Abstract
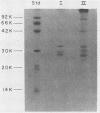
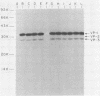
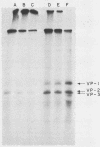
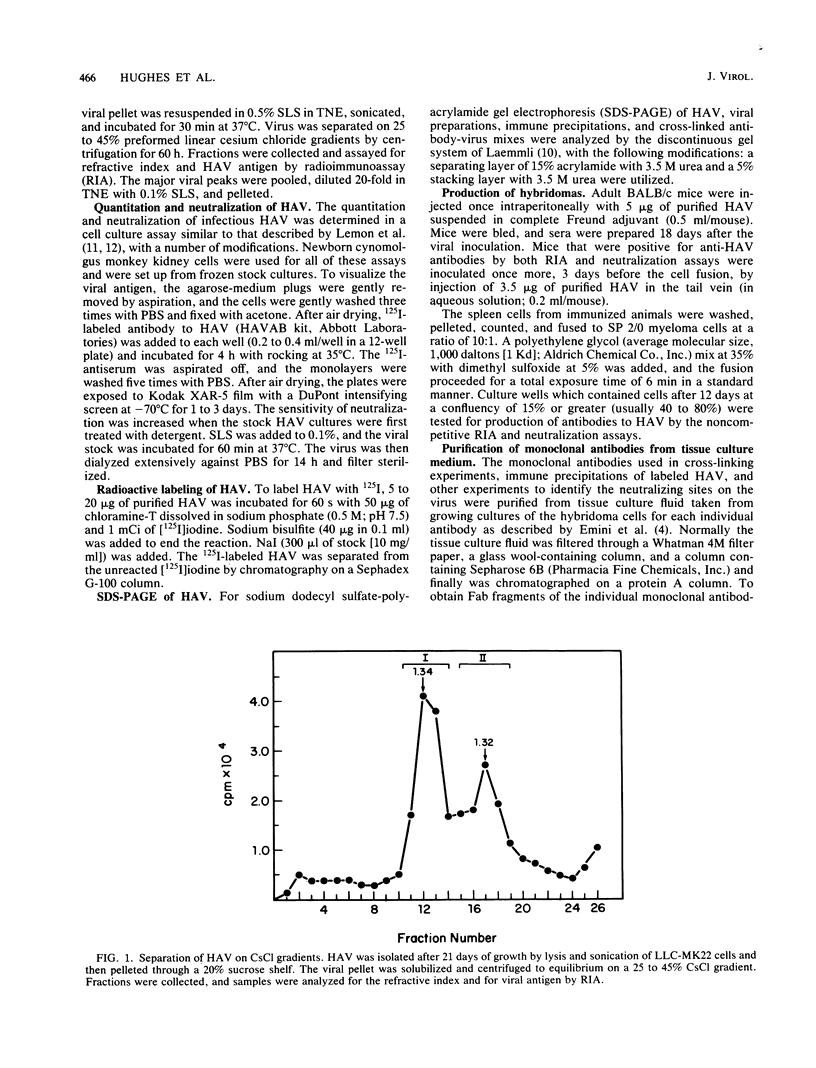
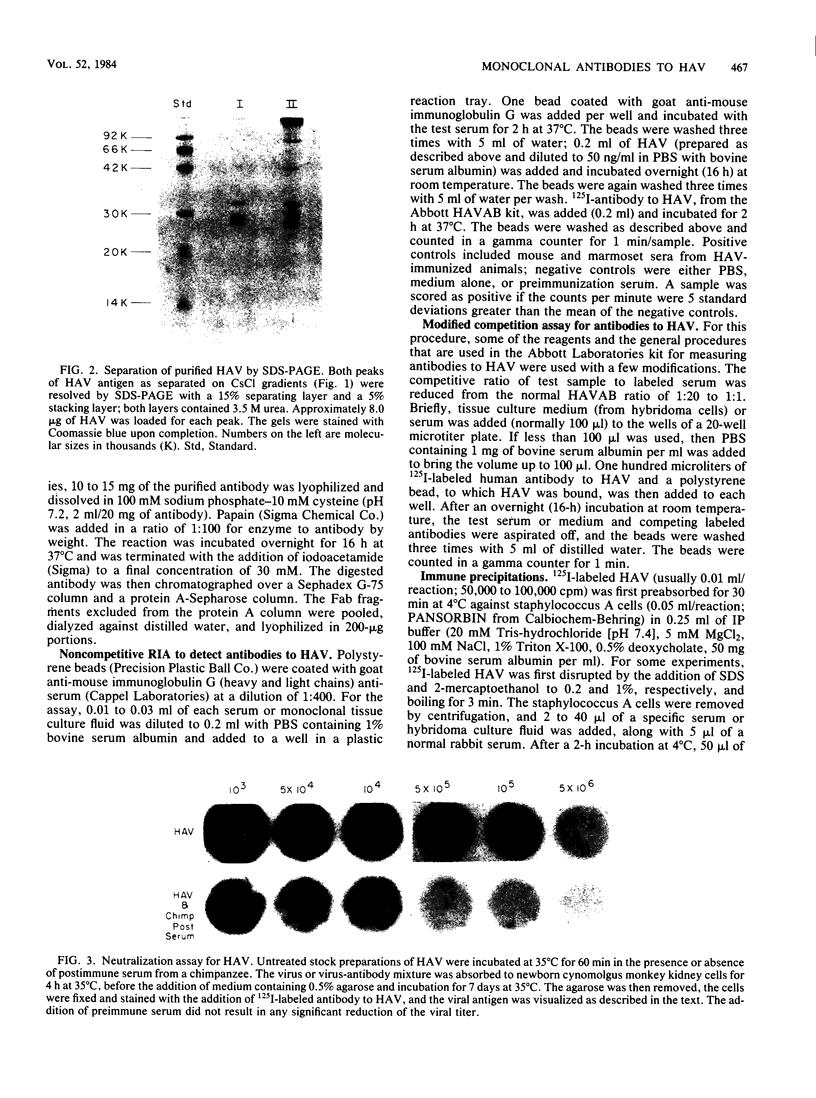
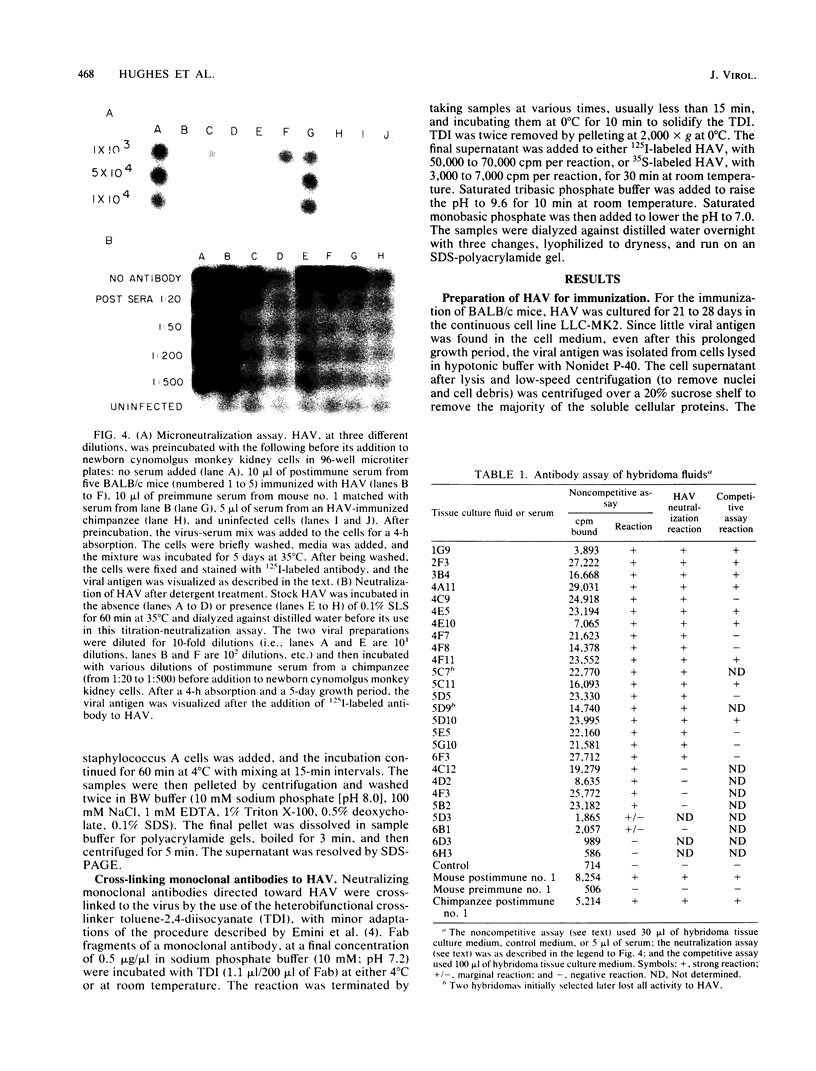
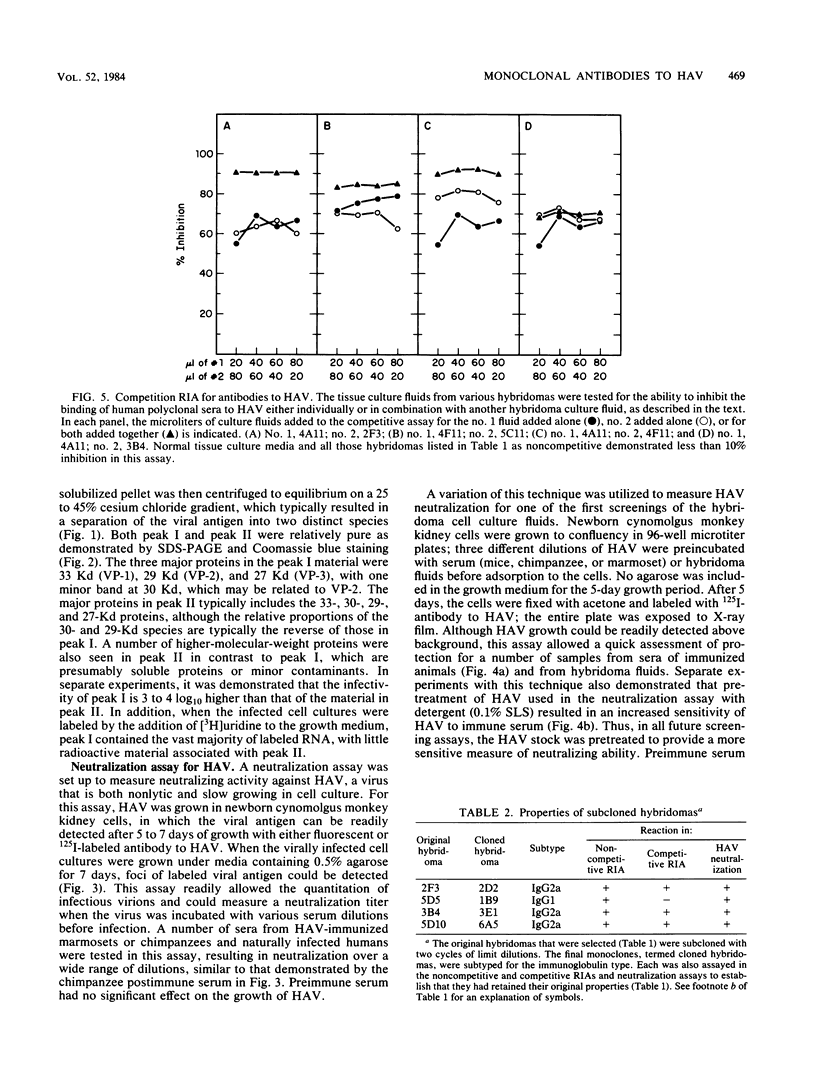
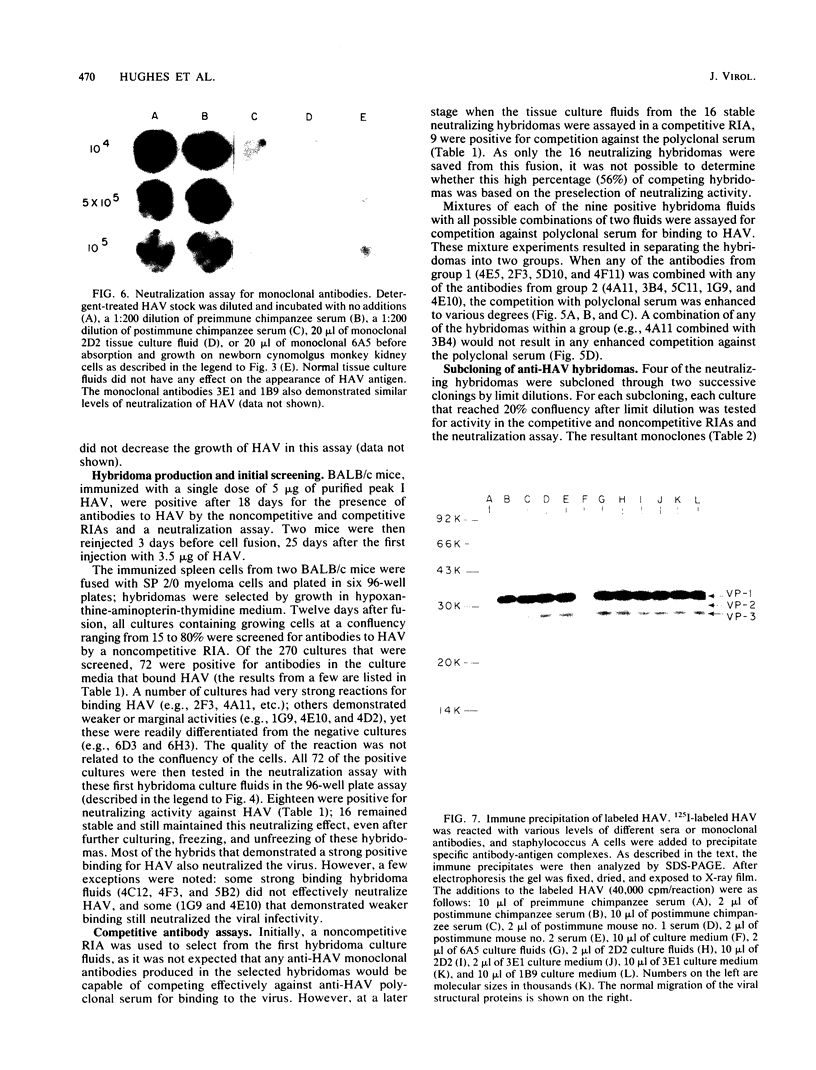
BALB/c mice were immunized with purified preparations of hepatitis A virus (HAV) isolated after 21 days of growth in LLC-MK2 cells. The HAV antigen was isolated from CsCl gradients and consisted primarily of the following three proteins as analyzed after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining: VP-1 at 33,000 daltons, VP-2 at 29,000 to 30,000 daltons, and VP-3 at 27,000 daltons. The spleen cells isolated from two BALB/c mice, immunized with two inoculations of HAV, were fused with SP 2/0 myeloma cells and grown in hypoxanthine-aminopterin-thymidine medium. Of 270 hybridomas initially screened, 72 were positive for binding HAV by a noncompetitive radioimmunoassay. All 72 were tested for the ability to neutralize the infectivity of HAV in an in vitro cell culture assay that was adapted for microtiter plates and that used detergent-treated virus for improved neutralization sensitivity and newborn cynomolgus monkey kidney cells for rapid growth. Eighteen hybridomas