Abstract
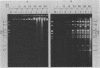
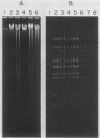
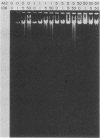
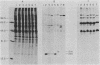
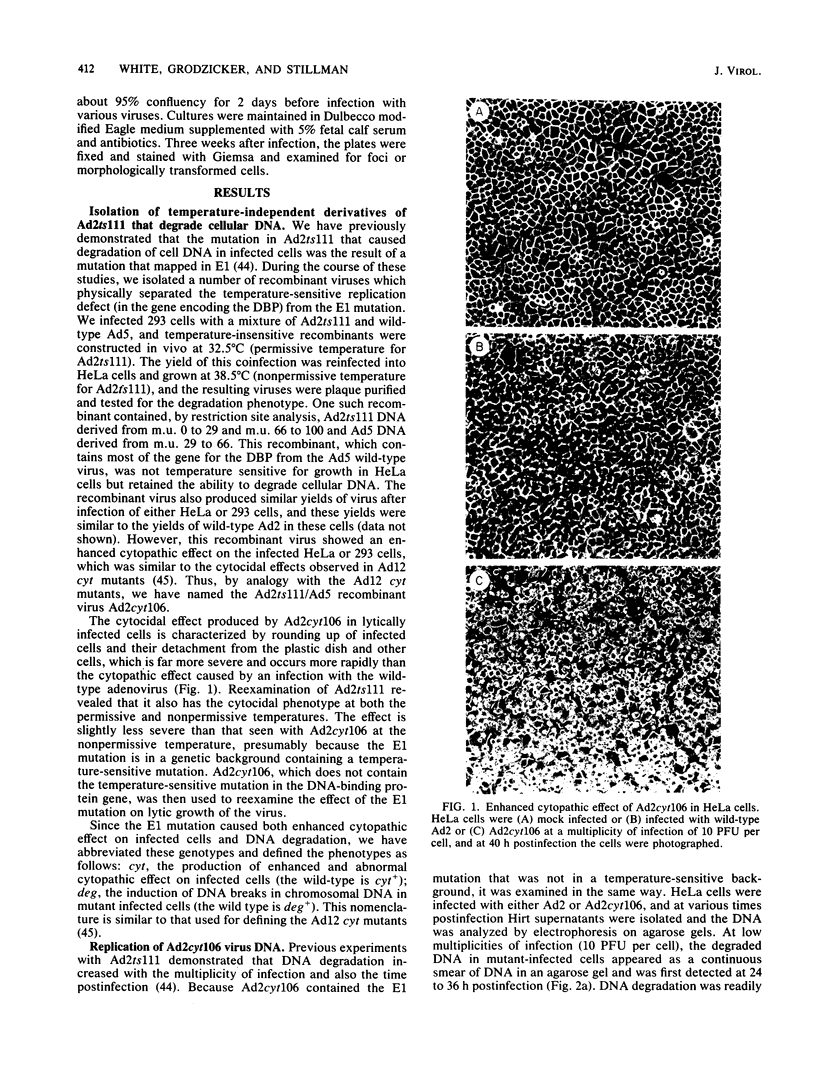
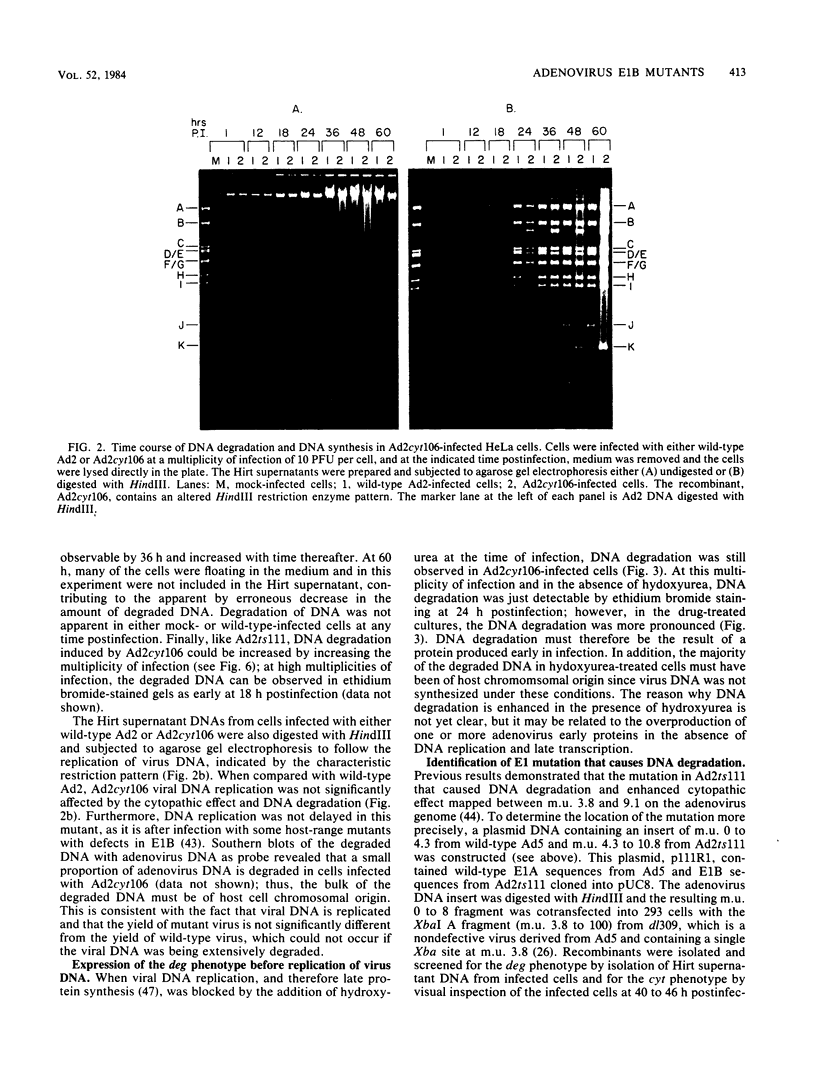
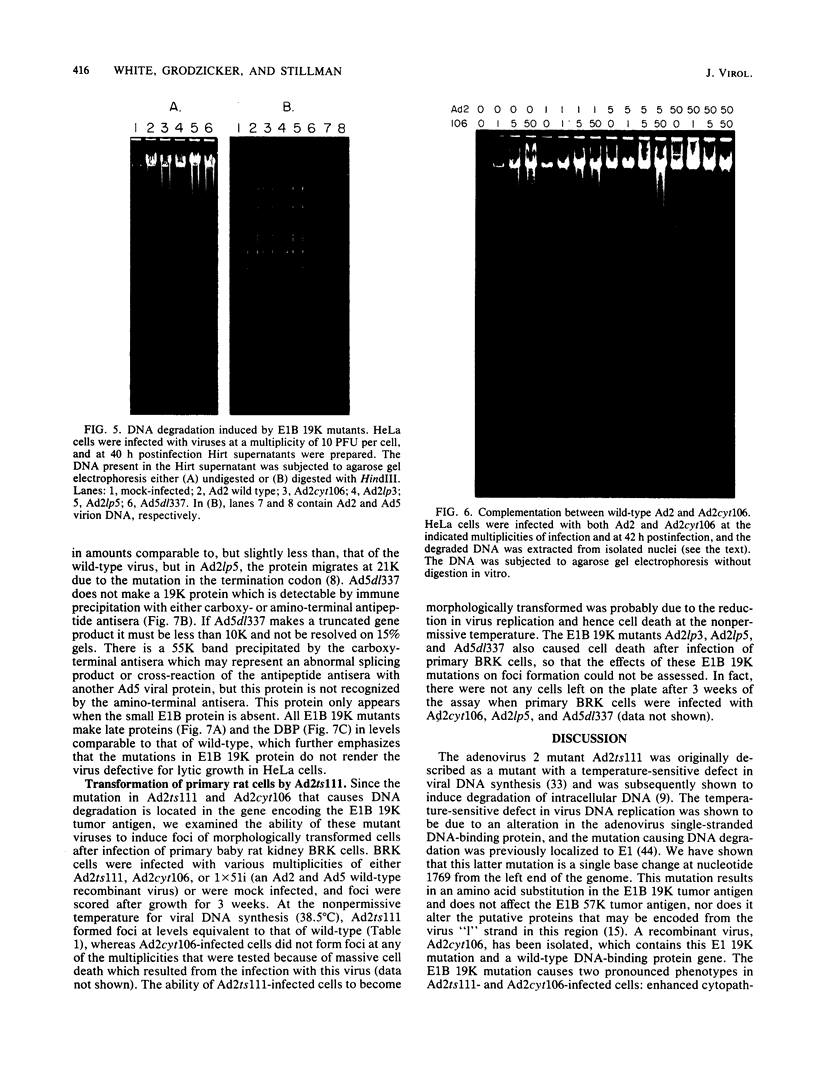
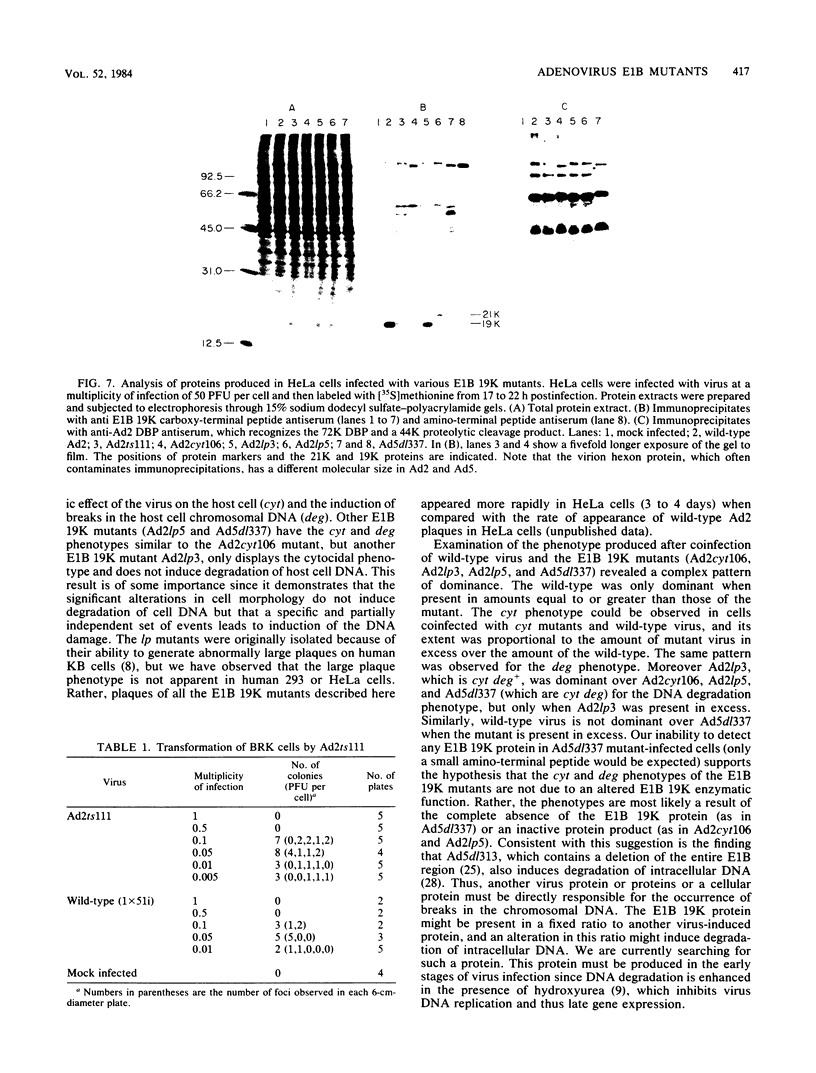
The adenovirus mutant Ad2ts111 has been previously shown to contain a mutation in the early region 2A gene encoding the single-stranded-DNA-binding protein that results in thermolabile replication of virus DNA and a mutation in early region 1 that causes degradation of intracellular DNA. A recombinant virus, Ad2cyt106, has been constructed which contains the Ad2ts111 early region 1 mutation and the wild-type early region 2A gene from adenovirus 5. This virus, like its parent Ad2ts111, has two temperature-independent phenotypes; first, it has the ability to cause an enhanced and unusual cytopathic effect on the host cell (cytocidal [cyt] phenotype) and second, it induces degradation of cell DNA (DNA degradation [deg] phenotype). The mutation responsible for these phenotypes is a single point mutation in the gene encoding the adenovirus early region 1B (E1B) 19,000-molecular-weight (19K) tumor antigen. This mutation causes a change from a serine to an asparagine in the 20th amino acid from the amino terminus of the protein. Three other mutants that affect the E1B 19K protein function have been examined. The mutants Ad2lp5 and Ad5dl337 have both the cytocidal and DNA degradation phenotypes (cyt deg), whereas Ad2lp3 has only the cytocidal phenotype and does not induce degradation of cell DNA (cyt