Abstract
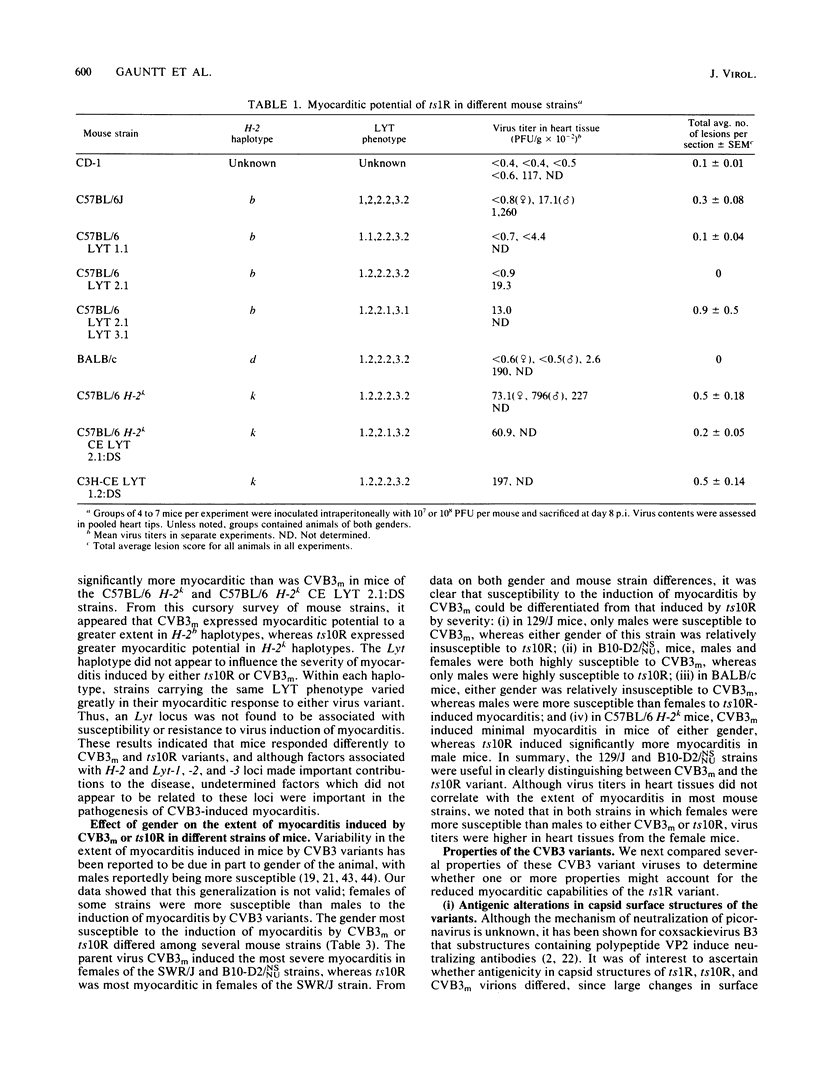
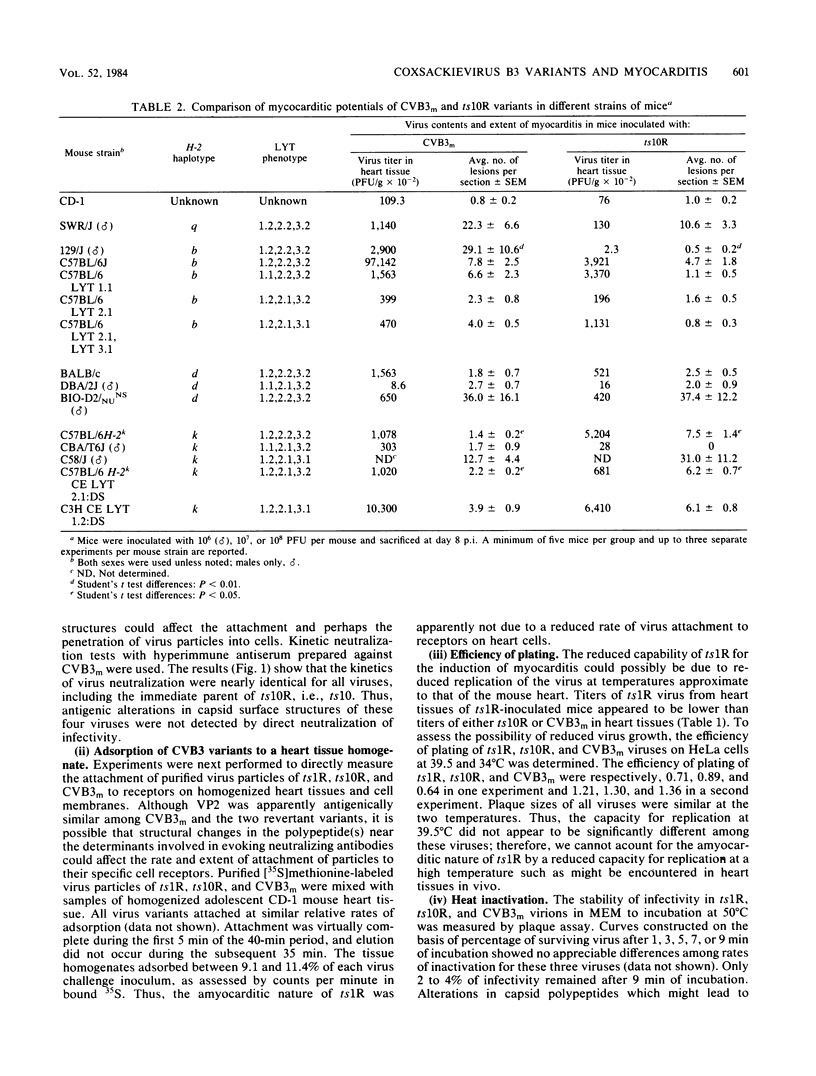
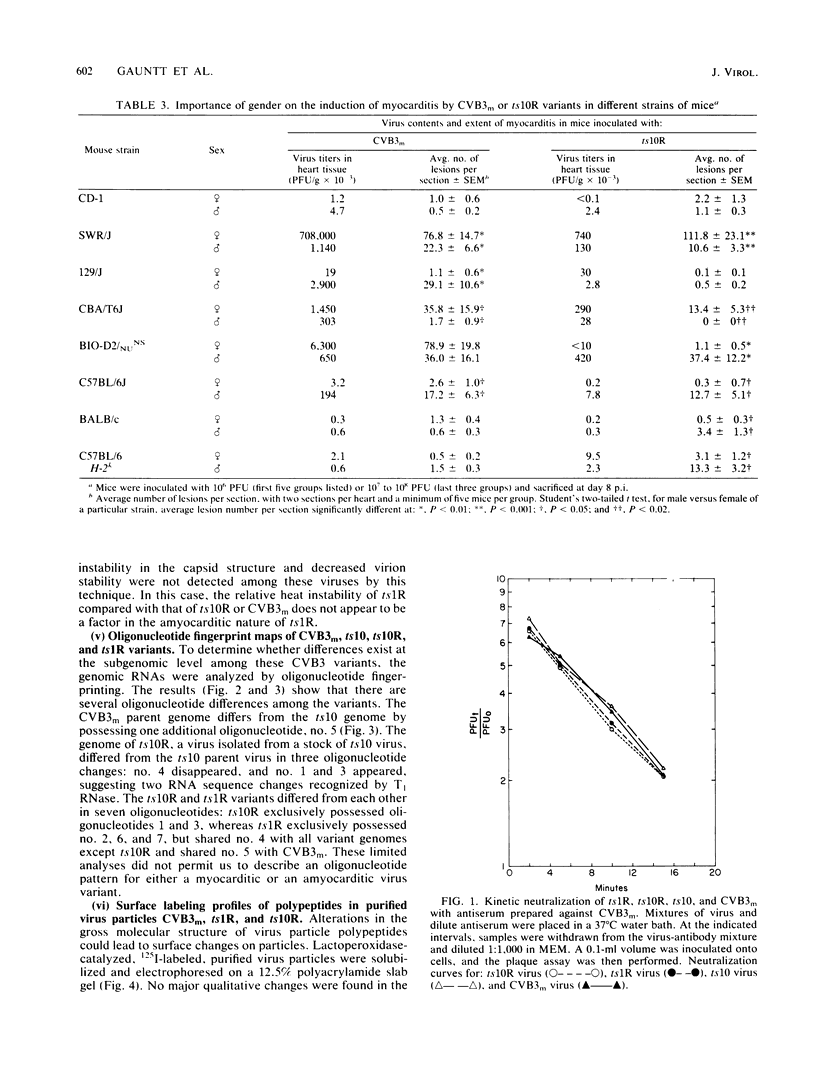
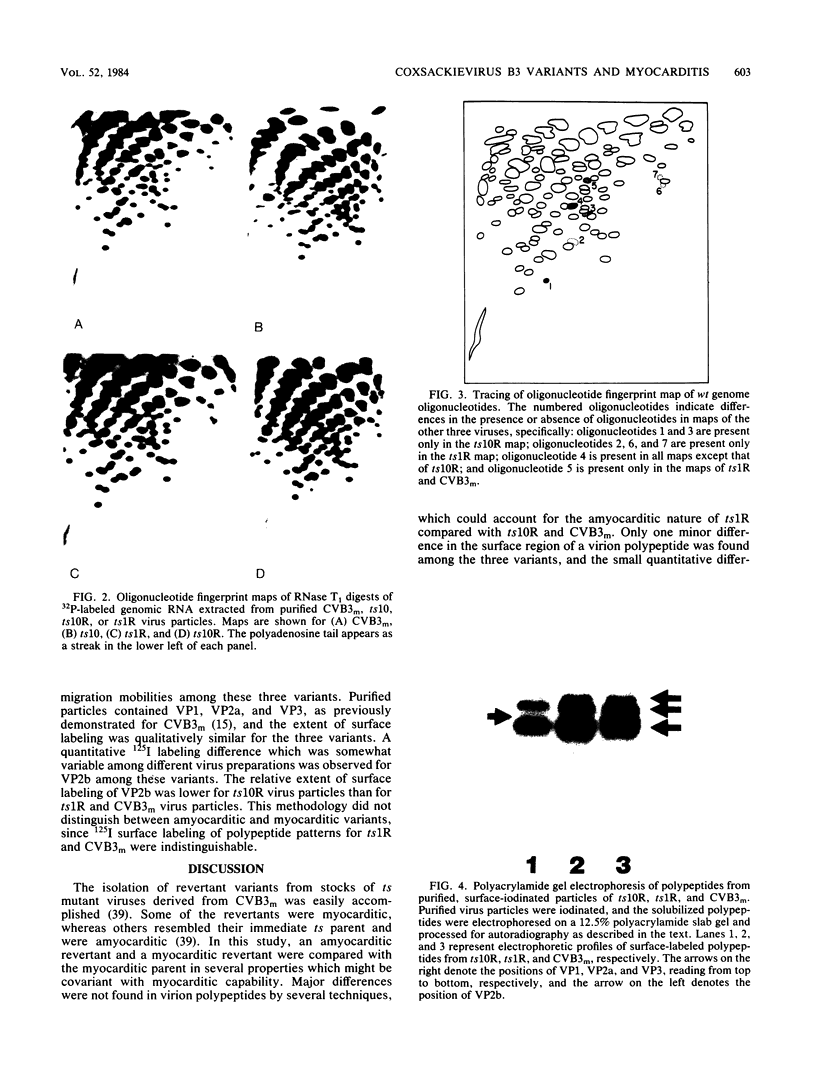
Two variants of coxsackievirus B3 (CVB3) were compared with the original myocarditic parent variant (CVB3m) for myocarditic properties in several strains of mice. The ts1R variant produced little to no myocarditis in any of the nine mouse strains examined. The ts10R variant and CVB3m could be differentiated on the basis of the extent of myocarditis induced in mice of selected H-2b and H-2k haplotypes and in the female versus the male responses of two other inbred strains. Virus quantities recovered from the hearts of myocarditic mice did not correlate with the extent of disease. The three variants could not be differentiated on the basis of: (i) rate and extent of adsorption to heart tissue homogenates, (ii) kinetic neutralization rates with antiserum directed against CVB3m, (iii) 125I labeling of surface regions of polypeptides on purified particles, or (iv) rates of heat inactivation of infectivity at 50 degrees C. These data suggest that differences in pathogenicity cannot be attributed to major alterations in capsid polypeptides. Oligonucleotide fingerprint maps of T1 RNase digests of the genomes of purified particles of the three CVB3 variants showed distinct differences. Thus, the extent of myocarditis induced by CVB3 variants in a mouse model is affected by some subtle expression of the genome, presumably not involving capsid polypeptides, as well as by the haplotype and sex of a given mouse host species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B. Genetics of resistance of animals to viruses: I. Introduction and studies in mice. Adv Virus Res. 1978;23:269–348. doi: 10.1016/S0065-3527(08)60102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatrice S. T., Katze M. G., Zajac B. A., Crowell R. L. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide, VP2. Virology. 1980 Jul 30;104(2):426–438. doi: 10.1016/0042-6822(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Ada G. L., Yap K. L. Functional and structural considerations in the recognition of virus-infected cells by cytotoxic T lymphocytes. Contemp Top Mol Immunol. 1978;7:319–371. doi: 10.1007/978-1-4757-0779-3_10. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D. The role of viruses in the production of heart disease. Am J Cardiol. 1972 Feb;29(2):231–240. doi: 10.1016/0002-9149(72)90634-0. [DOI] [PubMed] [Google Scholar]
- Cambridge G., MacArthur C. G., Waterson A. P., Goodwin J. F., Oakley C. M. Antibodies to Coxsackie B viruses in congestive cardiomyopathy. Br Heart J. 1979 Jun;41(6):692–696. doi: 10.1136/hrt.41.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. E., Loria R. M., Madge G. E. Coxsackievirus B cardiopathy and angiopathy in the hypercholesterolemic host. Atherosclerosis. 1978 Nov;31(3):295–306. doi: 10.1016/0021-9150(78)90065-5. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. A. High sensitivity to androgen as a contributing factor in sex differences in the immune response. Arthritis Rheum. 1979 Nov;22(11):1218–1233. doi: 10.1002/art.1780221109. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Hensley G. T., Rytel M. W. Post-coxsackie virus B3 myocardiopathy in mice. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):345–350. [PubMed] [Google Scholar]
