Abstract
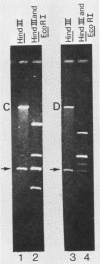
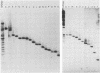
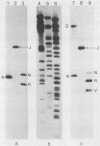
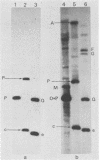
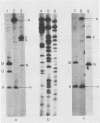
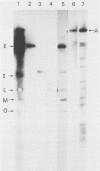
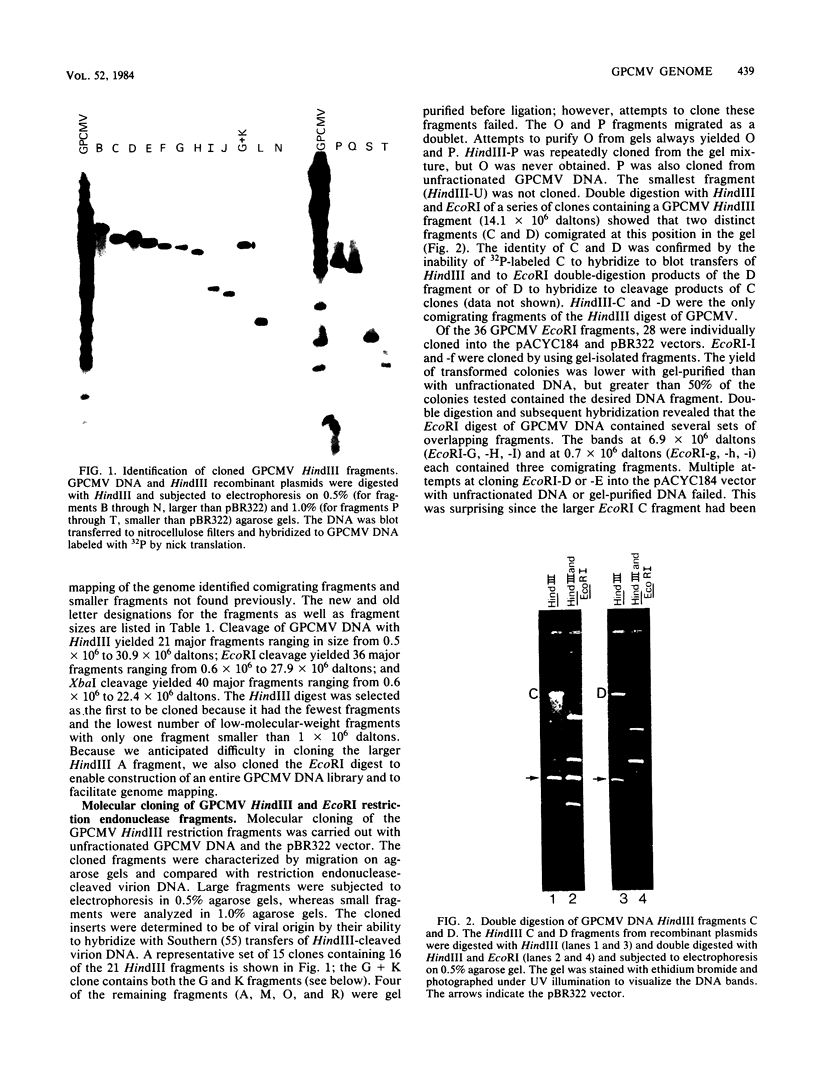
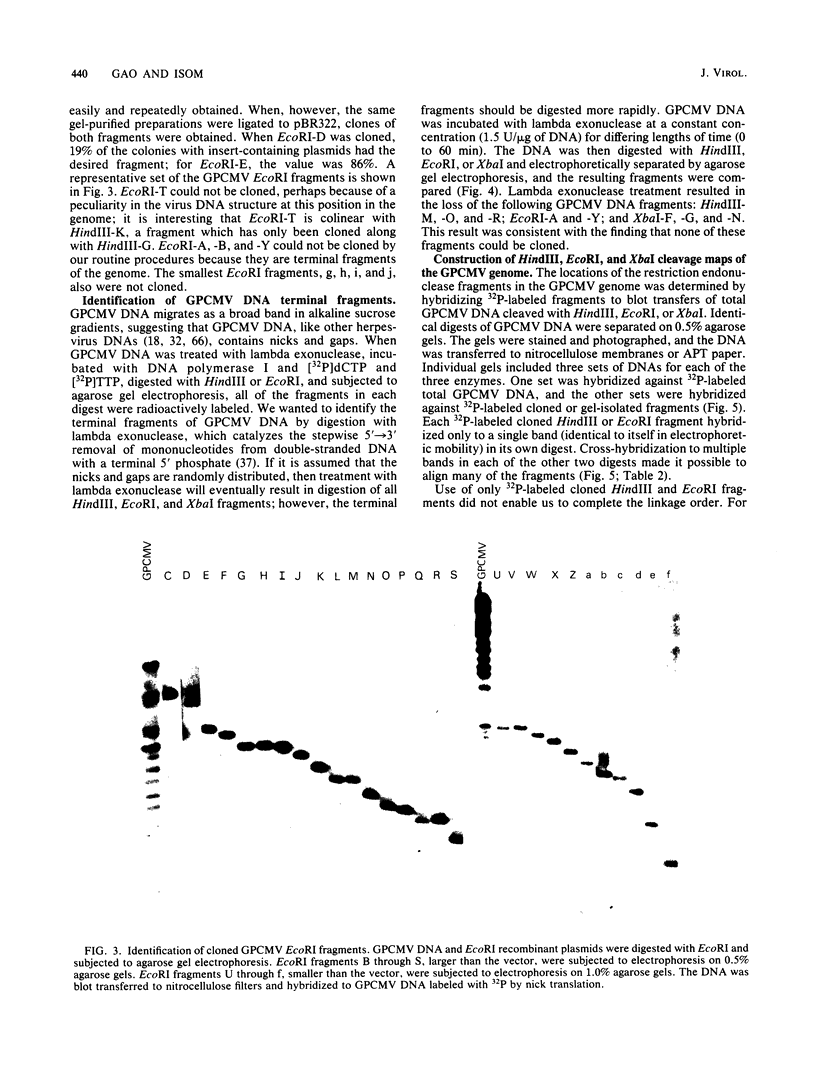
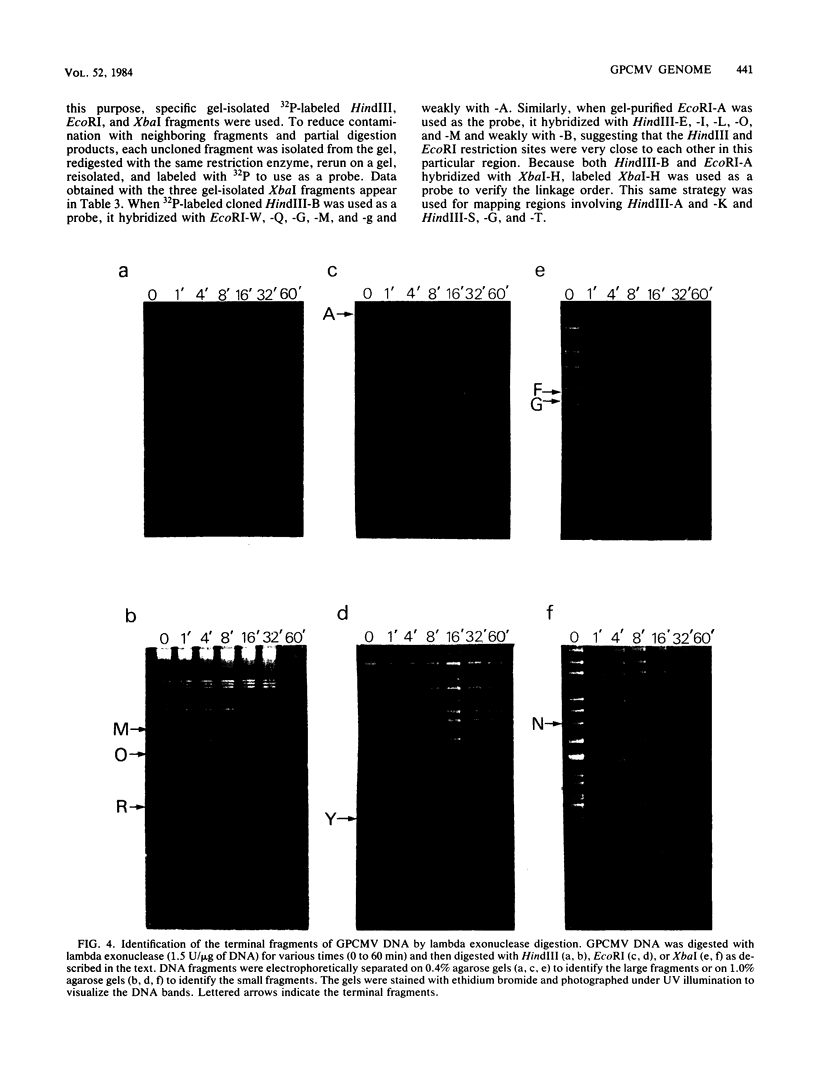
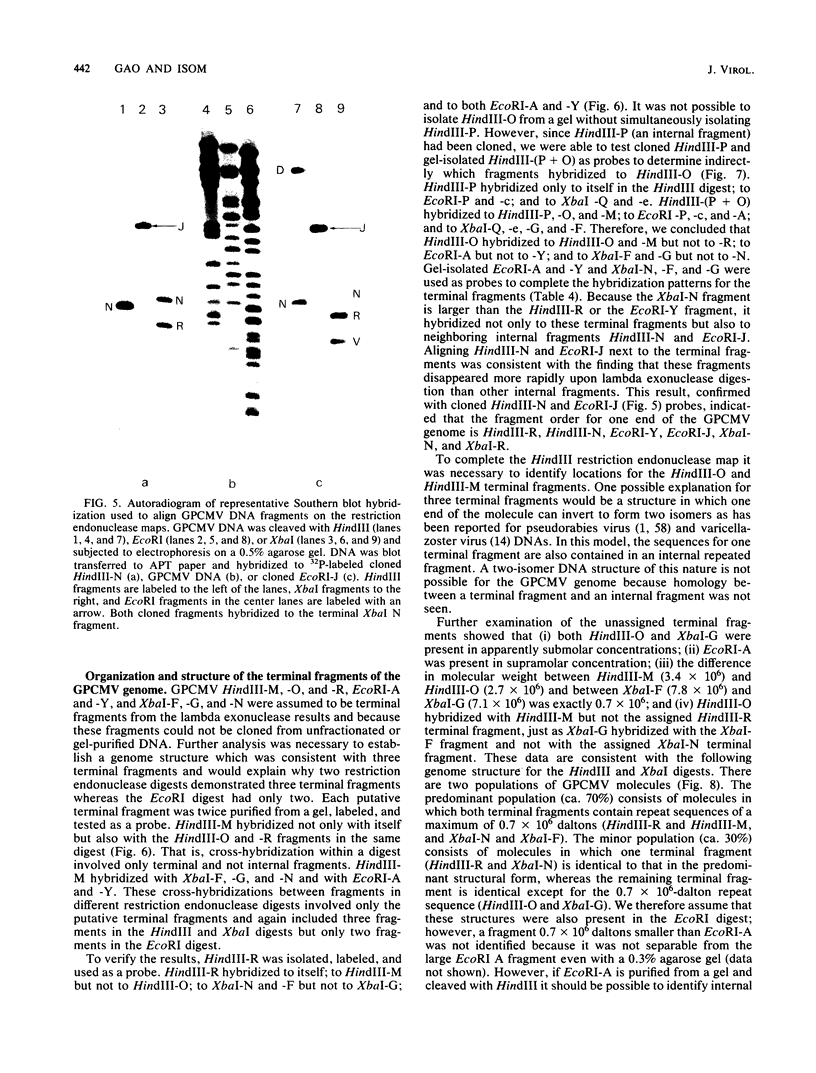
Fragments of guinea pig cytomegalovirus (GPCMV) DNA produced by HindIII or EcoRI restriction endonuclease digestion were cloned into vectors pBR322 and pACYC184, and recombinant fragments representing ca. 97% of the genome were constructed. Hybridization of 32P-labeled cloned and gel-purified HindIII, EcoRI, and XbaI fragments to Southern blots of HindIII-, EcoRI-, and XbaI-cleaved GPCMV DNA verified the viral origin of cloned fragments and allowed construction of HindIII, EcoRI, and XbaI restriction maps. On the basis of the cloning and mapping experiments, the size of GPCMV DNA was calculated to include 239 kilobase pairs, corresponding to a molecular weight of 158 X 10(6). No cross-hybridization between any internal fragments was seen. We conclude that the GPCMV genome consists of a long unique sequence with terminal repeat sequences but without internal repeat regions. In addition, GPCMV DNA molecules exist in two forms. In the predominant form, the molecules demonstrate sequence homology between the terminal fragments; in the