Abstract
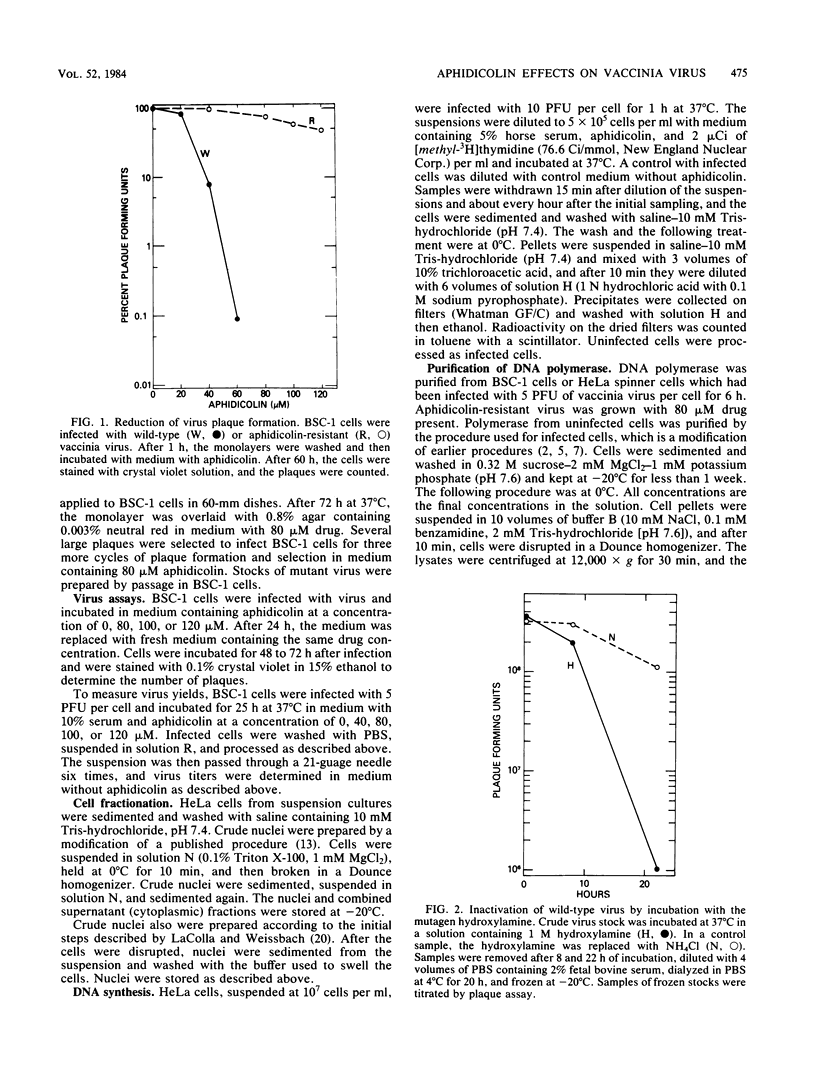
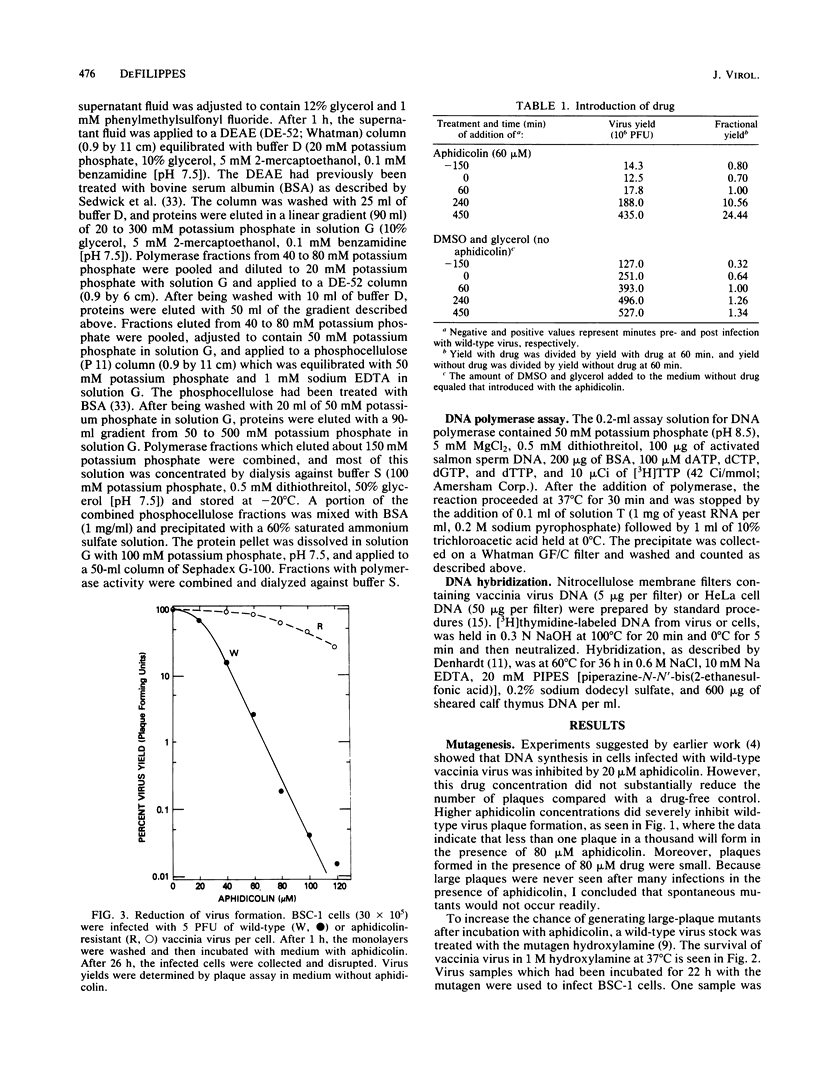
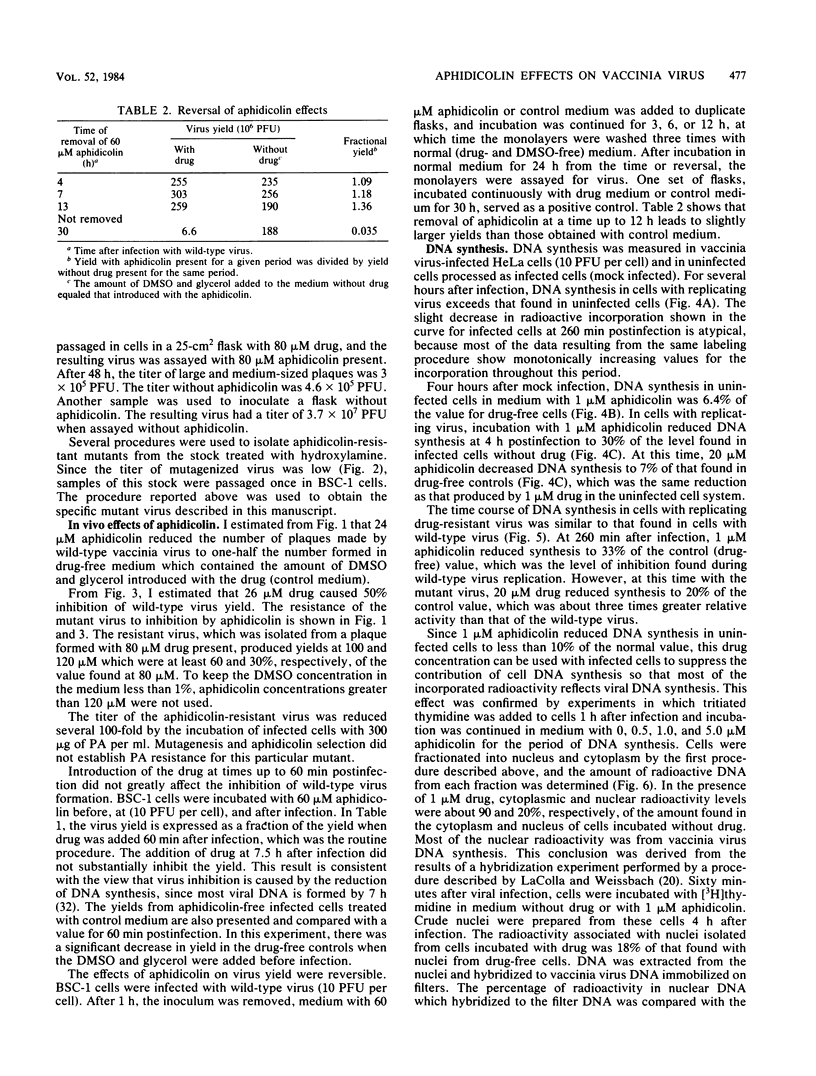
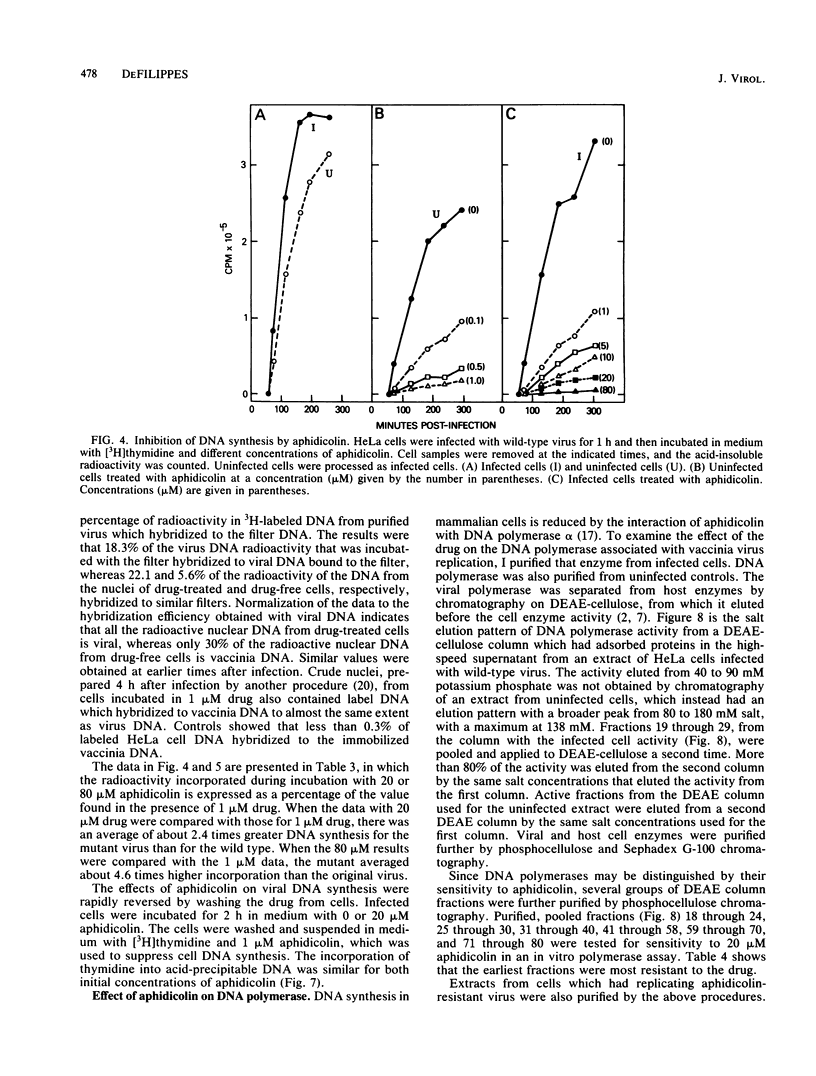
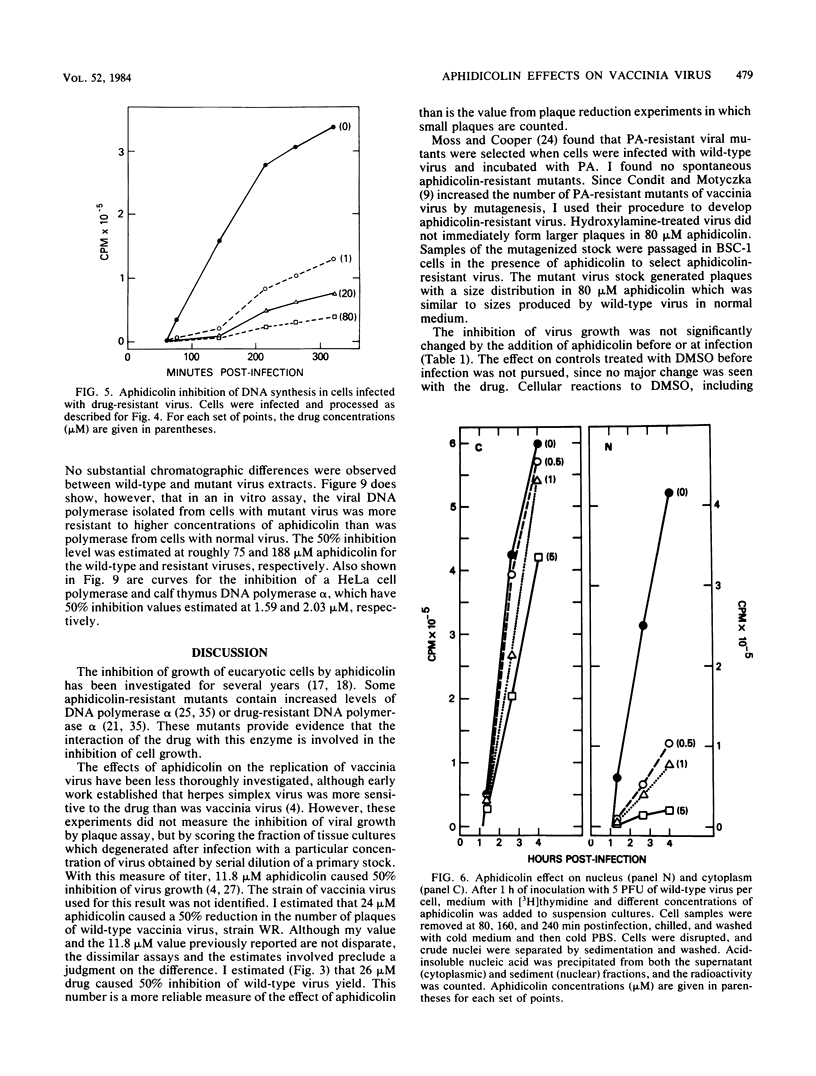
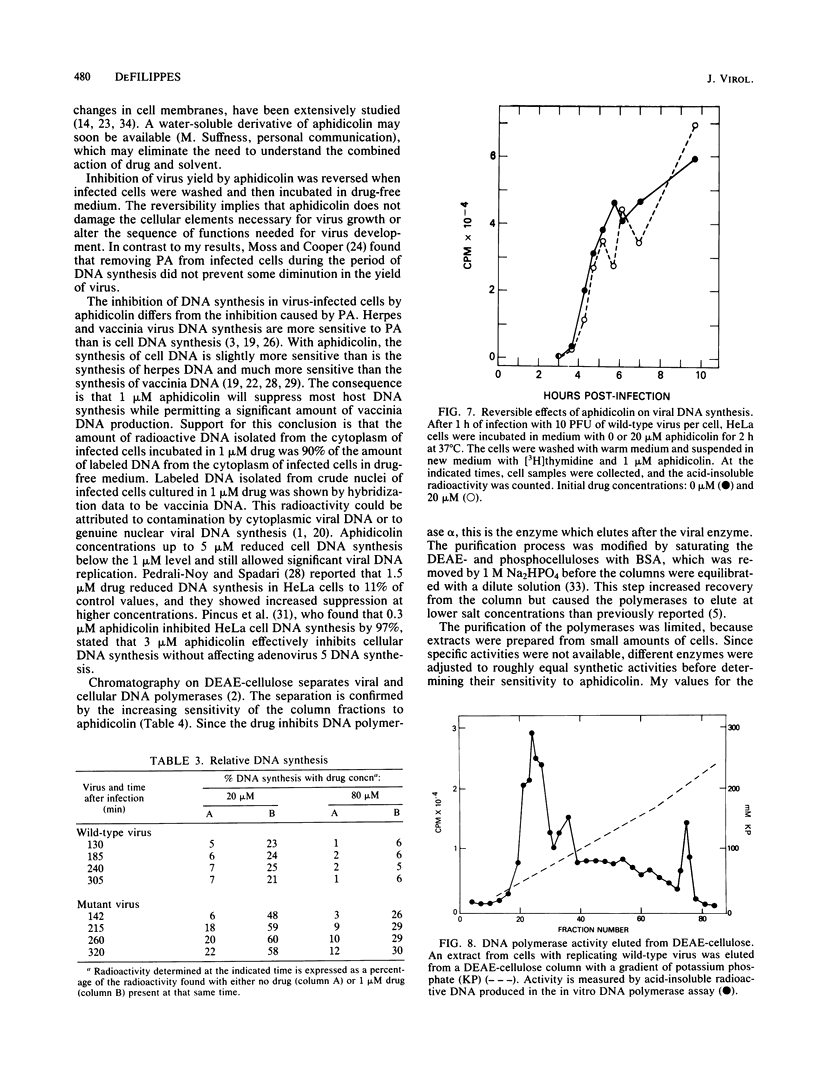
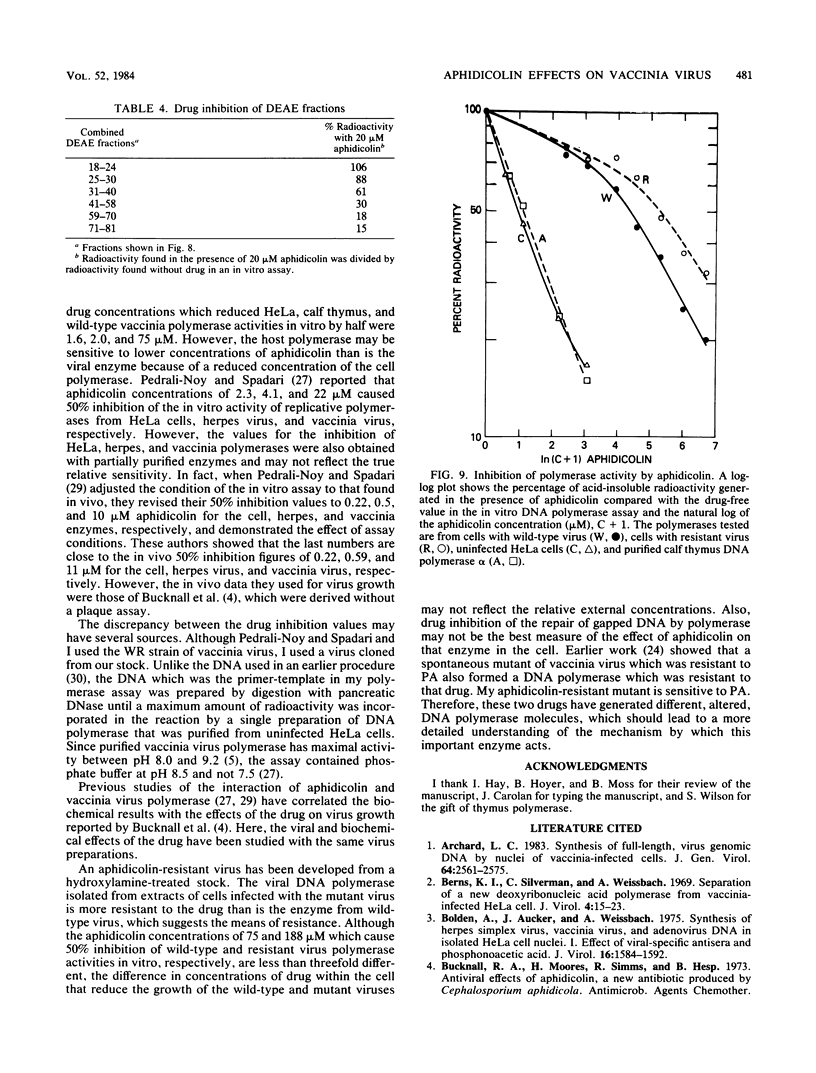
Vaccinia virus growth in BSC-1 and HeLa cells was inhibited by aphidicolin concentrations of 20 microM or more. Virus yield, which decreased only when the drug was added early in infection, was reduced several 100-fold by 80 microM aphidicolin. Viral inhibition was reversed by the suspension of the infected cells in drug-free medium. DNA synthesis in uninfected cells was reduced about 10-fold by 1 microM aphidicolin. In infected cells, aphidicolin concentrations over 10 microM were needed to reduce DNA synthesis to the same extent as in uninfected cells. Fractionation of infected cells which were incubated with 1 microM drug