were positive for neutralization; 16 remained stable. Of the 16, 9 were able to compete with labeled polyclonal serum for binding to HAV. The nine competing hybridomas could be separated into two groups which appear to be directed towards two different sites on HAV and could complement each other in the competitive radioimmunoassay against polyclonal sera. Of the original 16 neutralizing hybridomas, 4 were subcloned through two cycles of limit dilutions. All four monoclonal antibodies retained their original neutralizing and competitive properties; three were immunoglobulin G2a, and one was immunoglobulin G1. All four monoclonal antibodies readily precipitate whole 125I-labeled HAV but are not able to recognize the disrupted proteins of the virus (as tested by immune precipitations of heat- and detergent-disrupted virions or Western blot analyses). However, the heterobifunctional cross-linking reagent toluene-2,4-diisocyanate was used to cross-link purified Fab fragments of two different monoclonal antibodies (2D2 and 6A5) to HAV before disruption. This reagent demonstrated a specific reaction of the monoclonal antibodies to the VP-1 of HAV, suggesting this major surface protein contains at least one of the major neutralization sites for HAV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blondel B., Akacem O., Crainic R., Couillin P., Horodniceanu F. Detection by monoclonal antibodies of an antigenic determinant critical for poliovirus neutralization present on VP1 and on heat-inactivated virions. Virology. 1983 Apr 30;126(2):707–710. doi: 10.1016/s0042-6822(83)80027-0. [DOI] [PubMed] [Google Scholar]
- Chow M., Baltimore D. Isolated poliovirus capsid protein VP1 induces a neutralizing response in rats. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7518–7521. doi: 10.1073/pnas.79.23.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulepis A. G., Locarnini S. A., Gust I. D. Iodination of hepatitis A virus reveals a fourth structural polypeptide. J Virol. 1980 Aug;35(2):572–574. doi: 10.1128/jvi.35.2.572-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Wimmer E. Priming for and induction of anti-poliovirus neutralizing antibodies by synthetic peptides. Nature. 1983 Aug 25;304(5928):699–703. doi: 10.1038/304699a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Frösner G. G. Topology and immunoreactivity of capsid proteins in hepatitis A virus. Med Microbiol Immunol. 1983;172(2):101–106. doi: 10.1007/BF02124510. [DOI] [PubMed] [Google Scholar]