minor population, one terminal fragment is smaller by 0.7 X 10(6) daltons and is not homologous with the fragment at the other end of the physical map. The structural organization of GPCMV DNA is unique for a herpesvirus DNA, similar in its simplicity to the structure reported for murine cytomegalovirus DNA and quite dissimilar from that of human cytomegalovirus DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Rixon F. J., Blankenship M. L. Analysis of the structure of the genome of pseudorabies virus. Virology. 1979 Jun;95(2):285–294. doi: 10.1016/0042-6822(79)90484-7. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Hastings K., Hsiung G. D. Cytomegalovirus infection in guinea pigs. III. Persistent viruria, blood transmission, and viral interference. J Infect Dis. 1979 Dec;140(6):914–920. doi: 10.1093/infdis/140.6.914. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. C., Hsiung G. D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978 Aug;138(2):197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Blankenship M. L., Brown G. D., Kaplan A. S. Size and complexity of human cytomegalovirus DNA. Virology. 1978 Sep;89(2):643–646. doi: 10.1016/0042-6822(78)90209-x. [DOI] [PubMed] [Google Scholar]
- DeMarchi J. M. Post-transcriptional control of human cytomegalovirus gene expression. Virology. 1983 Jan 30;124(2):390–402. doi: 10.1016/0042-6822(83)90355-0. [DOI] [PubMed] [Google Scholar]