- Frisby D. P., Newton C., Carey N. H., Fellner P., Newman J. F., Harris T. J., Brown F. Oligonucleotide mapping of picornavirus RNAs by two-dimensional electrophoresis. Virology. 1976 Jun;71(2):379–388. doi: 10.1016/0042-6822(76)90365-2. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., Lee J. C., Paque R. E. Preliminary characterization of coxsackievirus B3 temperature-sensitive mutants. J Virol. 1983 Mar;45(3):1037–1047. doi: 10.1128/jvi.45.3.1037-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudvangen R. J., Duffey P. S., Paque R. E., Gauntt C. J. Levamisole exacerbates coxsackievirus B3-induced murine myocarditis. Infect Immun. 1983 Sep;41(3):1157–1165. doi: 10.1128/iai.41.3.1157-1165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman S. Z., Hammer G. S. Coxsackie virus myopericarditis. A microbiological and clinical review. Am J Cardiol. 1974 Aug;34(2):224–232. doi: 10.1016/0002-9149(74)90201-x. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Auld K. R., Woodruff J. F. Sex-related differences in the rapid production of cytotoxic spleen cells active against uninfected myofibers during Coxsackievirus B-3 infection. J Immunol. 1981 Apr;126(4):1336–1340. [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Lysis of infected myofibers by coxsackievirus B-3-immune T lymphocytes. Am J Pathol. 1980 Mar;98(3):681–694. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Sex-related differences in the pattern of coxsackievirus B-3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect Immun. 1981 Apr;32(1):68–73. doi: 10.1128/iai.32.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., Crowell R. L. Immunological studies of the group B coxsackieviruses by the sandwich enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation. J Gen Virol. 1980 Oct;50(2):357–367. doi: 10.1099/0022-1317-50-2-357. [DOI] [PubMed] [Google Scholar]
