Abstract
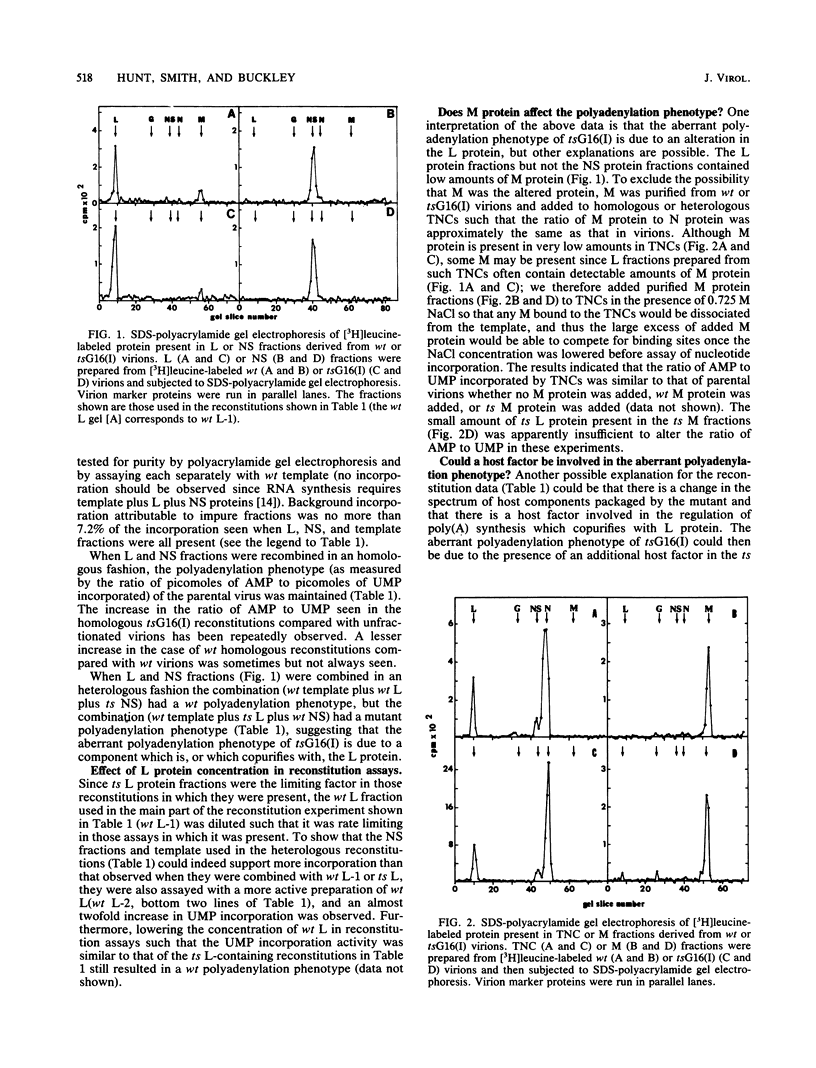
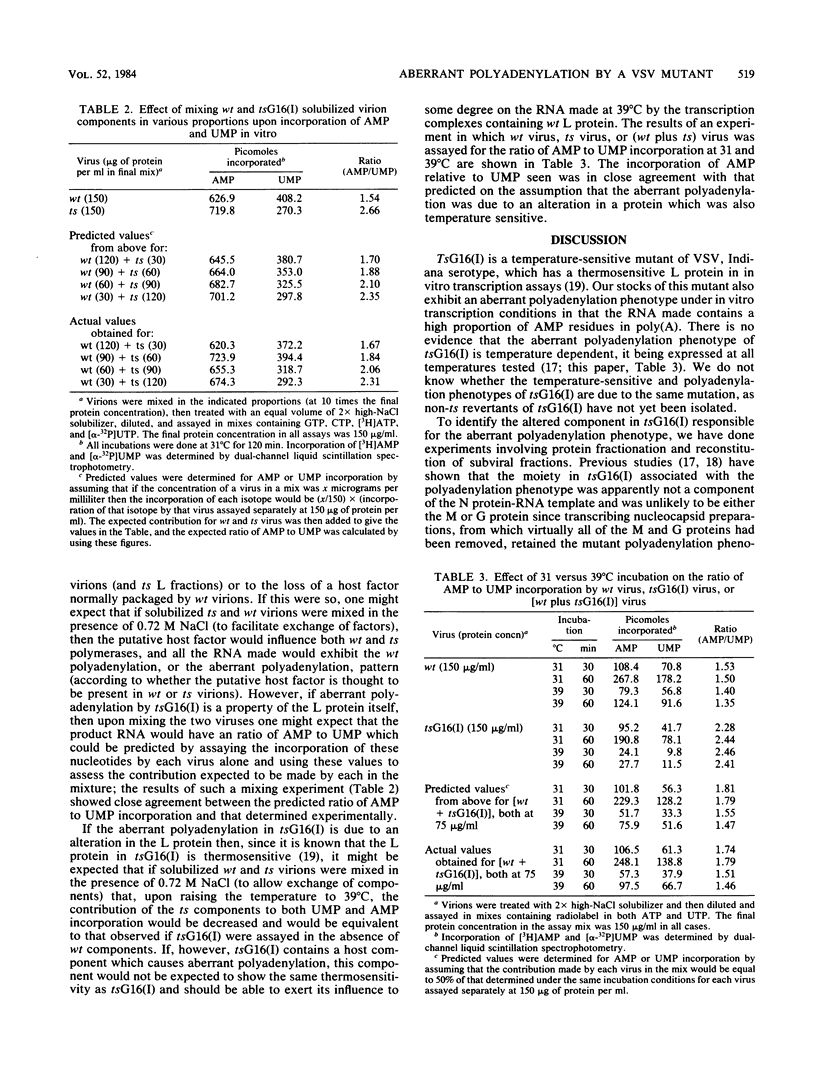
TsG16(I) is a temperature-sensitive mutant of vesicular stomatitis virus, Indiana serotype. Our stocks of this mutant overproduce polyadenylic acid in an in vitro transcription system. The overproduction of polyadenylic acid occurs at all temperatures tested (27, 31, 35, and 39 degrees C) and is apparently not due to an alternation in the N protein-RNA template. To characterize the altered moiety in tsG16(I) responsible for this phenotype, virions were fractionated and the polyadenylation phenotype in homologous and heterologous reconstitution assays was determined. The aberrant polyadenylation phenotype correlated with the presence of ts L protein but not ts NS or ts M protein fractions. Results of experiments in which solubilized tsG16(I) and wild-type virion components were mixed indicated that the altered moiety behaved as if present in stoichiometric amounts relative to active L protein. The effects of raising the temperature from 31 to 39 degrees C in such mixes were as would be predicted upon the assumption that the polyadenylation phenotype was associated with a thermosensitive transcriptase component [the L protein of tsG16(I) is known to be thermosensitive]. We conclude that the data strongly support the hypothesis that L is the altered protein responsible for the aberrant polyadenylation phenotype of tsG16(I).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch V., Banerjee A. K. Effect of temperature on the enzymatic activities present in purified virions of vesicular stomatitis virus. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1360–1367. doi: 10.1016/0006-291x(79)91130-6. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikami S. M., Moyer S. A. Host range mutants of vesicular stomatitis virus defective in in vitro RNA methylation. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7694–7698. doi: 10.1073/pnas.79.24.