- Delius H., Clements J. B. A partial denaturation map of herpes simplex virus type 1 DNA: evidence for inversions of the unique DNA regions. J Gen Virol. 1976 Oct;33(1):125–133. doi: 10.1099/0022-1317-33-1-125. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Ebeling A., Keil G. M., Knust E., Koszinowski U. H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983 Sep;47(3):421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci U S A. 1982 Jan;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. H., DiPaolo J. A. Neoplastic transformation of guinea pig fetal cells in culture induced by chemical carcinogens. Cancer Res. 1975 Apr;35(4):1035–1044. [PubMed] [Google Scholar]
- Fenoglio C. M., Oster M. W., Lo Gerfo P., Reynolds T., Edelson R., Patterson J. A., Madeiros E., McDougall J. K. Kaposi's sarcoma following chemotherapy for testicular cancer in a homosexual man: demonstration of cytomegalovirus RNA in sarcoma cells. Hum Pathol. 1982 Oct;13(10):955–959. doi: 10.1016/s0046-8177(82)80063-4. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982 Apr;18(1):39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Separation of the herpesvirus deoxyribonucleic acid duplex into unique fragments and intact strand on sedimentation in alkaline gradients. J Virol. 1972 Oct;10(4):565–572. doi: 10.1128/jvi.10.4.565-572.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen J. L., Walig C., Wertheim P., van der Noordaa J. Human cytomegalovirus DNA. I. Molecular weight and infectivity. J Virol. 