- Kawai C., Matsumori A., Kumagai N., Tokuda M. Experimental Coxsackie virus B-3 and B-4 myocarditis in mice. Jpn Circ J. 1978 Jan;42(1):43–47. doi: 10.1253/jcj.42.43. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lansdown A. B. Viral Infections and diseases of the heart. Prog Med Virol. 1978;24:70–113. [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Levine H. D. Virus myocarditis: a critique of the literature from clinical, electrocardiographic, and pathologic standpoints. Am J Med Sci. 1979 Mar-Apr;277(2):132–143. [PubMed] [Google Scholar]
- Morens D. M. Enteroviral disease in early infancy. J Pediatr. 1978 Mar;92(3):374–377. doi: 10.1016/s0022-3476(78)80422-3. [DOI] [PubMed] [Google Scholar]
- Morris P. J., Pietsch M. C. Letter: A possible association between paralytic poliomyelitis and multiple sclerosis. Lancet. 1973 Oct 13;2(7833):847–848. doi: 10.1016/s0140-6736(73)90891-x. [DOI] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J. Assessment of cell-mediated immunity against coxsackievirus B3-induced myocarditis in a primate model (Papio papio). Infect Immun. 1981 Jan;31(1):470–479. doi: 10.1128/iai.31.1.470-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Roesing T. G., Landau B. J., Crowell R. L. Limited persistence of viral antigen in coxsackievirus B3 induced heart disease in mice. Proc Soc Exp Biol Med. 1979 Mar;160(3):382–386. doi: 10.3181/00379727-160-40455. [DOI] [PubMed] [Google Scholar]
- Swierkosz J. E., Marrack P., Kappler J. W. The role of H-2-linked genes in helper T cell function. V. I-region control of helper T cell interaction with antigen-presenting macrophages. J Immunol. 1979 Aug;123(2):654–659. [PubMed] [Google Scholar]
