Abstract
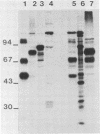
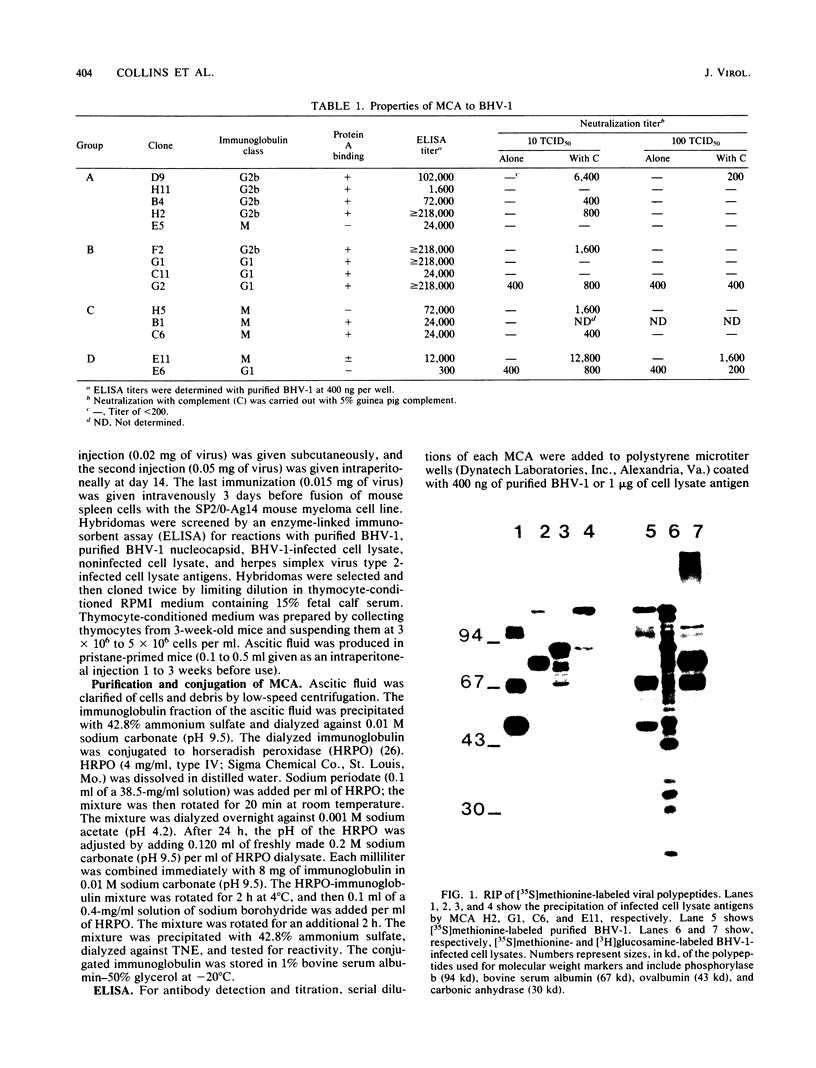
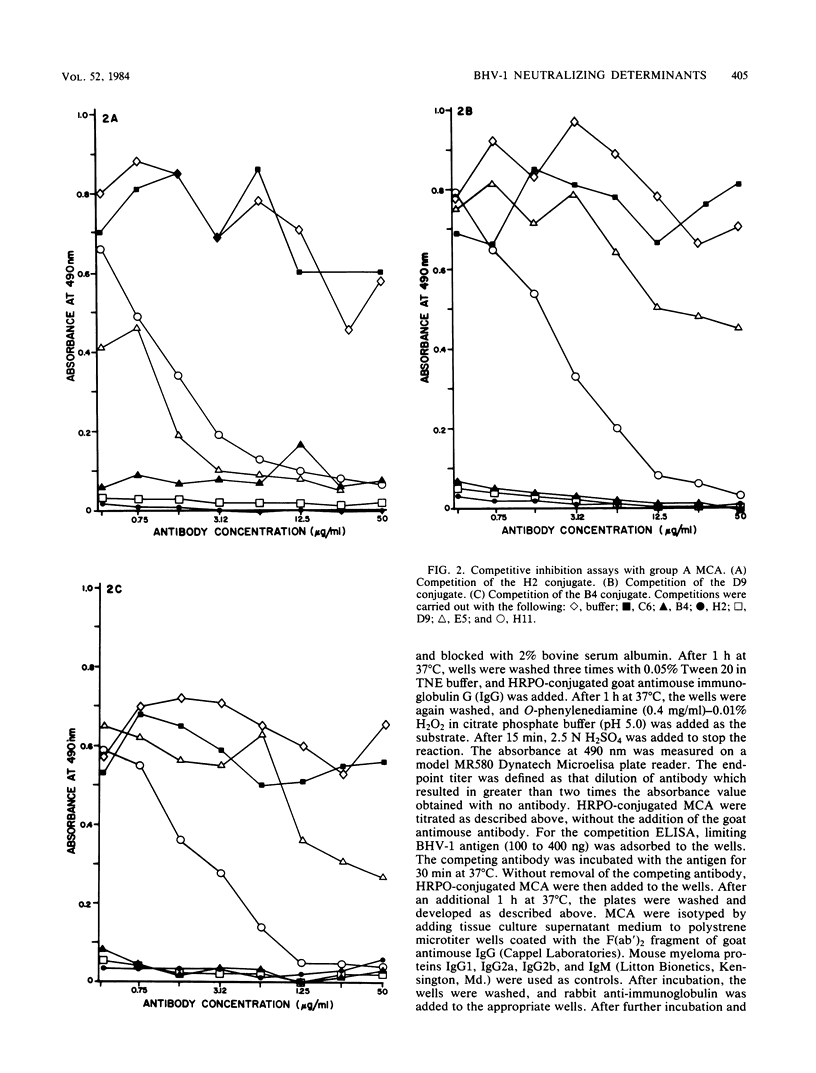
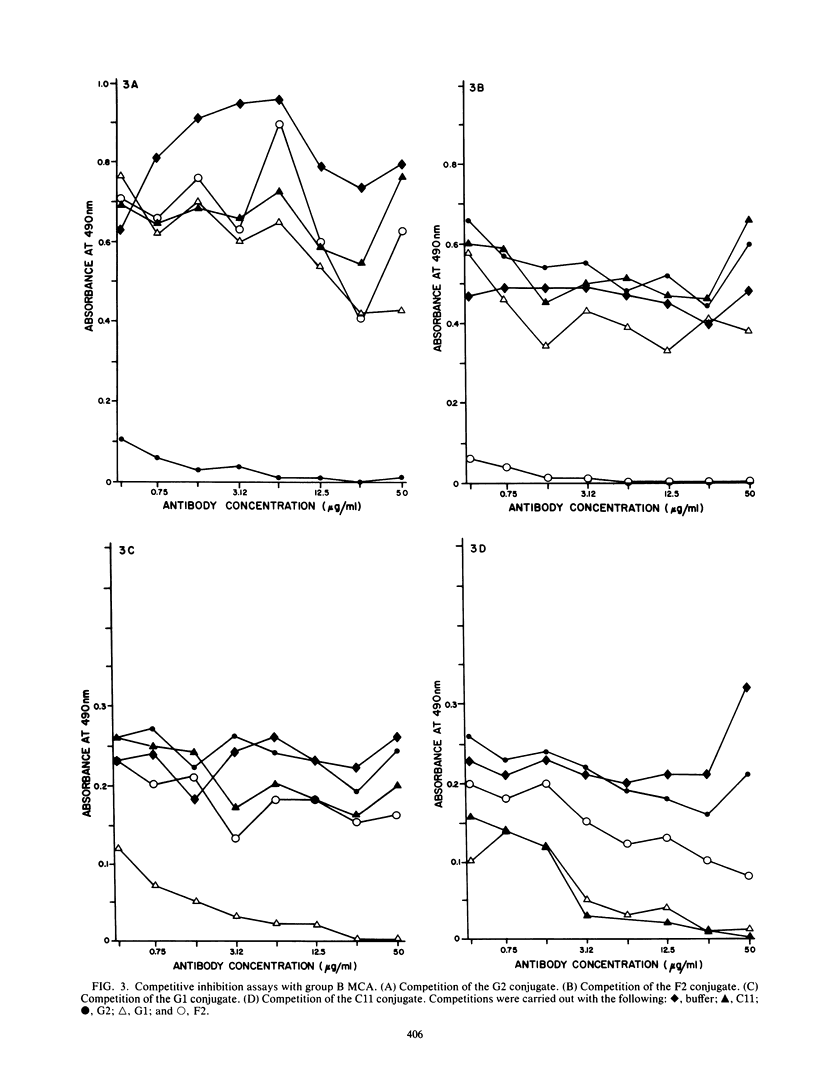
Monoclonal antibodies were used to study neutralizing determinants on polypeptides of bovine herpesvirus 1. Two of three monoclonal antibodies which recognized nonoverlapping epitopes on a glycoprotein of 82,000 daltons were found to neutralize. A second group of monoclonal antibodies that individually precipitated five viral glycopolypeptides ranging in size from 102,000 to 55,000 daltons also neutralized. Two monoclonal antibodies which were the most efficient in neutralization recognized a non-glycosylated protein of 115,000 daltons which was the major polypeptide on the virus. A fourth group of monoclonal antibodies precipitated a non-glycosylated polypeptide of 91,000 daltons and several smaller polypeptides, but these antibodies demonstrated only limited neutralizing activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton D. C., Zee Y. C., Ardans A. A. Identification of envelope and nucleocapsid proteins of infectious bovine rhinotracheitis virus by SDS-polyacrylamide gel electrophoresis. Vet Microbiol. 1983 Feb;8(1):57–68. doi: 10.1016/0378-1135(83)90019-6. [DOI] [PubMed] [Google Scholar]