1978 Jun;26(3):813–816. doi: 10.1128/jvi.26.3.813-816.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. VI. Mapping of the internal tandem reiteration. J Virol. 1979 Aug;31(2):315–324. doi: 10.1128/jvi.31.2.315-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Yee D., Griem K., Kieff E. DNA of Epstein-Barr virus. V. Direct repeats of the ends of Epstein-Barr virus DNA. J Virol. 1979 Jun;30(3):852–862. doi: 10.1128/jvi.30.3.852-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Oram J. D., Downing R. G., Patel K. Human cytomegalovirus DNA: BamHI, EcoRI and PstI restriction endonuclease cleavage maps. Gene. 1982 Jun;18(3):355–360. doi: 10.1016/0378-1119(82)90174-3. [DOI] [PubMed] [Google Scholar]
- Griffith B. P., Lucia H. L., Bia F. J., Hsiung G. D. Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun. 1981 May;32(2):857–863. doi: 10.1128/iai.32.2.857-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. R., Overall J. C., Jr Synergistic infection with murine cytomegalovirus and Pseudomonas aeruginosa in mice. J Infect Dis. 1978 Jun;137(6):775–782. doi: 10.1093/infdis/137.6.775. [DOI] [PubMed] [Google Scholar]
- Hanshaw J. B., Dudgeon J. A. Congenital cytomegalovirus. Major Probl Clin Pediatr. 1978;17:97–152. [PubMed] [Google Scholar]
- Hayward S. D., Nogee L., Hayward G. S. Organization of repeated regions within the Epstein-Barr virus DNA molecule. J Virol. 1980 Jan;33(1):507–521. doi: 10.1128/jvi.33.1.507-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D., Choi Y. C., Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978 Aug;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
