., Popko B. J. Expression and demethylation of germinally-transmitted BALB/c mouse mammary tumor virus DNA in Abelson MuLV B-lymphoid cell lines. Virus Res. 1984;1(5):381–400. doi: 10.1016/0168-1702(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Traina-Dorge V., Cohen J. C. Molecular genetics of mouse mammary tumor virus. Curr Top Microbiol Immunol. 1983;106:35–56. doi: 10.1007/978-3-642-69357-1_2. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]