- Gust I. D., Coulepis A. G., Feinstone S. M., Locarnini S. A., Moritsugu Y., Najera R., Siegl G. Taxonomic classification of hepatitis A virus. Intervirology. 1983;20(1):1–7. doi: 10.1159/000149367. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Gilbert S. F., Grieves J., Anderegg J., Rueckert R. A neutralizing monoclonal antibody against poliovirus and its reaction with related antigens. Virology. 1981 Nov;115(1):211–215. doi: 10.1016/0042-6822(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Kaaden O. R., Adam K. H., Strohmeier K. Induction of neutralizing antibodies and immunity in vaccinated guinea pigs by cyanogen bromide-peptides of VP3 of foot-and-mouth disease virus. J Gen Virol. 1977 Feb;34(2):397–400. doi: 10.1099/0022-1317-34-2-397. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis. 1983 Dec;148(6):1033–1039. doi: 10.1093/infdis/148.6.1033. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Ziola B. R., Salmi A., Scraba D. G. Structure of the Mengo virion. V. Distribution of the capsid polypeptides with respect to the surface of the virus particle. Virology. 1977 May 1;78(1):35–44. doi: 10.1016/0042-6822(77)90076-9. [DOI] [PubMed] [Google Scholar]
- MacGregor A., Kornitschuk M., Hurrell J. G., Lehmann N. I., Coulepis A. G., Locarnini S. A., Gust I. D. Monoclonal antibodies against hepatitis A virus. J Clin Microbiol. 