deg+). Thus, the DNA degradation is not caused by the altered cell morphology. Furthermore, the mutant Ad5dl337 does not make any detectable E1B 19K protein product, suggesting that the absence of E1B 19K protein function is responsible for the mutant phenotypes. A fully functional E1B 19K protein is not absolutely required for lytic growth of adenovirus 2 in HeLa cells, and its involvement in transformation of nonpermissive cells to morphological variants is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Schmitt R. C., Smart J. E., Lewis J. B. Early region 1B of adenovirus 2 encodes two coterminal proteins of 495 and 155 amino acid residues. J Virol. 1984 May;50(2):387–396. doi: 10.1128/jvi.50.2.387-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Fisher P. B., Ginsberg H. S. Deletion and insertion mutations in early region 1a of type 5 adenovirus that produce cold-sensitive or defective phenotypes for transformation. J Virol. 1984 Mar;49(3):731–740. doi: 10.1128/jvi.49.3.731-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Jochemsen A. G., Bernards R., Schrier P. I., van Ormondt H., van der Eb A. J. Deletion mutants of region E1a of AD12 E1 plasmids: effect on oncogenic transformation. Virology. 1983 Sep;129(2):393–400. doi: 10.1016/0042-6822(83)90178-2. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Allart C., Cousin C., Boulanger P. A., Martin G. R. Adenovirus early function required for protection of viral and cellular DNA. J Virol. 1979 Oct;32(1):61–71. doi: 10.1128/jvi.32.1.61-71.