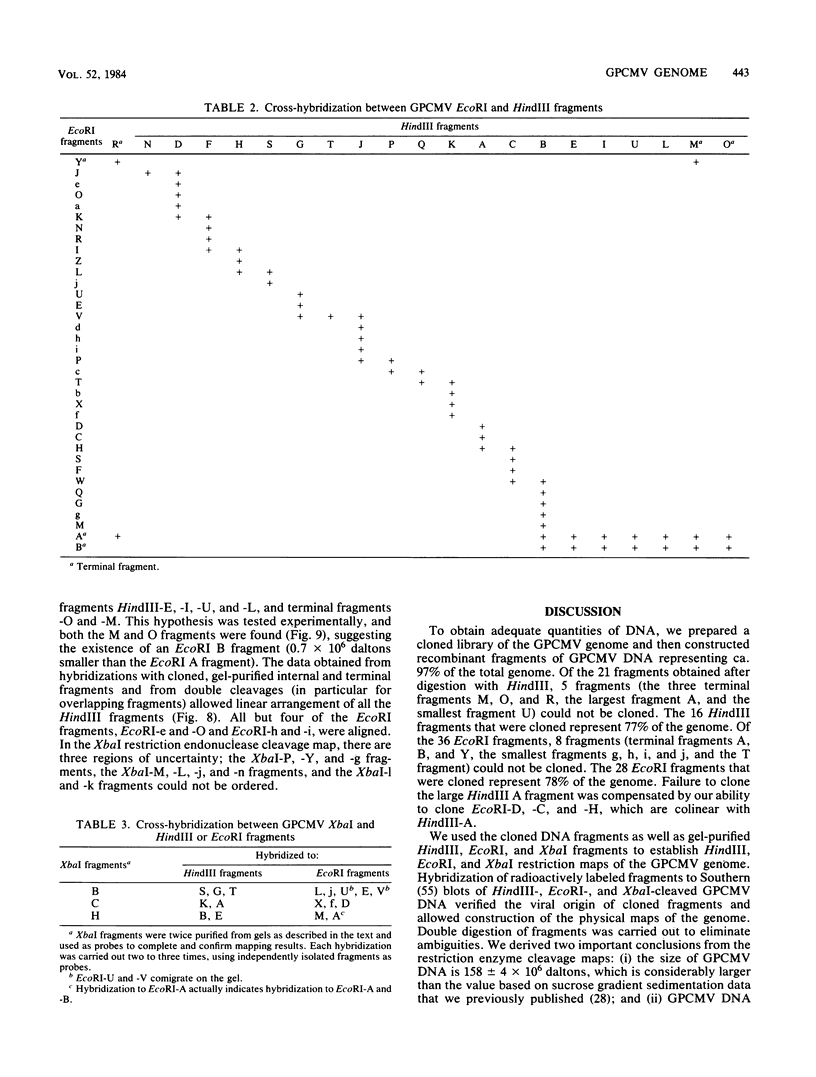
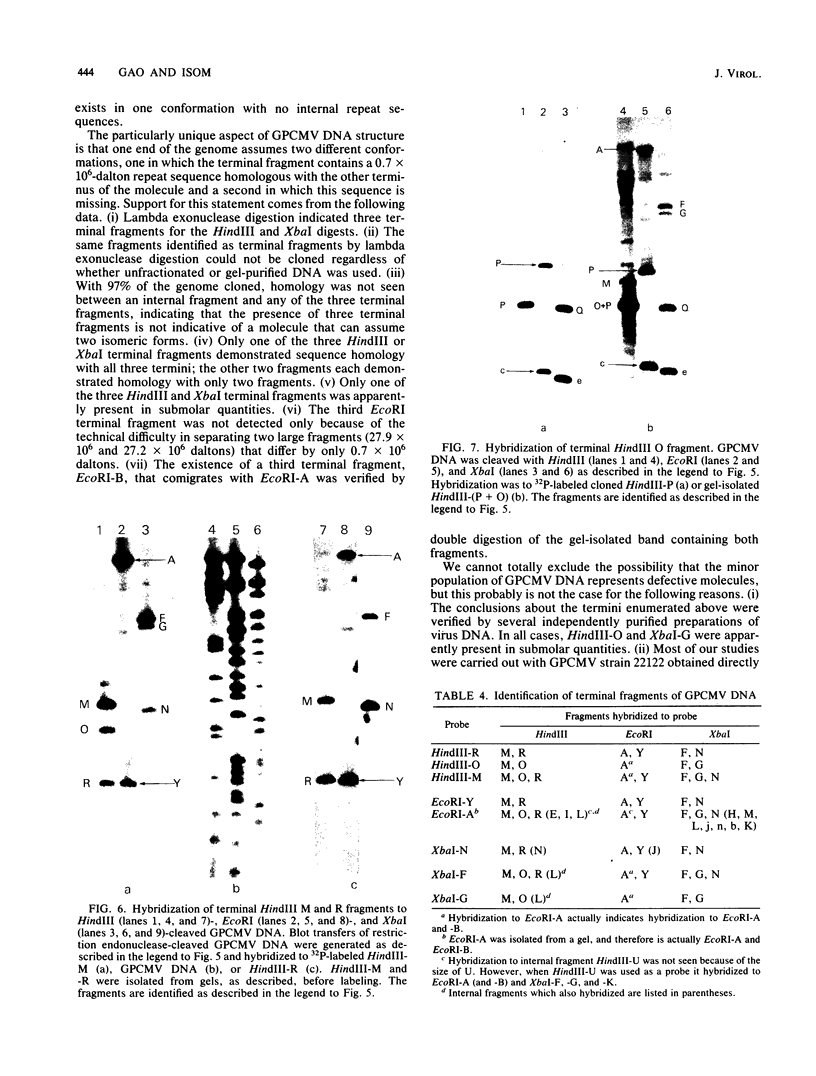
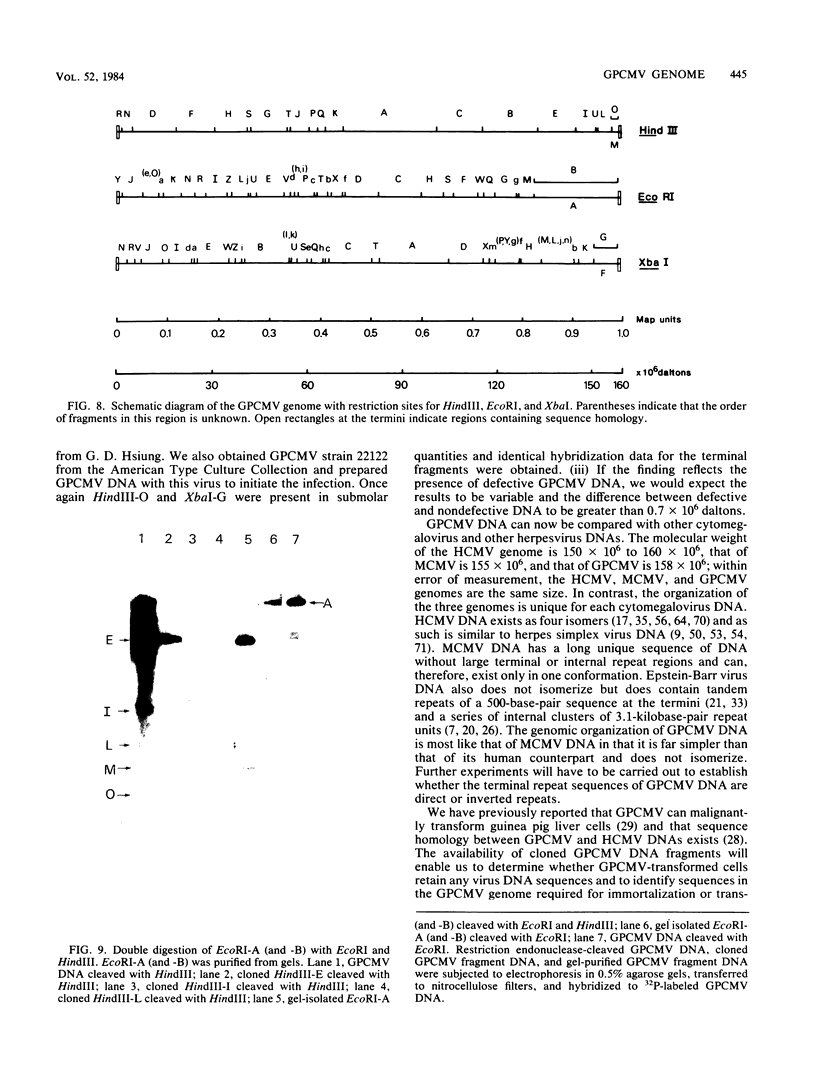
- Isom H. C., Gao M., Wigdahl B. Characterization of guinea pig cytomegalovirus DNA. J Virol. 1984 Feb;49(2):426–436. doi: 10.1128/jvi.49.2.426-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom H. C., Mummaw J., Kreider J. W. Malignant transformation of guinea pig cells after exposure to ultraviolet-irradiated guinea pig cytomegalovirus. Virology. 1983 Apr 30;126(2):693–700. doi: 10.1016/s0042-6822(83)80025-7. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey D. K., Olsen G. A., Overall J. C., Jr, Glasgow L. A. Alteration of host defense mechanisms by murine cytomegalovirus infection. Infect Immun. 1977 Dec;18(3):754–760. doi: 10.1128/iai.18.3.754-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. R., Sugden B. The structure of the termini of the DNA of Epstein-Barr virus. Cell. 1979 Jul;17(3):661–671. doi: 10.1016/0092-8674(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Kumar M. L., Nankervis G. A. Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis. 1978 Nov;138(5):650–654. doi: 10.1093/infdis/138.5.650. [DOI] [PubMed] [Google Scholar]
