Abstract
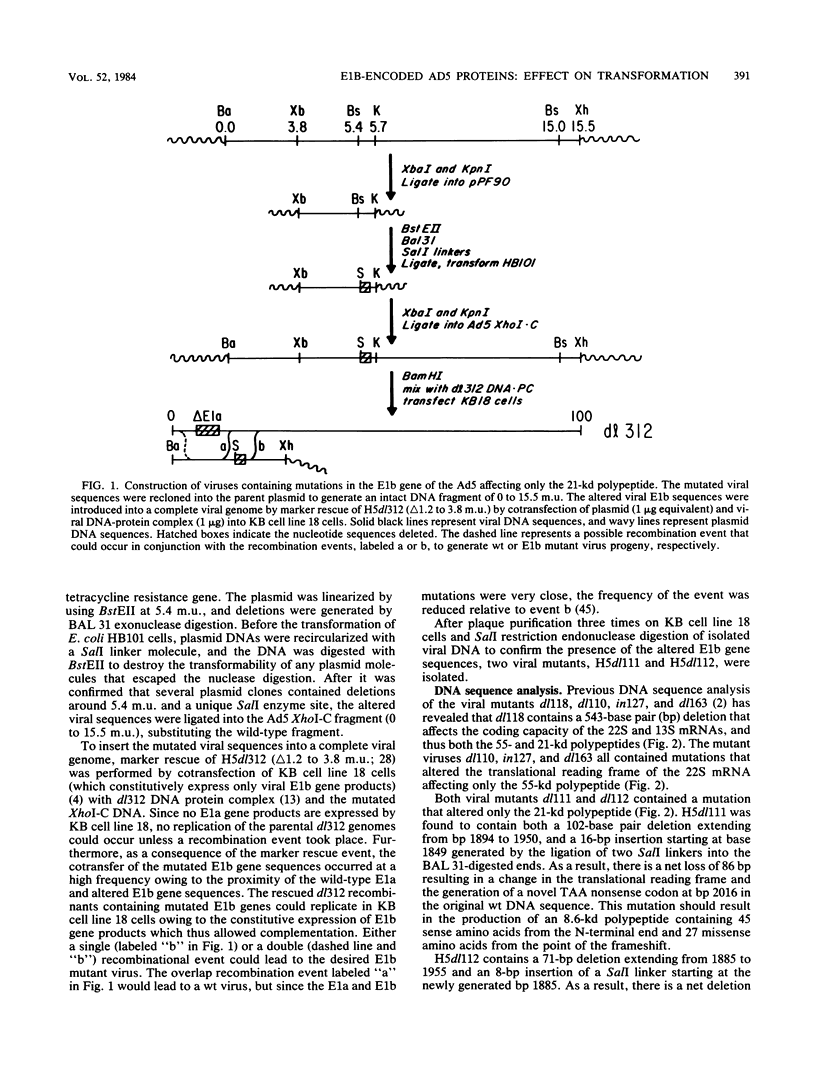
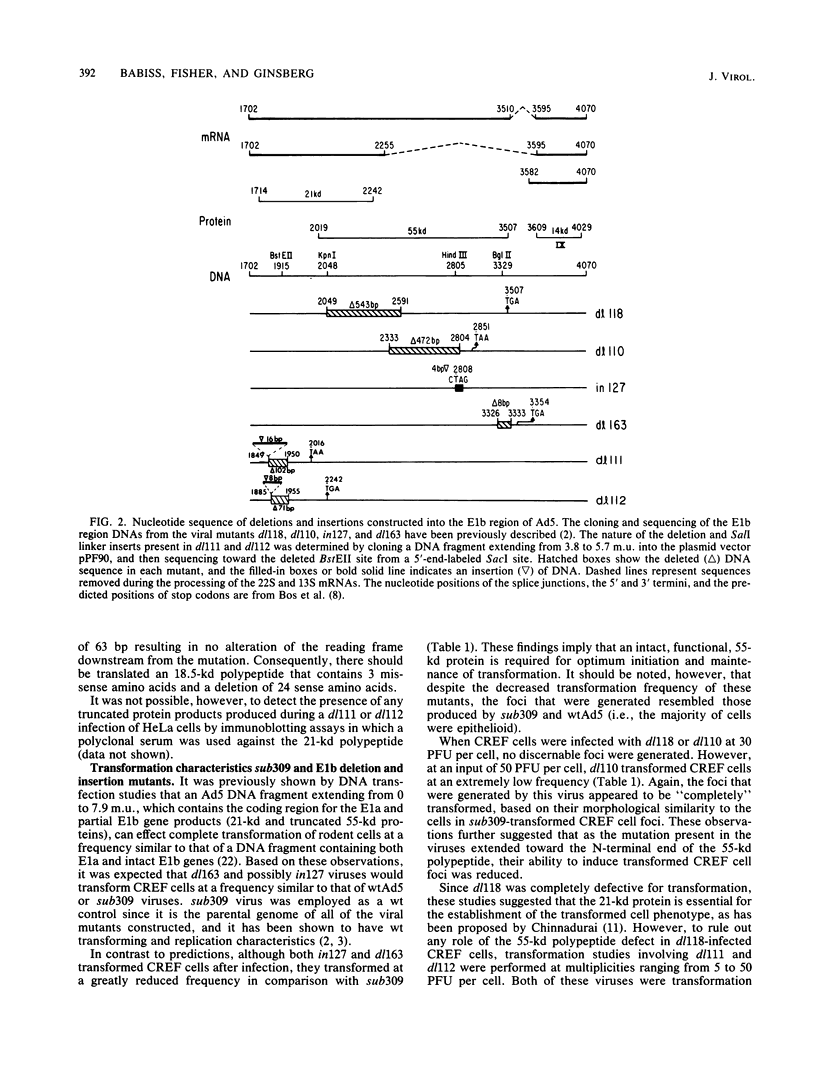
It is well established that the adenovirus 5 genes responsible for the initiation and maintenance of the transformed cell reside in the early region 1a and 1b genes, but it remains unclear how the polypeptides encoded in these genes mediate their functions. To probe the function of the early region 1b-encoded 55- and 21-kilodalton (kd) polypeptides during this process, a series of viral mutants was engineered so that they contained deletions or insertions at 5.4, 5.7, 7.9, or 9.6 map units. By means of either an overlap recombination procedure involving H5dl314 (delta 3.7 to 4.6 map units) cleaved with ClaI, or a marker rescue procedure involving H5dl312 (delta 1.2 to 3.8 map units), viral mutants were isolated by their ability to produce plaques on KB cell line 18 cells, which constitutively express only viral early region 1b functions. DNA sequence analysis confirmed that the series of mutants generated differed in their abilities to express the 21- or the 55-kd polypeptides, or both. Upon infection of cloned rat embryo fibroblast cells with viruses containing mutations affecting the 55-kd protein, the transformation frequency decreased as the size of the predicted truncated polypeptide decreased. Although all of the foci generated by the 55-kd protein mutants were indistinguishable from the foci induced by wild-type virus, they displayed an inefficient ability to grow in soft agar, again in relation to the size of the truncated polypeptide. In contrast, if cloned rat embryo fibroblast cells were transfected with viral DNA, the defectiveness in transformation observed after infection with virions was not as dramatic. However, all of the viruses containing 21-kd mutations were transformation defective, regardless of the mode by which the viral nucleic acid was introduced into the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Fisher P. B., Ginsberg H. S. Deletion and insertion mutations in early region 1a of type 5 adenovirus that produce cold-sensitive or defective phenotypes for transformation. J Virol. 