showed that cytoplasmic viral DNA synthesis was resistant to this aphidicolin concentration. The radioactivity associated with crude nuclei from these cells was estimated to be from vaccinia DNA synthesis. Spontaneous virus mutants which were resistant to 80 microM aphidicolin did not appear. However, after mutagenesis, mutants were generated which formed large plaques in medium with 80 microM drug. In cells with replicating aphidicolin-resistant virus, DNA synthesis was about four times more resistant to 80 microM aphidicolin than in cells with replicating wild-type virus. Chromatographic patterns of viral DNA polymerase isolated from cells with wild-type or resistant virus were similar. However, in an in vitro assay, 50% inhibition of enzyme activity was obtained with ca. 75 and 188 microM aphidicolin for the wild-type and resistant DNA polymerases, respectively. Viral enzymes were much more resistant to the drug than were the cell polymerases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C. Synthesis of full-length, virus genomic DNA by nuclei of vaccinia-infected HeLa cells. J Gen Virol. 1983 Dec;64(Pt 12):2561–2575. doi: 10.1099/0022-1317-64-12-2561. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Silverman C., Weissbach A. Separation of a new deoxyribonucleic acid polymerase from vaccinia-infected HeLa cells. J Virol. 1969 Jul;4(1):15–23. doi: 10.1128/jvi.4.1.15-23.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J Biol Chem. 1979 Aug 25;254(16):7812–7819. [PubMed] [Google Scholar]
- Chang C. C., Boezi J. A., Warren S. T., Sabourin C. L., Liu P. K., Glatzer L., Trosko J. E. Isolation and characterization of a UV-sensitive hypermutable aphidicolin-resistant Chinese hamster cell line. Somatic Cell Genet. 1981 Mar;7(2):235–253. doi: 10.1007/BF01567660. [DOI] [PubMed] [Google Scholar]