- Lakeman A. D., Osborn J. E. Size of infectious DNA from human and murine cytomegaloviruses. J Virol. 1979 Apr;30(1):414–416. doi: 10.1128/jvi.30.1.414-416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Lehman I. R., Kaiser A. D. An exonuclease induced by bacteriophage lambda. I. Preparation of the crystalline enzyme. J Biol Chem. 1967 Feb 25;242(4):672–678. [PubMed] [Google Scholar]
- Loh L., Hudson J. B. Immunosuppressive effect of murine cytomegalovirus. Infect Immun. 1980 Jan;27(1):54–60. doi: 10.1128/iai.27.1.54-60.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh L., Hudson J. B. Murine cytomegalovirus infection in the spleen and its relationship to immunosuppression. Infect Immun. 1981 Jun;32(3):1067–1072. doi: 10.1128/iai.32.3.1067-1072.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mayo D., Armstrong J. A., Ho M. Activation of latent murine cytomegalovirus infection: cocultivation, cell transfer, and the effect of immunosuppression. J Infect Dis. 1978 Dec;138(6):890–896. doi: 10.1093/infdis/138.6.890. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Marks J. R., Spector D. H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology. 1983 Aug;129(1):94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Reeves W., Ray G., Flournoy N., Lerner K. G., Sale G. E., Thomas E. D. A prospective analysis interstitial pneumonia and opportunistic viral infection among recipients of allogeneic bone marrow grafts. J Infect Dis. 1977 Dec;136(6):754–767. doi: 10.1093/infdis/136.6.754. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Galloway D. A., McDougall J. K. Transformation of NIH 3T3 cells with cloned fragments of human cytomegalovirus strain AD169. J Virol. 1982 Jul;43(1):83–91. doi: 10.1128/jvi.43.1.83-91.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Jahn G., Galloway D. A., McDougall J. K. Structure of the transforming region of human cytomegalovirus AD169. J Virol. 1984 Jan;49(1):109–115. doi: 10.1128/jvi.49.1.109-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Akrigg A., Dollery A. A., Duggleby C. J., Wilkinson G. W., Greenaway P. J. Use of recombinant plasmids to investigate the structure of the human cytomegalovirus genome. J Gen Virol. 1982 Mar;59(Pt 1):111–129. doi: 10.1099/0022-1317-59-1-111. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Tolkoff-Rubin N. E., Russell P. S., Hirsch M. S. Infectious disease syndromes attributable to cytomegalovirus and their significance among renal transplant recipients. Transplantation. 1977 Dec;24(6):458–464. doi: 10.1097/00007890-197712000-00010. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. C. Structure and function of herpesvirus genomes. II. EcoRl, Sbal, and HindIII endonuclease cleavage sites on herpes simplex virus. Virology. 1977 Feb;76(2):581–595. doi: 10.1016/0042-6822(77)90240-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S. Inverted repetition in the chromosome of pseudorabies virus. J Virol. 