1984 Mar;49(3):731–740. doi: 10.1128/jvi.49.3.731-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S., Fisher P. B. Cold-sensitive expression of transformation by a host range mutant of type 5 adenovirus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1352–1356. doi: 10.1073/pnas.80.5.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Young C. S., Fisher P. B., Ginsberg H. S. Expression of adenovirus E1a and E1b gene products and the Escherichia coli XGPRT gene in KB cells. J Virol. 1983 May;46(2):454–465. doi: 10.1128/jvi.46.2.454-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Brackmann K. H., Green M., Wold W. S., Cartas M., Matsuo T., Hashimoto S. Identification and peptide mapping of human adenovirus type 2-induced early polypeptides isolated by two-dimensional gel electrophoresis and immunoprecipitation. J Biol Chem. 1980 Jul 25;255(14):6772–6779. [PubMed] [Google Scholar]
- Carlock L., Jones N. C. Synthesis of an unspliced cytoplasmic message by an adenovirus 5 deletion mutant. Nature. 1981 Dec 10;294(5841):572–574. doi: 10.1038/294572a0. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Green M. Enhanced infectivity of adenovirus type 2 DNA and a DNA-protein complex. J Virol. 1978 Apr;26(1):195–199. doi: 10.1128/jvi.26.1.195-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Babiss L. E., Weinstein I. B., Ginsberg H. S. Analysis of type 5 adenovirus transformation with a cloned rat embryo cell line (CREF). Proc Natl Acad Sci U S A. 1982 Jun;79(11):3527–3531. doi: 10.1073/pnas.79.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Jochemsen H., Daniëls G. S., Hertoghs J. J., Schrier P. I., van den Elsen P. J., van der EB A. J. Identification of adenovirus-type 12 gene products involved in transformation and oncogenesis. Virology. 1982 Oct 15;122(1):15–28. doi: 10.1016/0042-6822(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Westphal H. The structure of adenovirus 2 early nuclear and cytoplasmic RNAs. J Mol Biol. 1980 Feb 15;137(1):23–48. doi: 10.1016/0022-2836(80)90155-2. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Tumor antigens of human Ad5 in transformed cells and in cells infected with transformation-defective host-range mutants. Cell. 1979 Nov;18(3):781–791. doi: 10.1016/0092-8674(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Lupker J. H., Davis A., Jochemsen H., van der Eb A. J. In vitro synthesis of adenovirus type 5 T antigens. I. Translation of early region 1-specific rna from lytically infected cells. J Virol. 1981 Jan;37(1):524–529. doi: 10.1128/jvi.37.1.524-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Wold W. S., Hashimoto S., Rankin A., Symington J., Green M. Polypeptides encoded by transforming region E 1b of human adenovirus 2: immunoprecipitation from transformed and infected cells and cell-free translation of E 1b-specific mRNA. Virology. 1982 Apr 30;118(2):456–465. doi: 10.1016/0042-6822(82)90366-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Bacchetti S., Graham F. L. Tn5 mutagenesis of the transforming genes of human adenovirus type 5. Gene. 1982 Jul-Aug;19(1):33–42. doi: 10.1016/0378-1119(82)90186-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Transformation of rodent cells by DNA extracted from transformation-defective adenovirus mutants. J Virol. 1983 Jun;46(3):1039–1044. doi: 10.1128/jvi.46.3.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Sullivan C. A., Levine A. J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1b-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982 Jul 30;120(2):510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Maruyama K., Saito I., Fukui Y., Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. doi: 10.1128/jvi.38.3.1048-1054.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. An adenovirus mutant defective in splicing RNA from early region 1A. Nature. 1981 Jun 11;291(5815):508–510. doi: 10.1038/291508a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J., van Ormondt H., Schrier P. I., Lupker J. H., Jochemsen H., van den Elsen P. J., DeLeys R. J., Maat J., van Beveren C. P., Dijkema R. Structure and function of the transforming genes of human adenoviruses and SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):383–399. doi: 10.1101/sqb.1980.044.01.043. [DOI] [PubMed] [Google Scholar]