- Citarella R. V., Muller R., Schlabach A., Weissbach A. Studies on vaccinia virus-directed deoxyribonucleic acid polymerase. J Virol. 1972 Oct;10(4):721–729. doi: 10.1128/jvi.10.4.721-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. Restriction enzyme digests of rapidly renaturing fragments of vaccinia virus DNA. J Virol. 1975 Jan;17(1):227–238. doi: 10.1128/jvi.17.1.227-238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E., Dube D. K., Martin G. M., Loeb L. A. Mammalian mutator mutant with an aphidicolin-resistant DNA polymerase alpha. Proc Natl Acad Sci U S A. 1983 Feb;80(3):797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman G. H., Preisler H. D., Papahadjopoulos D. Membrane action of DMSO and other chemical inducers of Friend leukaemic cell differentiation. Nature. 1976 Jul 29;262(5567):361–363. doi: 10.1038/262360a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Cooper N. Genetic evidence for vaccinia virus-encoded DNA polymerase: isolation of phosphonoacetate-resistant enzyme from the cytoplasm of cells infected with mutant virus. J Virol. 1982 Aug;43(2):673–678. doi: 10.1128/jvi.43.2.673-678.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Yasuda H., Ikegami S., Ohashi M., Yamada M. Aphidicolin resistant mutant of which DNA polymerase alpha is induced by this drug. Biochem Biophys Res Commun. 1979 Dec 14;91(3):939–945. doi: 10.1016/0006-291x(79)91970-3. [DOI] [PubMed] [Google Scholar]
- Noy G. P., Weissbach A. HeLa cell DNA polymerases: the effect of cycloheximide in vivo and detection of a new form of DNA polymerase alpha. Biochim Biophys Acta. 1977 Jul 5;477(1):70–83. doi: 10.1016/0005-2787(77)90161-7. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Aphidicolin allows a rapid and simple evaluation of DNA-repair synthesis in damaged human cells. Mutat Res. 1980 May;70(3):389–394. doi: 10.1016/0027-5107(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Effect of aphidicolin on viral and human DNA polymerases. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1194–1202. doi: 10.1016/0006-291x(79)91106-9. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S., Robertson W., Rekosh D. Characterization of the effect of aphidicolin on adenovirus DNA replication: evidence in support of a protein primer model of initiation. Nucleic Acids Res. 1981 Oct 10;9(19):4919–4938. doi: 10.1093/nar/9.19.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. The DNA polymerases of KB cells. Methods Enzymol. 1974;29:89–102. doi: 10.1016/0076-6879(74)29012-8. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Sugino A., Nakayama K. DNA polymerase alpha mutants from a Drosophila melanogaster cell line. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7049–7053. doi: 10.1073/pnas.77.12.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]