- Braun D. K., Pereira L., Norrild B., Roizman B. Application of denatured, electrophoretically separated, and immobilized lysates of herpes simplex virus-infected cells for detection of monoclonal antibodies and for studies of the properties of viral proteins. J Virol. 1983 Apr;46(1):103–112. doi: 10.1128/jvi.46.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Chesebro B. Synthesis, processing, and cell surface expression of friend and xenotropic murine leukemia virus gp70 antigens on friend virus-induced erythroleukemia cell clones. J Immunol. 1980 Sep;125(3):1325–1331. [PubMed] [Google Scholar]
- Engels M., Steck F., Wyler R. Comparison of the genomes of infectious bovine rhinotracheitis and infectious pustular vulvovaginitis virus strains by restriction endonuclease analysis. Arch Virol. 1981;67(2):169–174. doi: 10.1007/BF01318601. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J. A. Bovine herpesvirus IBR-IPV. Strain differences. Cornell Vet. 1972 Jul;62(3):431–453. [PubMed] [Google Scholar]
- Kahrs R. F. Infectious bovine rhinotracheitis: a review and update. J Am Vet Med Assoc. 1977 Nov 15;171(10):1055–1064. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kida H., Brown L. E., Webster R. G. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982 Oct 15;122(1):38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Kingsford L., Ishizawa L. D., Hill D. W. Biological activities of monoclonal antibodies reactive with antigenic sites mapped on the G1 glycoprotein of La Crosse virus. Virology. 