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Ezoe H., Fatt R. B., Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981 Oct;40(1):20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Lucher L. A., Symington J. S., Kramer T. A. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983 Dec;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S., Büttner W. Integration and transcription of group C human adenovirus sequences in the DNA of five lines of transformed rat cells. J Mol Biol. 1981 Sep 25;151(3):337–366. doi: 10.1016/0022-2836(81)90001-2. [DOI] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucher L. A., Brackmann K. H., Symington J. S., Green M. Antibody directed to a synthetic peptide encoding the NH2-terminal 16 amino acids of the adenovirus type 2 E1B-53K tumor antigen recognizes the E1B-20K tumor antigen. Virology. 1984 Jan 15;132(1):217–221. doi: 10.1016/0042-6822(84)90106-5. [DOI] [PubMed] [Google Scholar]
- Lupker J. H., Davis A., Jochemsen H., van der Eb A. J. In vitro synthesis of adenovirus type 5 T antigens. I. Translation of early region 1-specific rna from lytically infected cells. J Virol. 1981 Jan;37(1):524–529. doi: 10.1128/jvi.37.1.524-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I., Mak S. Transformation of rat cells by cyt mutants of adenovirus type 12 and mutants of adenovirus type 5. J Virol. 1983 Mar;45(3):1107–1117. doi: 10.1128/jvi.45.3.1107-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Cousin C., D'Halluin J. C., Boulanger P. A. Isolation and phenotypic characterization of human adenovirus type 2 temperature-sensitive mutants. J Gen Virol. 1978 Nov;41(2):303–314. doi: 10.1099/0022-1317-41-2-303. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M., Mandal N. C. A simple procedure for large-scale preparation of pure plasmid DNA free from chromosomal DNA from bacteria. Anal Biochem. 1983 Sep;133(2):265–270. doi: 10.1016/0003-2697(83)90080-5. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Transformation of rodent cells by DNA extracted from transformation-defective adenovirus mutants. J Virol. 1983 Jun;46(3):1039–1044. doi: 10.1128/jvi.46.3.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Lewis J. B., Mathews M. B., Harter M. L., Anderson C. W. Adenovirus type 2 early proteins: assignment of the early region 1A proteins synthesized in vivo and in vitro to specific mRNAs. Virology. 1981 Jul 30;112(2):703–713. doi: 10.1016/0042-6822(81)90315-9. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., White E., Grodzicker T. Independent mutations in Ad2ts111 cause degradation of cellular DNA and defective viral DNA replication. J Virol. 1984 May;50(2):598–605. doi: 10.1128/jvi.50.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. D. Genetic studies with tumorigenic adenoviruses. II. Heterogeneity of cyt mutants of adenovirus type 12. Virology. 1969 May;38(1):8–15. doi: 10.1016/0042-6822(69)90122-6. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Van den Elsen P., Houweling A., Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983 Jul 30;128(2):377–390. doi: 10.1016/0042-6822(83)90264-7. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- van Ormondt H., Hesper B. Comparison of the nucleotide sequences of early region E1b DNA of human adenovirus types 12, 7 and 5 (subgroups A, B and C). Gene. 1983 Mar;21(3):217–226. doi: 10.1016/0378-1119(83)90005-7. [DOI] [PubMed] [Google Scholar]