- Trent D. W., Clewley J. P., France J. K., Bishop D. H. Immunochemical and oligonucleotide fingerprint analyses of Venezuelan equine encephalomyelitis complex viruses. J Gen Virol. 1979 May;43(2):365–381. doi: 10.1099/0022-1317-43-2-365. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Nealon T., Gauntt C. J. Assessment of coxsackievirus B3 ts mutants for induction of myocarditis in a murine model. Infect Immun. 1979 Feb;23(2):486–495. doi: 10.1128/iai.23.2.486-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C., Gelsthorpe K., Doughty R. W. A relation between HLA antigens and clinical features in patients with acquired valvular heart disease. Br Med J. 1976 Jun 19;1(6024):1499–1501. doi: 10.1136/bmj.1.6024.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Madge G. E. The role of host genetics in the pathogenesis of coxsackievirus infection in the pancreas of mice. J Infect Dis. 1980 Jan;141(1):47–54. doi: 10.1093/infdis/141.1.47. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. III. Role of sex. J Immunol. 1977 Aug;119(2):591–597. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus tb-3 infection. II. Characterization of effector cells and demonstration cytotoxicity against viral-infected myofibers1. J Immunol. 1977 Apr;118(4):1165–1169. [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Dunlop M. B., Blanden R. V., Doherty P. C., Shreffler D. C. H-2 compatibility requirement for virus-specific T-cell-mediated cytolysis. Evaluation of the role of H-2I region and non-H-2 genes in regulating immune response. J Exp Med. 1976 Aug 1;144(2):519–532. doi: 10.1084/jem.144.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]