1983 Sep;129(2):443–455. doi: 10.1016/0042-6822(83)90182-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Good P. J., VanOort H. J., Campbell A. R., Reed D. E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain). J Virol. 1983 Jul;47(1):259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Babiuk L. A., Darcel C. L. Analysis of bovine herpes virus-type 1 isolates by restriction endonuclease fingerprinting. Arch Virol. 1983;76(4):341–354. doi: 10.1007/BF01311201. [DOI] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Gilchrist J. E., Weinmaster G., Qualtiere L., Van den Hurk S., Babiuk L. A. Herpesvirus-induced "early" glycoprotein: characterization and possible role in immune cytolysis. J Virol. 1982 Sep;43(3):1046–1054. doi: 10.1128/jvi.43.3.1046-1054.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Monath T. P. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology. 1983 Feb;125(1):8–17. doi: 10.1016/0042-6822(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens L. T., Zee Y. C. Purification and buoyant density of infectious bovine rhinotracheitis virus. Proc Soc Exp Biol Med. 1976 Jan;151(1):132–135. doi: 10.3181/00379727-151-39159. [DOI] [PubMed] [Google Scholar]
- Volk W. A., Synder R. M., Benjamin D. C., Wagner R. R. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus: comparative neutralizing activity. J Virol. 1982 Apr;42(1):220–227. doi: 10.1128/jvi.42.1.220-227.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]