1977 Apr;22(1):232–234. doi: 10.1128/jvi.22.1.232-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol. 1979 Jul;31(1):231–239. doi: 10.1128/jvi.31.1.231-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: pathogenesis and persistence in experimentally infected animals. Infect Immun. 1976 Mar;13(3):934–940. doi: 10.1128/iai.13.3.934-940.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Tyms A. S. Diseases of the fetus and neonate due to human cytomegalovirus: a laboratory perspective. Med Lab Sci. 1982 Jul;39(3):275–286. [PubMed] [Google Scholar]
- Wadsworth S., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. V. Terminally repetitive sequences. J Virol. 1976 Feb;17(2):503–512. doi: 10.1128/jvi.17.2.503-512.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- Weststrate M. W., Geelen J. L., van der Noordaa J. Human cytomegalovirus DNA: physical maps for restriction endonucleases BglII, hindIII and XbaI. J Gen Virol. 1980 Jul;49(1):1–21. doi: 10.1099/0022-1317-49-1-1. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M. Physical maps for Herpes simplex virus type 1 DNA for restriction endonucleases Hind III, Hpa-1, and X. bad. J Virol. 1976 Oct;20(1):222–233. doi: 10.1128/jvi.20.1.222-233.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourtee E. L., Bia F. J., Griffith B. P., Root R. K. Neutrophil response and function during acute cytomegalovirus infection in guinea pigs. Infect Immun. 1982 Apr;36(1):11–16. doi: 10.1128/iai.36.1.11-16.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]