1983 Nov;18(5):1237–1243. doi: 10.1128/jcm.18.5.1237-1243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Bootman J., Evans D. M., Ferguson M., Reeve P., Spitz M., Stanway G., Cann A. J., Hauptmann R. Location and primary structure of a major antigenic site for poliovirus neutralization. Nature. 1983 Feb 24;301(5902):674–679. doi: 10.1038/301674a0. [DOI] [PubMed] [Google Scholar]
- Osterhaus A. D., van Wezel A. L., van Steenis B., Drost G. A., Hazendonk T. G. Monoclonal antibodies to polioviruses. Production of specific monoclonal antibodies to the Sabin vaccine strains. Intervirology. 1981;16(4):218–224. doi: 10.1159/000149270. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Poliovirus empty capsid morphogenesis: evidence for conformational differences between self- and extract-assembled empty capsids. J Virol. 1982 Mar;41(3):792–800. doi: 10.1128/jvi.41.3.792-800.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G., Gauss-Müller V., Tratschin J. D., Deinhardt F. The physicochemical properties of infectious hepatitis A virions. J Gen Virol. 1981 Dec;57(Pt 2):331–341. doi: 10.1099/0022-1317-57-2-331. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Siegl G., Frösner G. G., Deinhardt F. Characterization and classification of virus particles associated with hepatitis A. III. Structural proteins. J Virol. 1981 Apr;38(1):151–156. doi: 10.1128/jvi.38.1.151-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]