Abstract
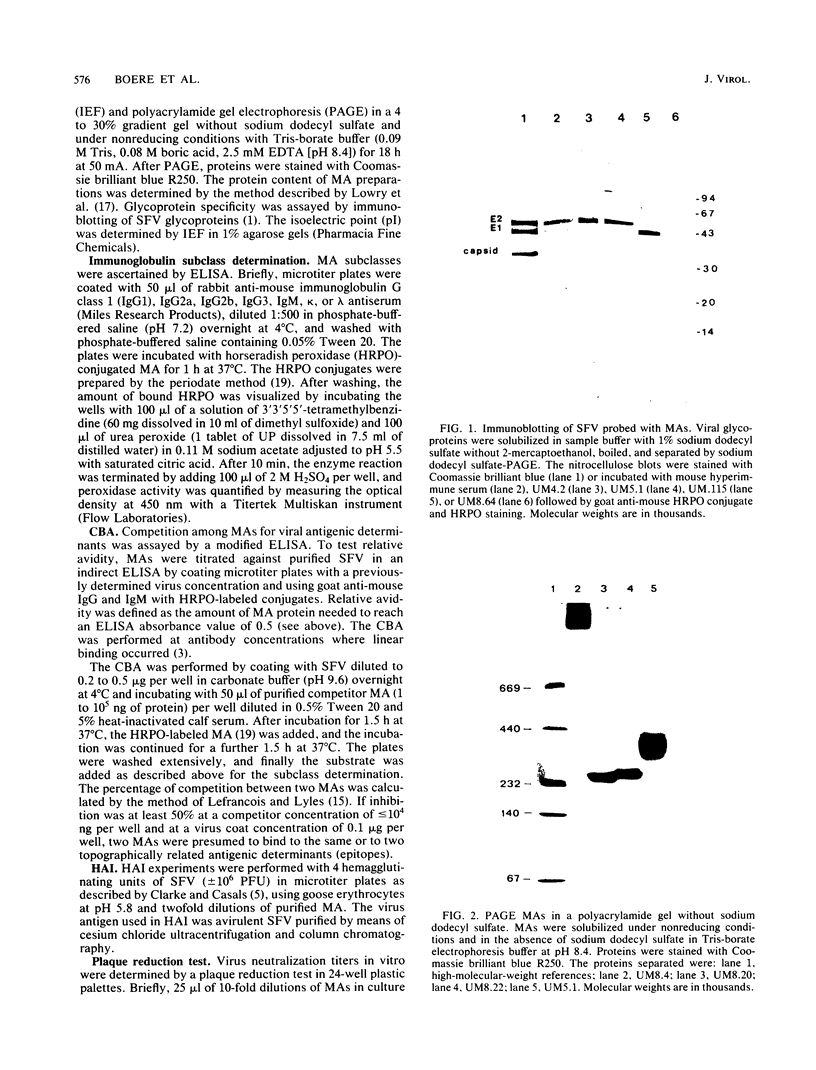
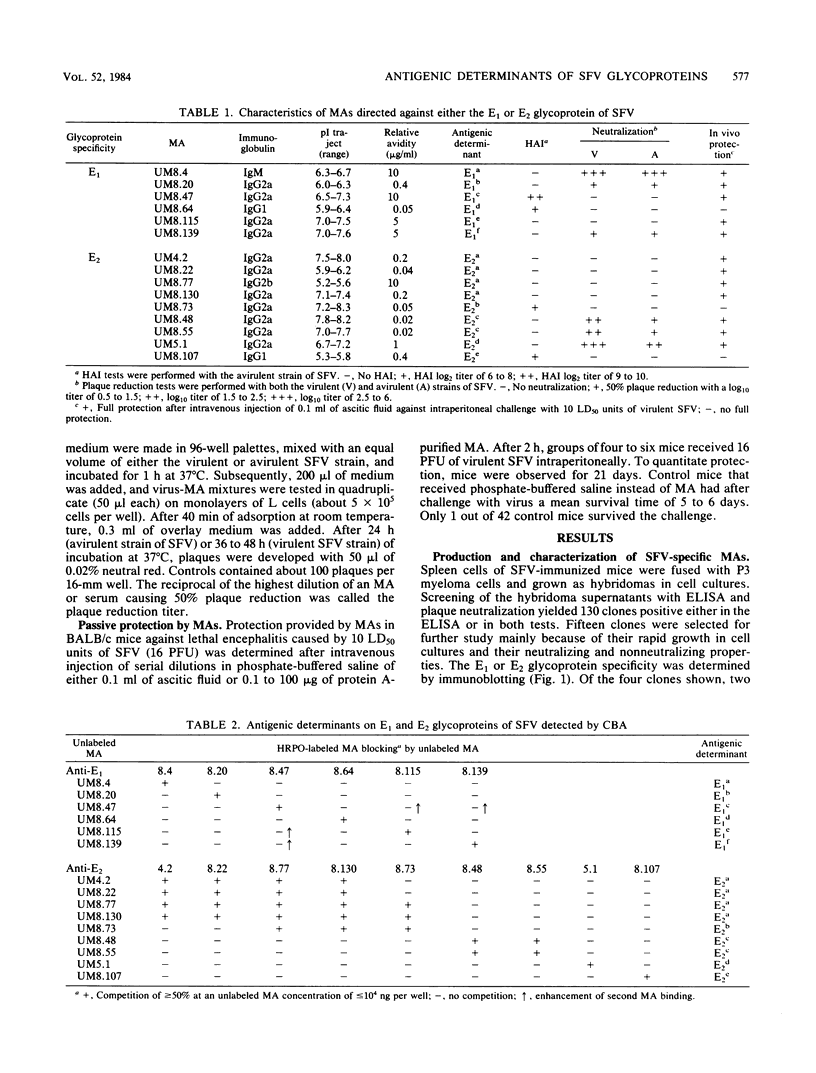
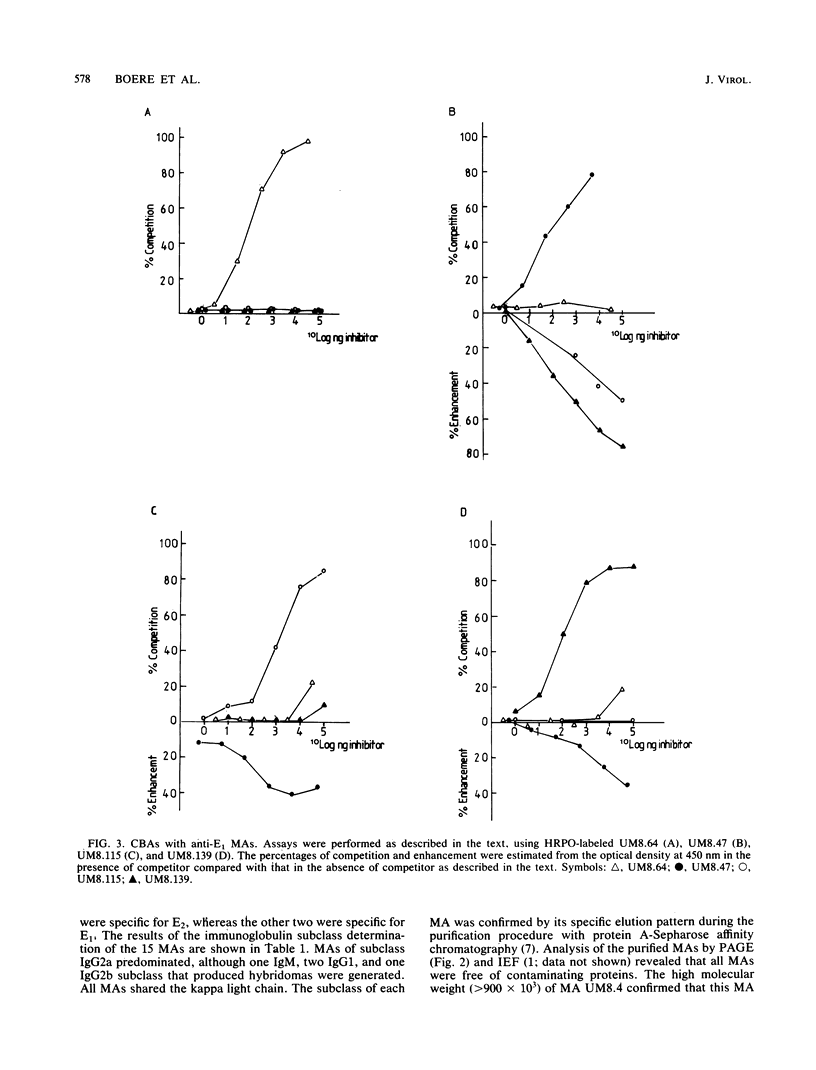
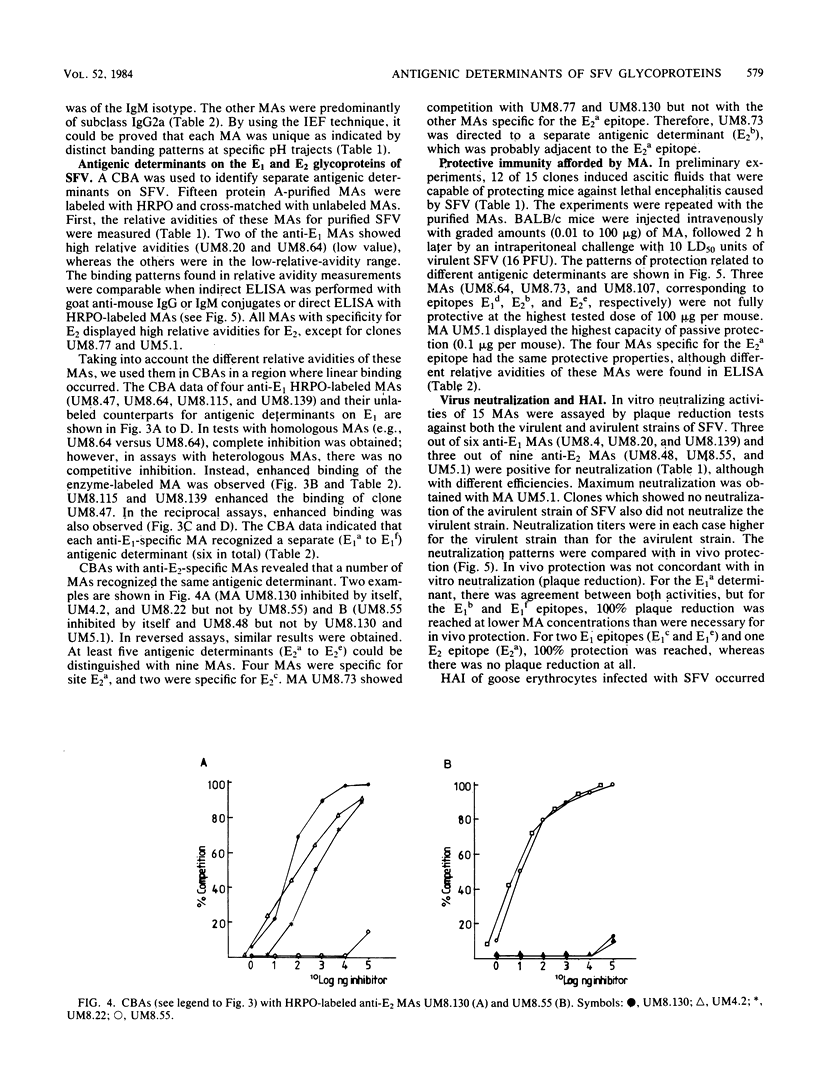
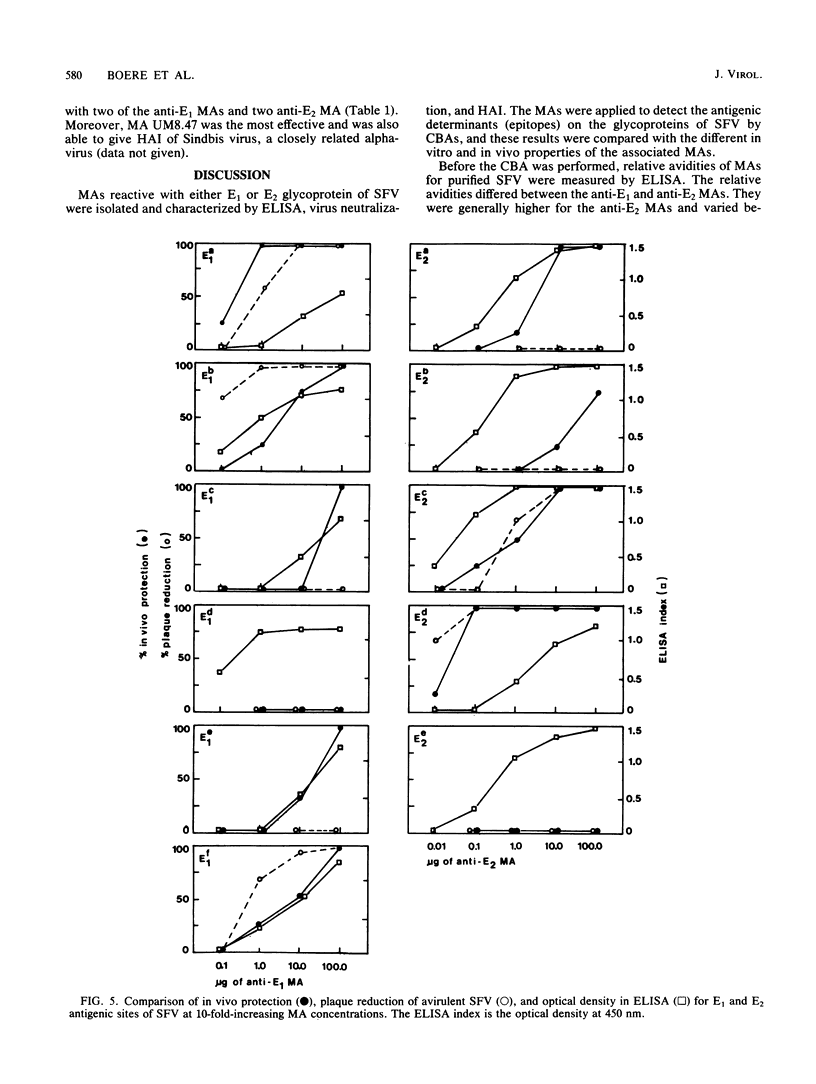
Fifteen monoclonal antibodies (MAs) directed against either the E1 or E2 glycoprotein of Semliki Forest virus (SFV) were characterized by immunoglobulin subclass, pI traject, hemagglutination inhibition, neutralization of infectious virus, and protection against virulent infection in mice. All MAs except UM8.4 (immunoglobulin M [IgM]) belonged to various subclasses of IgG and predominantly to IgG2a, but all were unique as indicated by their banding patterns in isoelectric focusing. Competitive binding assays with these MAs revealed the presence of at least six distinct antigenic determinants (epitopes) on the E1 glycoprotein and five epitopes on the E2 glycoprotein. Two of the epitopes on E1, as defined by the properties of the MAs, were associated with hemagglutination inhibition (E1c and E1d), three were associated with neutralization (E1a, E1b, and E1f), and five were associated in various degrees with protection (E1a, E1b, E1c, E1e, and E1f) of mice against virulent SFV infection. With the MAs against E2, the epitopes on E2 were similarly defined. Epitopes E2b and E2e were associated with hemagglutination inhibition, E2c and E2d were associated with neutralization, and three epitopes were associated with in vivo protection (E2a, E2c, and E2d). Furthermore, for each MA the relative avidity to purified SFV was determined with an enzyme-linked immunosorbent assay. The binding of some MAs to purified SFV was enhanced by a second MA. The relative avidities of individual MAs did not correlate with their neutralizing capacities. From the results, we suggest that the amino acid sequence which makes up determinant E2d and is recognized by the highly protective MA UM5.1 is an excellent candidate for the production of a synthetic vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., ter Meulen V. Relationships between monoclonal antibody-binding sites on the measles virus haemagglutinin. J Gen Virol. 1982 Nov;63(Pt 1):113–120. doi: 10.1099/0022-1317-63-1-113. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. H., Moyle W. R., Moustafa Z. A., Canfield R. E. Mixing two monoclonal antibodies yields enhanced affinity for antigen. J Immunol. 1982 Jun;128(6):2709–2713. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Riedel H. Structure and assembly of alphaviruses. Curr Top Microbiol Immunol. 1982;99:1–50. doi: 10.1007/978-3-642-68528-6_1. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Metselaar D., Kirya G. B., Timms G. L. Investigations into yellow fever virus and other arboviruses in the northern regions of Kenya. Bull World Health Organ. 1970;42(5):787–795. [PMC free article] [PubMed] [Google Scholar]
- Kingsford L., Ishizawa L. D., Hill D. W. Biological activities of monoclonal antibodies reactive with antigenic sites mapped on the G1 glycoprotein of La Crosse virus. Virology. 1983 Sep;129(2):443–455. doi: 10.1016/0042-6822(83)90182-4. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Harmsen M., Khader Boutahar-Trouw B. Cellular immunity against Semliki Forest virus in mice. Infect Immun. 1979 Feb;23(2):213–218. doi: 10.1128/iai.23.2.213-218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Harmsen M., Khader Boutahar-Trouw B. Delayed-type hypersensitivity against Semliki Forest virus in mice. Infect Immun. 1979 Feb;23(2):219–223. doi: 10.1128/iai.23.2.219-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. G., Adler W. H. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. I. Passive transfer of protection with immune serum and immune cells. J Immunol. 1973 May;110(5):1345–1353. [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Identification of infected cell-specific monoclonal antibodies and their role in host resistance to ocular herpes simplex virus type 1 infection. J Gen Virol. 1984 Mar;65(Pt 3):657–661. doi: 10.1099/0022-1317-65-3-657. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Kokubun K. M., Cole G. A. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983 Oct 15;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]