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Emerson S. U., Wagner R. R. RNA- temperature-sensitive mutants of vesicular stomatitis virus: L-protein thermosensitivity accounts for transcriptase restriction of group I mutants. J Virol. 1976 May;18(2):596–603. doi: 10.1128/jvi.18.2.596-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M. Vesicular stomatitis virus mutant with altered polyadenylic acid polymerase activity in vitro. J Virol. 1983 Jun;46(3):788–799. doi: 10.1128/jvi.46.3.788-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Location of the transcription defect in group I temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):28–35. doi: 10.1128/jvi.13.1.28-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. D., Emerson S. U. Use of a hybrid infectivity assay to analyze primary transcription of temperature-sensitive mutants of the New Jersey serotype of vesicular stomatitis virus. J Virol. 1982 Jul;43(1):37–40. doi: 10.1128/jvi.43.1.37-40.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. F., Lazzarini R. A. Synthesis of viral mRNA and polyadenylate by a ribonucleoprotein complex from extracts of VSV-infected cells. Cell. 1974 Sep;3(1):77–84. doi: 10.1016/0092-8674(74)90043-9. [DOI] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Keene J. D. Sequential synthesis of small capped RNA transcripts in vitro by vesicular stomatitis virus. Virology. 1983 Feb;125(1):206–218. doi: 10.1016/0042-6822(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Two methyltransferase activities in the purified virions of vesicular stomatitis virus. J Virol. 1977 Dec;24(3):786–793. doi: 10.1128/jvi.24.3.786-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Synthesis of poly(A) in vitro by purified virions of vesicular stomatitis virus. Nat New Biol. 1973 Nov 7;246(149):17–19. doi: 10.1038/newbio246017a0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]



